Abstract
Introduction
The clinical efficiency of cefoperazone/sulbactam (CPZ/SUL) against Escherichia coli bacteremia was unknown. This study aimed to explore the relationship between CPZ/SUL MIC values and clinical outcomes in Escherichia coli bacteremia.
Methods
A multicenter, retrospective, observational cohort study was conducted in Taiwan between January 2015 and December 2020. Patients treated with CPZ/SUL for E. coli bacteremia were enrolled in the analysis. The CPZ/SUL MICs were determined by using the agar dilution method. The primary outcome was 30-day mortality.
Results
Among 247 isolates, 160 (64.8%) isolates were susceptible, 8 (3.2%) were intermediate, and 79 (32.0%) were resistant to cefoperazone. The activity of cefoperazone against cefoperazone-non-susceptible E. coli (n = 87) was restored upon combination with sulbactam, with susceptibility ranging from 0% to 97.7%. The 30-day mortality was 4.5% (11/247) and overall clinical success rate was 91.9% (227/247). Multivariate Cox proportional-hazards model revealed that heart failure [adjusted relative risk (ARR), 5.49; 95% confidence interval (CI) 1.31–23.02; p = 0.020], malignancy (ARR 7.50; 95% CI 2.02–27.80; p = 0.003), SOFA score (ARR 1.29; 95% CI 1.09–1.52; p = 0.003), and CPZ/SUL MIC ≥ 64 mg/L (ARR 11.31; 95% CI 1.34–95.52; p = 0.026) were independently associated with 30-day mortality. No statistically significant differences in 30-day mortality were found between groups with or without cefoperazone susceptibility (3.4% vs. 5.0%, p = 0.751, respectively).
Conclusions
Patients with E. coli bacteremia who were treated with CPZ/SUL had a favorable outcome when the MICs of the isolates were ≤ 16 mg/L and a high risk of mortality with MICs ≥ 64 mg/L.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The clinical efficiency of cefoperazone/sulbactam (CPZ/SUL) against Escherichia coli bacteremia was unknown |
What was learned from the study? |
The activity of cefoperazone against cefoperazone-non-susceptible E. coli was restored upon combination with sulbactam, with susceptibility ranging from 0% to 97.7% |
The overall clinical success rate was 91.9% (227/247) and the clinical success rate was 92.2% (226/245) among patients infected with E. coli bacteremia with CPZ/SUL MIC ≤16 mg/L |
Heart failure, malignancy, SOFA score, and CPZ/SUL MIC ≥ 64 mg/L were independently associated with 30-day mortality |
CPZ/SUL had a consistent clinical efficiency among patients with third-generation cephalosporin-non-susceptible E. coli bacteremia |
Introduction
Escherichia coli is not only a common commensal inhabitant of the gastrointestinal tract but is also one of the most important human pathogens. E. coli is one of the leading causes of both community-onset and nosocomial bloodstream infections, with incidence rates ranging from 31.9% to 81.0% among those of major gram-negative species in 28 European countries [1, 2]. Moreover, the increasing incidence of multidrug-resistant E. coli strains is of great concern. In a recent report from the World Health Organization, E. coli is included as a high-priority pathogen that causes infections in humans: within a community, in hospitals, or transmitted through the food chain [3]. This report highlighted third-generation cephalosporin (3GC) resistance (36.6%) for E. coli bloodstream infection, which limits empiric treatments. A recent report from the Taiwan Surveillance of Multicenter Antimicrobial Resistance also showed that 3GC-resistant E. coli increased community-onset bacteremia from 0.5% in 2001–2002 to 27.6% in 2019 [4, 5]. For patients infected with drug-resistant E. coli, adequate antimicrobial therapy might be easily delayed, leading to appreciable morbidity and mortality [6].
Cefoperazone is a 3GC with broad-spectrum antibacterial activity against Enterobacterales and Pseudomonas aeruginosa, when no β-lactamase is present in the bacteria [7, 8]. Sulbactam, a semisynthetic β-lactamase inhibitor, inhibits the hydrolytic activity of Ambler class A penicillinases (such as TEM-1, TEM-2, and SHV-1), plasmid-mediated extended-spectrum β-lactamases (ESBL), and chromosomal AmpC β-lactamases [9]. The combination of cefoperazone and sulbactam markedly restored the activity against β-lactamase-producing strains [7, 10,11,12]. Previous studies have reported the clinical effects of cefoperazone/sulbactam (CPZ/SUL) in the treatment of febrile neutropenia, intra-abdominal infections, and multidrug-resistant Acinetobacter baumannii infections [13,14,15,16]. A recent multicenter study in Taiwan also demonstrated that CPZ/SUL, comparable to piperacillin-tazobactam (PTZ), is a viable choice for empirical antibiotic therapy for elderly patients with severe community-acquired pneumonia or hospital-acquired pneumonia or ventilator-associated pneumonia [17]. However, no validated MIC interpretive breakpoints for cefoperazone (with or without sulbactam) against Enterobacterales have been provided by the Clinical and Laboratory Standards Institute (CLSI) [18].
