Abstract
Introduction
This study aimed to understand the impact of the coronavirus disease 2019 (COVID-19) epidemic on the distribution and antibiotic resistance of pathogenic bacteria isolated from the lower respiratory tract of children in our hospital.
Methods
Antimicrobial susceptibility tests were performed on bacteria isolated clinically from the lower respiratory tracts of children in our hospital from 2018 to 2021 by the Kirby–Bauer method and automated systems.
Results
From 2018 to 2021, the top three lower respiratory tract clinical isolates in our hospital were Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae. These three species showed obvious seasonal epidemic patterns, and their numbers decreased significantly during the COVID-19 epidemic, from 4559 in 2019 to 1938 in 2020. Bacterial resistance to antibiotics also changed before and after the COVID-19 epidemic. The annual proportions of methicillin-resistant S. aureus (MRSA) were 41%, 37.4%, 26.2%, and 29.8%. The resistance rates of Klebsiella pneumoniae to ceftriaxone were 40.5%, 51.9%, 35.3%, and 53.3%, and the detection rates of carbapenem-resistant K. pneumoniae (CRKP) were 2.7%, 11.1%, 5.9%, and 4.4%. The detection rates of β-lactamase-producing H. influenzae were 51.9%, 59.2%, 48.9%, and 55.3%. The rate of MRSA, ceftriaxone-resistant K. pneumoniae, CRKP, and β-lactamase-producing H. influenzae decreased significantly in 2020 compared with 2019, whereas that of carbapenem-resistant P. aeruginosa and carbapenem-resistant A. baumannii increased. The detection rates of β-lactamase-negative ampicillin-resistant H. influenzae (BLNAR) gradually increased over the 4 years.
Conclusions
Protective measures against COVID-19, including reduced movement of people, hand hygiene, and surgical masks, may block the transmission of S. pneumoniae, H. influenzae, and M. catarrhalis and reduce the detection rate of MRSA, ceftriaxone-resistant K. pneumoniae, CRKP, and β-lactamase-producing H. influenzae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
According to reports, lower respiratory tract infections ranked second of the top ten causes of disability-adjusted life-years in children under 10 in 2019. Our previous surveillance results showed that S. pneumoniae, H. influenzae, and M. catarrhalis have been the most frequently detected in our hospital. |
The aim of this study is to understand the regularity of the prevalence of common lower respiratory tract infections among children in Shenzhen, and the impact of the strict protection in Shenzhen against COVID-19 on the distribution and resistance of these bacteria. |
What was learned from the study? |
S. pneumoniae, M. catarrhalis, and H. influenzae have obvious seasonal epidemic patterns, and, during the COVID-19 epidemic in 2020, their number dropped significantly compared to 2019 (S. pneumoniae: 801 vs. 1 545; M. catarrhalis: 783 vs. 1 273; H. influenzae: 354 vs. 1 741), the detection rates of β-lactamase-positive H. influenzae, ceftriaxone-resistant K. pneumoniae, MRSA, and CRKP also decreased. However, compared to 2020, antimicrobial resistance of most bacteria in 2021 still continue to rise, despite normalized prevention and control measures. |
We can block the spread of common bacteria through protective measures during the epidemic season of community-acquired lower respiratory tract. However, to block the spread of nosocomial-acquired lower respiratory tract infection bacteria (including multidrug-resistant bacteria), it is still necessary to strengthen the prevention and control of nosocomial infection and the rational use of antibiotics for a long time. |
Introduction
Since the coronavirus disease 2019 (COVID-19) outbreak in late 2019, the global public health system has faced unprecedented challenges, and different countries have instituted different anti-epidemic policies. China implemented strict anti-epidemic policies; for example, Wuhan’s lockdown measures from January 23 to April 8, 2020 blocked large-scale spread of the COVID-19 epidemic in China. Shenzhen also invoked strong epidemic prevention and control measures, including strict implementation of temperature monitoring, working remotely from home, suspending school classes, staying home unless otherwise necessary, washing hands frequently, and wearing surgical masks. The above measures may effectively block the transmission of infectious diseases and change the distribution of pathogenic bacteria. As the only large-scale public children's hospital in Shenzhen, we have observed that the distribution and antibiotic resistance of pathogens isolated from the lower respiratory tract in our hospital has changed since the outbreak. Therefore, we here report data for the periods January 2018–December 2019 (before the COVID-19 epidemic), January 2020–May 2020, and May 2021–June 2021 (during the COVID-19 epidemic), and June 2020–April 2021 and July 2021–December 2021 (normalized COVID-19 epidemic prevention and control) to help understand and block the spread of bacteria causing lower respiratory tract infections in children.