Our study aimed to investigate the relationship between CPZ/SUL MIC values and clinical outcomes against E. coli bacteremia.
Methods
Study Design and Population
This study had a multicenter, retrospective, observational cohort design and evaluated the correlation between MICs of CPZ/SUL (the formulation CPZ/SUL of 1:1, TTY Biopharm Company, Taiwan) and the treatment outcomes of patients with E. coli bacteremia. The study was conducted in eight academic medical centers located in Taiwan: Chang Gung Memorial Hospital, Linkou (CGMH-LK; 3700 beds), Taipei Veterans General Hospital (TVGH; 2808 beds), and Tri-Service General Hospital (TSGH; 1900 beds) in northern Taiwan. China Medical University Hospital (CMUH; 2076 beds) and Taichung Veterans General Hospital (TC-TVGH; 1624 beds) in central Taiwan. Chi Mei Medical Hospital (CMMH; 1284 beds), Kaohsiung Medical University Hospital (KMUH; 1481 beds), and Kaohsiung Chang Gung Memorial Hospital (KCGMH; 2683 beds) in southern Taiwan. The Institutional Review Boards of KMUH (IRB no. KMUHIRB-E(I)-20210154) and all other participating hospitals reviewed and approved this study.
Adult patients (≥ 20 years) who had at least one positive blood culture report for E. coli isolates between January 2015 and December 2020 were enrolled. Eligible patients fulfilled the following criteria: (1) clinical symptoms or signs were compatible with sepsis and (2) CPZ/SUL was administered for at least 3 days. Patients were excluded if they (1) had polymicrobial bacteremia, (2) died before the date that the species was identified, or (3) received combination antibiotic therapy for more than 48 h. Patient demographics, clinical and microbiological characteristics, antibiotic treatment, and clinical outcomes were retrieved from the medical records using a standardized case-record form and reviewed.
Microbiological Studies
All isolates were identified by the clinical microbiology laboratory located at each participating study site. MICs of antibiotics were determined in the central laboratory of KMUH. CPZ/SUL MICs were determined using the standard agar dilution method in accordance with the guidelines recommended by the CLSI [18]. We determined the concentration ratio of cefoperazone to sulbactam with 1:1 ratio and a serial twofold dilution ranging from 0.06/0.06 to 64/64 mg/L in cation-adjusted Mueller–Hinton agar (Becton Dickinson). E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used for quality control. In the absence of CLSI interpretive criteria for CPZ/SUL, we proposed CPZ/SUL breakpoints as susceptibility (≤ 16 mg/L), intermediate susceptibility (32 mg/L), and resistance (≥ 64 mg/L). The antibiograms of the other antimicrobial agents were obtained using the Vitek 2 system (bioMérieux, Marcy l'Etoile, France). For the CPZ/SUL-resistant isolates, the multiplex PCRs of ESBLs (blaTEM, blaSHV, and blaCTX-M) and AmpC (blaMOX, blaLAT, blaCMY, blaACC, blaDHA, and so on) were performed to investigate the resistance mechanisms [19, 20].
Variable Definition and Outcome
Infection onset date was considered to be when the index blood culture was collected. Sources of bacteremia were established according to the criteria of the Centers for Disease Control and Prevention [21]. Comorbidities were assessed by using the Charlson comorbidity index [22]. The clinical severity of infection was assessed by using the sequential organ failure assessment (SOFA) and Pitt bacteremia scores when CPZ/SUL was initiated [23, 24]. CPZ/SUL was administered at a standard dose of 2/2 g intravenously every 12 h with dose adjustments based on estimated creatinine clearance (Cockroft–Gault equation), according to the manufacturer’s recommendations [25, 26]. 3GC non-susceptibility was defined as the non-susceptibility of E. coli isolates to cefoperazone. A successful clinical outcome was defined as the complete resolution of clinical signs and symptoms and lack of persistent or recurrent bacteremia. The primary outcome was 30-day mortality.
Statistical Analysis
Statistical analyses were conducted using IBM SPSS version 20 (IBM Corp., Armonk, NY, USA), SAS software version 9.4 (SAS Institute), and R software program version 2.12 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were compared using the chi-squared test or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean ± standard deviation and compared using the Student’s t test.
All variables with a P value of less than 0.05 in the univariate model were further entered into the multivariate analysis. Relative risk (RR) with 95% confidence intervals (CI) and their P values were calculated with the use of a Cox proportional-hazards model. We also calculated the effect size, a similar result, with the use of an exact method (PHREG Procedure, Ties = exact) of multivariate Cox proportional-hazards model. All tests were two-sided, and statistical significance was set at p < 0.05.
Results
MIC Values of Cefoperazone Alone and CPZ/SUL Against E. coli Isolates
A total of 247 E. coli isolates were enrolled in this study. The antimicrobial susceptibility profile of E. coli is listed in Table 1. The MIC values of cefoperazone alone and in combination with sulbactam against E. coli isolates are shown in Table 2. The MICs of cefoperazone, including the MIC range, MIC50, and MIC90, were reduced after the addition of sulbactam. Among 247 isolates, 160 (64.8%) isolates were susceptible, 8 (3.2%) were intermediate, and 79 (32.0%) were resistant to cefoperazone. A total of 245 (99.2%) isolates were susceptible and 2 (0.8%) were resistant to CPZ/SUL. The activity of cefoperazone against cefoperazone-non-susceptible E. coli (n = 87) was restored upon combination with sulbactam, with susceptibility ranging from 0 to 97.7% (Table 2). The distribution of CPZ/SUL MICs among E. coli isolates is shown in Fig. 1.
Among the cefoperazone-non-susceptible isolates, two isolates were found to be resistant to CPZ/SUL (Table 2). The PCR detections for the two isolates revealed that isolate No. 22001 harbored blaCTX-M-G1 and blaCMY-2 genes; isolate No. 22022 harbored blaCTX-M-G9 gene.
Patient Demographics, Clinical Characteristics, and Relationship Between MIC Values and Clinical Outcomes
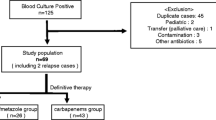
A total of 247 patients met the eligibility criteria (Fig. 2) and their patient characteristics are shown in Table 3. The mean age was 73.0 ± 13.6 years, and the most common comorbidities were hypertension (61.5%), diabetes (40.9%), and malignancy (24.3%). The common sources of bacteremia were the urinary tract (46.2%) and primary bacteremia (23.9%). The mean duration of CPZ/SUL therapy was 7.9 ± 3.5 days.
Flowchart of patient selection. CPZ/SUL cefoperazone/sulbactam, CGMH-LK Chang Gung Memorial Hospital, CMMH Chi Mei Medical Hospital, CMUH China Medical University Hospital, KCGMH Kaohsiung Chang Gung Memorial Hospital, KMUH Kaohsiung Medical University Hospital, TSGH Tri-Service General Hospital, TVGH Taipei Veterans General Hospital, TC-TVGH Taichung Veterans General Hospital
In total, 11 patients (4.5%) died within 30 days of bacteremia diagnosis. The overall clinical success rate was 91.9% (227/247) and the clinical success rate was 92.2% (226/245) among patients infected with E. coli bacteremia with MIC ≤ 16 mg/L. The association between clinical outcome and CPZ/SUL is shown in Fig. 3. In the multivariate Cox proportional-hazards analysis, heart failure [adjusted relative risk (ARR), 5.49; 95% confidence interval (CI) 1.31–23.02; p = 0.020], malignancy (ARR 7.50; 95% CI 2.02–27.80; p = 0.003), SOFA score (ARR 1.29; 95% CI 1.09–1.52; p = 0.003), and CPZ/SUL MIC ≥ 64 mg/L (ARR 11.31; 95% CI 1.34–95.52; p = 0.026) were independently associated with 30-day mortality (Table 4). Eighty-seven (35.2%) patients were infected with 3GC-non-susceptible E. coli in the study cohort. The proportions of 30-day mortality in both groups were not different (5.0% vs. 3.4%, p = 0.751) (Table 5).