Methods
Collection and Identification of Strains
Culture-positive clinical isolates isolated from lower respiratory tract specimens submitted for clinical inspection in our hospital from January 2018 to December 2021 were collected. All patients included in this study were not co-infected with COVID-19. Repeat isolates of the same species from the same patient were excluded. The samples collected as part of the study standard care/procedure, and the study protocol was approved by the Ethical Committee of Shenzhen Children’s Hospital (number: 201601304). Specimen collection and transportation and bacterial culture procedures were performed according to the Clinical Microbiology Procedures Handbook [1]. Bacterial identification was performed using a VITEK 2-Compact and VITEK MS from bioMérieux, France.
Antimicrobial Susceptibility Test Materials
Antibiotic disks were purchased from OXOID, UK. Penicillin E test strips were purchased from Antu Bio, China. The VITEK 2-Compact uses the companion GN13, GN334, GN335, GP67, GP639, and GP68 antimicrobial susceptibility cards produced by bioMérieux, France. Mueller–Hinton (MH) agar containing 5% defibrillated sheep blood and HTM agar were purchased from Guangzhou Dijing.
Antimicrobial Susceptibility Test Methods
Antimicrobial susceptibility testing for Streptococcus pneumoniae, Streptococcus agalactiae, Staphylococcus aureus, Enterobacteriaceae, and nonfermenting Gram-negative bacilli was carried out using the VITEK 2-Compact automated system, the Kirby–Bauer method, or the E test method as a supplement. The MIC value of S. pneumoniae for penicillin was determined with an E test strip. Antimicrobial susceptibility testing for Haemophilus influenzae and Moraxella catarrhalis was according to the Kirby–Bauer method. Extended-spectrum lactamases (ESBL) of Klebsiella pneumoniae were detected using a VITEK 2-Compact, and β-lactamases of H. influenzae were detected using the nitrocefin-based test. Antimicrobial susceptibility testing and result interpretation were conducted according to Clinical and Laboratory Standards Institute standards [2, 3], and according to the US Food and Drug Administration breakpoint standard [4], to interpret the results of tigecycline antimicrobial susceptibility test, and cefoperazone–sulbactam results were interpreted according to the breakpoint standard of cefoperazone [5]. Carbapenem-resistant Enterobacteriaceae (CRE) is defined as resistance to imipenem, meropenem, or ertapenem, while carbapenem-resistant Morganella spp. Proteus spp., and Providencia spp. are defined as resistance to carbapenems, except for imipenem [6].
Quality Control Strains
S. aureus ATCC 25923 (Kirby-Bauer method) and ATCC 29213 (automated systems), Escherichia coli ATCC 25922 and ATCC 35218, Pseudomonas aeruginosa ATCC 27853, S. pneumoniae ATCC 49619, H. influenzae ATCC 49247 and ATCC 49766, Enterococcus faecalis ATCC 29212, and K. pneumoniae ATCC 700603 were used as quality control strains.
Statistical Analysis
Statistical analysis of the data obtained was performed using WHONET 5.6 software.
Results
Bacterial Distribution
From 2018 to 2021, a total of 18,965 clinical strains were isolated from lower respiratory tract samples in our hospital, with only 2293 isolated in 2020; among them, 11,645 strains (61.4%) were isolated from male children and 7320 strains (38.6%) were isolated from female children; 15,862 strains (83.6%) were detected in 0–3 year olds, and 2486 strains (13.1%) were detected in 3–6 year olds; 1385 (7.3%) were isolated from outpatient and emergency cases, 852 (4.5%) from intensive care unit (ICU) cases, and 16,728 strains (88.2%) from non-ICU hospitalization cases.