Discussion
In this multicenter center study cohort, we included 247 patients who received CPZ/SUL monotherapy for the treatment of E. coli bacteremia and evaluated the clinical outcomes among patient groups acquiring isolates with different CPZ/SUL MICs. Excellent outcomes against E. coli bacteremia isolates with MICs ≤ 16 mg/L were revealed; however, there was a high risk of failure against isolates with MICs ≥ 64 mg/L. This study also showed that CPZ/SUL had a consistent clinical efficiency against 3GC-non-susceptible isolates.
Currently, in the absence of CLSI criteria for CPZ/SUL for Enterobacterales, in vitro studies have often used cefoperazone breakpoints (CLSI susceptibility, ≤ 16 mg/L; intermediate susceptibility = 32 mg/L; and resistance, ≥ 64 mg/L) to report the susceptibility results for CPZ/SUL [11, 27, 28]. Whether these proposed breakpoints are too liberal or too conservative with respect to clinical outcomes remains undetermined. Our study indicated that high CPZ/SUL MICs (≥ 64 mg/L) are associated with significantly increased mortality and acceptable clinical success rate (92.2%) among patients infected with E. coli bacteremia with MIC ≤ 16 mg/L, suggesting that the clinical efficiency of CPZ/SUL treatment for E. coli bacteremia is correlated with the proposed MIC breakpoint.
Previous reports have shown that the addition of sulbactam to cefoperazone can significantly enhance antimicrobial activity and overcome the bacterial inoculum effect against cefoperazone [7, 10, 11, 29]. Our study indicated that the susceptibility of E. coli to CPZ/SUL formulations was augmented upon combination with sulbactam (Table 1). This finding is consistent with the report of two large-scale surveillance studies: a Chinese surveillance study enrolling 1022 E. coli isolates with CPZ/SUL (2:1) susceptibility rate from 68% to 86.5% between 2010 and 2018 [30] and a recent global epidemiological survey showing a better CPZ/SUL (1:1) susceptibility (92.8%) against E. coli isolates in 2015–2016 [31].
Our in vitro data showed that the activity of cefoperazone against cefoperazone-non-susceptible E. coli (n = 87) was restored upon combination with sulbactam, which is consistent with the result of a previous study [32]. Thus, we investigated the clinical efficiency of 3GC susceptibility or not in this cohort. The acceptable outcomes were demonstrated regardless of the 3GC susceptibility (Table 5). 3GC resistance in Enterobacterales is typically mediated by bacterial production of ESBLs or AmpC β-lactamases [33, 34]. Two CPZ/SUL resistance isolates (No. 22001 and No. 220222) harbored blaCTX-M-G1, blaCMY-2 genes, and blaCTX-M-G9 gene, respectively. Further study on the CPZ/SUL resistance mechanisms is needed.
The incidence of infections with 3GC-resistant gram-negative bacilli is emerging in Asia, as almost 50% of E. coli isolates are ESBL-producing [35]. Although the recommended first-line regimens for treating these infections are usually carbapenems, they may facilitate the spread of carbapenem-resistant organisms with the increasing consumption of carbapenems [36, 37]. Therefore, carbapenem-sparing regimens are being investigated. β-lactamase/β-lactamase inhibitor combinations may represent appropriate carbapenem-sparing regimens; however, it remains undecided whether these combinations are as effective as carbapenems for treating ESBL-Enterobacterales infections. In the MERINO trial, PTZ was not non-inferior to meropenem as a definitive regimen for treating ceftriaxone-resistant Enterobacterales infection [38]. However, a post hoc analysis based on a reference broth microdilution method revealed that significant error rates were identified in PTZ susceptibility, which accounted for some of the differences in mortality between meropenem- and PTZ-treated groups. The between-group mortality difference was less pronounced than in the original MERINO trial analysis when PTZ-non-susceptible strains were excluded (30-day mortality rates in the PTZ and meropenem groups were 9% and 8%, respectively) [39]. The efficacy of CPZ/SUL against ESBL-producing Enterobacterales has rarely been studied in clinical settings [40]. A study enrolling limited numbers of cases revealed that clinical success rate was 70.6% (12/17) and sepsis-related mortality was 5.9% (1/17) in patients receiving CPZ/SUL (2:1) therapy for the treatment of BSIs caused by ESBL-producing Enterobacterales [39]. The current study revealed that the clinical success and 30-day mortality rates in patients with 3GC-non-susceptibility E. coli infection receiving CPZ/SUL (1:1) therapy were 90.8% and 3.4%, respectively. It is supposed that CPZ/SUL at a 1:1 ratio has a higher susceptibility rate against ESBL-producing Enterobacterales [32]. However, further investigation is needed to compare CPZ/SUL (1:1) and carbapenem for the treatment of BSIs caused by ESBL-producing Enterobacterales.