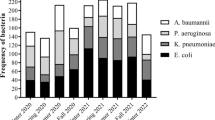
The top five bacteria with the most significant number of clinical isolates in the 4 years were S. pneumoniae, M. catarrhalis, H. influenzae, S. aureus, and P. aeruginosa (Table 1). The peak period of the number of lower respiratory tract pathogenic bacteria isolated every year was November, December, or January, and the number of isolated strains in February was significantly lower than that in January and March. Except for 2020, the trough period in the other 3 years was in July, August, or September. The number of isolates of S. pneumoniae, M. catarrhalis, and H. influenzae decreased significantly from January 2020 to May 2020 (during the COVID-19 epidemic).
Compared with 2018 and 2019, the number of lower respiratory tract isolates during the new wave of the COVID-19 outbreak in Shenzhen from May to June 2021 was significantly lower. The number of M. catarrhalis isolates detected began to increase rapidly in September every year, reached a peak in November or December, decreased slowly, and then dropped to the lowest level in August of the following year. Compared with M. catarrhalis, the number of H. influenzae isolates detected increased slightly later: it began to rise in December every year, reached a first peak in January of the following year, declined in February, reached a second peak in March, April or May, and then began to decline, reaching the lowest point in September, October, or November. The trend for S. pneumoniae was similar to that for H. influenzae and M. catarrhalis but was more complex, exhibiting a greater number of peaks (Fig. 1).
Resistance Rates of Common Bacteria to Antibiotics
Based on the bacterial distribution described above, we then determined antimicrobial resistance rates for the main or notable pathogens detected.
Antimicrobial Resistance Rates of Streptococcus pneumoniae and Staphylococcus aureus
The resistance rates of S. pneumoniae to erythromycin, clindamycin, and tetracycline were maintained at a high level (> 85%) over the 4 years; no penicillin-, moxifloxacin-, ertapenem-, vancomycin- or linezolid-resistant strains were detected, and the resistance rates detected in each year to all the antibiotics tested were the same (Table 2). In contrast, the detection rates of methicillin-resistant S. aureus (MRSA) among S. aureus strains were 41%, 37.4%, 26.2%, and 29.8% for each of the 4 years. The resistance rate of S. aureus to penicillin G, oxacillin and erythromycin in 2020 was lower than that in the other 3 years, that to erythromycin and clindamycin among MRSA in 2020 was lower than that in the other 3 years, and that of MRSA in 2020 to rifampicin was higher than that in the other 3 years. Additionally, methicillin-sensitive S. aureus (MSSA) showed similar resistance rates to various antibiotics over the 4 years, with no S. aureus strains isolated being found to be resistant to vancomycin or linezolid (Table 3).
Antimicrobial Resistance Rates of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii
The rates of ESBL detection in K. pneumoniae in our hospital were 35.1%, 44.4%, 28.1%, and 48.8% for 2018, 2019, 2020, and 2021, respectively; the rates of resistance to ceftriaxone were 40.5%, 51.9%, 35.3%, and 53.3%, and the rates of carbapenem-resistant K. pneumoniae (CRKP) detection were 2.7%, 11.1%, 5.9%, and 4.4%. The rates of K. pneumoniae resistance to cefuroxime and ceftriaxone were both > 35%, but resistance rates to β-lactam/β-lactamase inhibitor combinations were all < 40%, and those to carbapenems were all < 20%. The rates of K. pneumoniae resistance detected in 2020 to tested antibiotics other than amikacin were significantly lower than those in 2019 (Table 4).
Detection rates of carbapenem-resistant P. aeruginosa (CRPA) and carbapenem-resistant A. baumannii (CRAB) among isolates of these species were 10.2%, 6.6%, 14.5%, 11.3%, and 11.4%, 5.9%, 11.1%, and 21.2% in each of the 4 years, and that of CRAB in 2021 increased significantly compared with that in 2020. The resistance rates of P. aeruginosa and A. baumannii to all tested antibiotics were < 15% and < 25%, respectively (Table 5).
Antimicrobial Resistance Rates of Haemophilus influenzae and Moraxella catarrhalis
Rates of β-lactamase-producing H. influenzae detection were 51.9%, 59.2%, 48.9%, and 55.3% in the 4 years evaluated. The detection rates of β-lactamase-negative ampicillin-resistant H. influenzae (BLNAR) increased slowly each year over the 4 years, at 12.8%, 13.9%, 17.8%, and 18.5%. The resistance rates of H. influenzae to ampicillin and trimethoprim/sulfamethoxazole were approximately 70%, to ampicillin–sulbactam and cefuroxime approximately 50%, and to cefotaxime < 10%. Rates of nonsusceptibility to azithromycin among β-lactamase-producing H. influenzae strains were significantly higher than those among non-β-lactamase-producing strains. One and three levofloxacin-nonsusceptible H. influenzae strains were detected in 2020 and 2021, respectively (Table 6). The nonsusceptibility rates of M. catarrhalis to erythromycin were approximately 20–30%, and those to trimethoprim-sulfamethoxazole and tetracycline were both < 5%; no amoxicillin-clavulanate acid resistant strains were detected (Table 7).
Discussion
To explore how COVID-19 measures may have impacted pathogen distribution, we examined lower respiratory tract specimens from pediatric patients at our hospital for the presence of various bacteria. According to reports [7], infectious diseases accounted for six of the top ten causes of disability-adjusted life-years in children under 10 in 2019, of which lower respiratory tract infections ranked second. New infectious disease pathogens often emerge, and previous infectious disease pathogens often resurface. Since 2017, we have been working on the distribution of clinically isolated bacteria in children in Shenzhen as well as on monitoring antimicrobial resistance. Previous research has shown that S. pneumoniae, H. influenzae, and M. catarrhalis have always been among our hospital's top three bacterial isolates [8, 9]. The present study indicates that these three bacteria are still common pathogens causing community-acquired lower respiratory tract infections in children in Shenzhen, with apparent seasonal patterns. From January 2020 to May 2020 (during the COVID-19 epidemic) and May 2021 to June 2021 (a new round of the COVID-19 epidemic in Shenzhen), the previous epidemic patterns of the three bacteria were broken, with all showing their lowest values over 4 years. This may be because the strengthened prevention against COVID-19 in Shenzhen (including working remotely from home, suspending school classes, staying home unless otherwise necessary, washing hands frequently, and wearing surgical masks) blocked the spread of these three bacteria. Among them, since January–May 2020 (during the COVID-19 epidemic) is the peak season of H. influenzae in previous years, the number of H. influenzae (354) detected in 2020 has dropped to the lowest level in 4 years, but the peak period of the prevalence of S. pneumoniae and M. catarrhalis is not during this period. As a result, the proportion of H. influenzae detected in 2020 is 15.4%, which is significantly lower than that in 2018, 2019, and 2021 (29.1–35.2%). Related studies of some viruses and Mycoplasma pneumoniae infection have reported similar results [10]. The number of pathogenic bacteria isolated from the lower respiratory tract in February in each of the 4 years evaluated was significantly lower than that in January. The main reason may be that the traditional Spring Festival in China tends to occur in February. Shenzhen is a large immigrant city, and many people return to their hometowns during the traditional Spring Festival, and, because many families leave Shenzhen for the holiday, there are fewer children, including those with respiratory tract infections, in the city during this period. During the COVID-19 epidemic, the detection rates of P. aeruginosa and A. baumannii, which mainly cause nosocomial pneumonia [11], did not decrease significantly. Previous studies have shown that these two bacteria are often colonized in hospital plumbing and medical environments, spreading through contact to cause nosocomial infections in ICU patients [12, 13]. Respiratory protection measures against COVID-19 are usually not feasible in ICU patients, and standard hand hygiene, healthcare environment disinfection, and contact isolation measures may be more critical for reducing nosocomial infections of these two bacteria. The distribution of bacteria indicates that, during the COVID-19 epidemic, strict implementation of adequate protective measures may have effectively reduced the spread of bacteria common in community-acquired lower respiratory infections, but that these measures may not have been effective against nosocomially-transmitted pathogens.
Bacteria are important components of infectious disease pathogens, and their antibiotic resistance is of concern. Additionally, developing new antibiotics usually requires considerable time and cost, which constrains clinicians with regard to treating many patients infected with multidrug-resistant bacteria. Due to the toxic side effects of antibiotics, the use of antibiotics in pediatrics is limited, and treating multidrug-resistant infections in children is more challenging than it is in adults. The results of our study show that S. pneumoniae in children with lower respiratory tract infections in Shenzhen maintains high sensitivity to penicillin, which can be used as the first choice for clinical treatment. The detection rate of MRSA also exhibited a downward trend, similar to the China antimicrobial surveillance network (CHINET) report [14], at less than 30% in the past 2 years. The resistance mechanism of H. influenzae to β-lactam antibiotics is mainly due to TEM or BOR-producing β-lactamase and decreased affinity caused by mutation of the ftsI gene, which encodes penicillin-binding protein 3 [15, 16]. The proportion of β-lactamase-producing H. influenzae isolated in our hospital was approximately 50–60%, and the resistance rate to ampicillin exceeded 60%. However, BLNAR, which exploits the decrease in affinity caused by ftsI gene mutation as the β-lactam antibiotic resistance mechanism, is resistant to a variety of β-lactam antibiotics [15, 16], and its detection rate increased slowly from 12.8% in 2018 to 18.5% in 2021. The resistance mechanism of M. catarrhalis to β-lactam antibiotics is mainly the production of BRO β-lactamase [17], and no amoxicillin-clavulanic acid-resistant strains were detected.
The situation of antibiotic resistance among the Enterobacteriaceae and nonfermenting Gram-negative bacilli is severe. The Centers for Disease Control and Prevention report on the threat of antibiotic resistance listed CRE and CRAB as urgent threats and ESBL-producing Enterobacteriaceae, multidrug-resistant P. aeruginosa, and MRSA as serious threats in 2019 [18]. The surveillance results of CHINET showed that the resistance rates in China of K. pneumoniae and A. baumannii to imipenem increased from 3% and 32.9% in 2005 to 25% and 77.1% in 2018, respectively, and that of P. aeruginosa to imipenem was also approximately 30% [14]. From the perspective of bacterial resistance changes in our hospital, rates of β-lactamase-producing H. influenzae, ceftriaxone-resistant K. pneumoniae, MRSA, and CRKP detection in 2020 were lower than those in 2019, which may be related to the COVID-19 epidemic; however, rates of CRPA and CRAB detection were higher in 2020 than in 2019 (14.5% vs. 6.6% and 11.1% vs. 5.9%), and the rate of CRAB detection continued to rise to 21.2% in 2021. Although the detection rates of CRKP, CRPA, and CRAB were significantly lower than those reported by CHINET [14], those of β-lactamase-producing H. influenzae, ceftriaxone-resistant K. pneumoniae, MRSA, BLNAR, and CRAB in 2021 all increased compared with 2020.
Due to the limitation of conditions, we could not simultaneously detect lower respiratory tract infection-related pathogens (such as viruses, mycoplasma, etc.) other than common bacteria during the COVID-19 epidemic. Due to the small number of detections of K. pneumoniae, P. aeruginosa, and A. baumannii in each year from 2018 to 2021, our study chose to analyze the antimicrobial resistance of common bacteria by year, instead of separate analyses during the COVID-19 epidemic period and the normalized COVID-19 epidemic prevention period.
Conclusions
We observed that, during the COVID-19 epidemic, due to effective protective measures, the detected numbers of some common bacteria causing lower respiratory tract infections in children in Shenzhen decreased, and the drug resistance of some bacteria decreased, but the overall trend still showed a slow increase. We should continue to strengthen the rational use of antibiotics, monitor bacterial resistance, strengthen the prevention and control of nosocomial infections, and implement a multipronged approach to prevent further development of bacterial resistance.
References
Leber AL, editor. 2016 update: clinical microbiology procedures handbook. 4th ed. Washington, DC: ASM Press; 2016.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. M100 28th edition. Wayne: Clinical and Laboratory Standards Institute; 2021.
Clinical and Laboratory Standards Institute (CLSI). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. M45 3rd edition. Wayne: Clinical and Laboratory Standards Institute; 2016.
U.S. Food and Drug Administration. FDA-identified interpretive criteria [S/OL]. [2022-10-30]. https://www.fda.gov/drugs/development-resources/tigecycline-injection-products.
Jones RN, Barry AL, Packer RR, Gregory WW, Thornsberry C. In vitro antimicrobial spectrum, occurrence of synergy, and recommendations for dilution susceptibility testing concentrations of the cefoperazone–sulbactam combination. J Clin Microbiol. 1987;25(9):1725–9.
Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE)-November 2015 update CRE toolkit[S/OL]. [2022-10-30]. https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–22.
Meng Q, Chen YS, Cui XY, Chen HY, Sun LF, Yang QB, Hu FP. Distribution and antibiotic resistance surveillance of clinical isolates in Shenzhen Children’s Hospital during 2017. Chin J Infect Chemother. 2019;19(04):417–24.
Meng Q, Cui XY, Zhou LT, Yan HM, Jiang HF, Ye BJ, Chen HY, Sun LF, Chen YS. The distribution and antibiotic resistance profiles of clinically isolated bacteria in Shenzhen Children’s Hospital from 2018 to 2020. Chin J Infect Chemother. 2022;22(03):314–21.
Li L, Wang H, Liu A, Wang R, Zhi T, Zheng Y, Bao Y, Chen Y, Wang W. Comparison of 11 respiratory pathogens among hospitalized children before and during the COVID-19 epidemic in Shenzhen, China. Virol J. 2021;18(1):202.
Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36(11):1999–2006.
Roberts LW, Forde BM, Hurst T, Ling W, Nimmo GR, Bergh H, George N, Hajkowicz K, McNamara JF, Lipman J, Permana B, Schembri MA, Paterson D, Beatson SA, Harris PNA. Genomic surveillance, characterization and intervention of a polymicrobial multidrug-resistant outbreak in critical care. Microb Genom. 2021;7(3):mgen000530.
Knoester M, de Boer MG, Maarleveld JJ, Claas EC, Bernards AT, de Jonge E, van Dissel JT, Veldkamp KE. An integrated approach to control a prolonged outbreak of multidrug-resistant Pseudomonas aeruginosa in an intensive care unit. Clin Microbiol Infect. 2014;20(4):O207–15.
Hu F, Guo Y, Yang Y, Zheng Y, Wu S, Jiang X, Zhu D, Wang F, China Antimicrobial Surveillance Network (CHINET) Study Group. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–81.
Needham CA. Haemophilus influenzae: antibiotic susceptibility. Clin Microbiol Rev. 1988;1(2):218–27.
Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20(2):368–89.
Khan MA, Northwood JB, Levy F, Verhaegh SJ, Farrell DJ, Van Belkum A, Hays JP. Bro {beta}-lactamase and antibiotic resistances in a global cross-sectional study of Moraxella catarrhalis from children and adults. J Antimicrob Chemother. 2010;65(1):91–7.
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019 [EB/OL]. [2022-10-30]. https://www.cdc.gov/drugresistance/biggest-threats.html.
Acknowledgements
We would like to thank Professor Fupin Hu and Professor Demei Zhu from the Institute of Antibiotics of Huashan Hospital affiliated to Fudan University for their guidance and advice.
Funding
This work and the journal’s rapid service fee were funded by Guangdong High-level Hospital Construction Fund, Guangdong Provincial High-level Hospital Construction Special Fund (YNKT2021-ZZ10) to Heping Wang and the Research Grants for Postdocs Settle in Shenzhen from Shenzhen Government to Wujiao Li.
Author Contributions
Qing Meng and Lintao Zhou contributed in the conception and design of the study, analysis and interpretation of data and drafting the article. Wujiao Li, Hanfang Jiang, Huimin Yan and Binjun Ye contributed in the acquisition of data and revising the article. Yunsheng Chen participated in the analysis and interpretation of data and revision the article. All authors gave their final approval of the version to be submitted.
Disclosures
Qing Meng, Wujiao Li, Hanfang Jiang, Huimin Yan, Heping Wang, Binjun Ye, Lintao Zhou and Yunsheng Chen have no conflicts to declare.
Compliance with Ethics Guidelines
The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval for this study was obtained from the Ethical Committee of Shenzhen Children’s Hospital (Shenzhen, Guangdong Province, China) under registration number 2016013. All experiments were performed under the relevant guidelines and regulations. Guardians of all children included in this study provided written informed consent to participate.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Meng, Q., Li, W., Jiang, H. et al. Comparison of the Distribution and Changes in the Antibiotic Resistance of Clinical Bacterial Isolates from the Lower Respiratory Tract of Children in Shenzhen Before the Epidemic, During the Epidemic, and During the Period of Normalized Prevention and Control of COVID-19. Infect Dis Ther 12, 563–575 (2023). https://doi.org/10.1007/s40121-022-00751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00751-4