There were some limitations to this study. First, as a result of the study’s retrospective design, the microbiologic evidence of recurrent and/or persistent bacteremia would not have been obtained on the basis of a preplanned culture collection schedule but rather the practice used in each study site according to the patient’s clinical condition. Therefore, the clinical success rate may be overestimated. Second, although the agar dilution has been known as the gold standard method for determination of MICs, it is not commonly used in most clinical laboratories because it is time-consuming and labor-intensive. Additional studies (i.e., the broth microdilution) using a large number of clinical isolates may be necessary to further substantiate the results. Third, the current study revealed that high CPZ/SUL MICs (≥ 64 mg/L) were associated with significantly increased mortality. Because only two patients had resistant E coli in contrast to the susceptible category, this would markedly skew the statistical applications. Forth, ESBL genes were only confirmed in two CPZ/SUL resistance isolates. There is a lack of data on the distribution of drug-resistant mechanisms among cefoperazone-non-susceptible isolates in the current study. However, resistance to 3GCs is a suitable surrogate indicator for identifying ESBL-producing or AmpC E. coli strains [33,34,35, 39]. Nevertheless, this reflects that the drug-resistant mechanisms of 3GC-resistant E. coli are not available in clinical practice. Our data revealed that CPZ/SUL treatment for 3GC-resistant E. coli bacteremia was excellent, with a clinical success rate of 90.8%. Finally, only patients with E. coli bacteremia were enrolled in the current study. Therefore, we cannot assume that the findings would translate for all other Enterobacterales infections.
Conclusion
Patients with E. coli bacteremia treated with a CPZ/SUL therapy had favorable outcomes when the MICs of isolates were ≤ 16 mg/L and a high risk of mortality with MICs of isolates ≥ 64 mg/L. In addition, CPZ/SUL showed acceptable clinical efficiency against 3GC-non-susceptible E. coli isolates.
References
Brown GW. Discriminant analysis. Am J Dis Child. 1984;138:395–400.
Greenberger PA, Halwig JM, Patterson R, Wallemark CB. Emergency administration of radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1986;77:630–4.
World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report. https://www.who.int/publications/i/item/9789240027336. Accessed Jan 13, 2022.
Liu PY, Lee YL, Lu MC, et al. National surveillance of antimicrobial susceptibility of bacteremic gram-negative bacteria with emphasis on community-acquired resistant isolates: report from the 2019 surveillance of multicenter antimicrobial resistance in Taiwan (SMART). Antimicrob Agents Chemother. 2020;64:e01089-e1120.
Sun HY, Chen SY, Chang SC, Pan SC, Su CP, Chen YC. Community-onset Escherichia coli and Klebsiella pneumoniae bacteremia: influence of health care exposure on antimicrobial susceptibility. Diagn Microbiol Infect Dis. 2006;55:135–41.
Nguyen ML, Toye B, Kanji S, Zvonar R. Risk factors for and outcomes of bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species at a Canadian tertiary care hospital. Can J Hosp Pharm. 2015;68:136–43.
Jones RN, Barry AL, Thornsberry C, Wilson HW. The cefoperazone-sulbactam combination. in vitro qualities including beta-lactamase stability, antimicrobial activity, and interpretive criteria for disk diffusion tests. Am J Clin Pathol. 1985;84:496–504.
Li JT, Lu Y, Hou J, et al. Sulbactam/cefoperazone versus cefotaxime for the treatment of moderate-to-severe bacterial infections: results of a randomized, controlled clinical trial. Clin Infect Dis. 1997;24:498–505.
Bhattacharjee A, Sen MR, Prakash P, Anupurba S. Role of beta-lactamase inhibitors in enterobacterial isolates producing extended-spectrum beta-lactamases. J Antimicrob Chemother. 2008;61:309–14.
Williams JD. Beta-lactamase inhibition and in vitro activity of sulbactam and sulbactam/cefoperazone. Clin Infect Dis. 1997;24:494–7.
Chang PC, Chen CC, Lu YC, et al. The impact of inoculum size on the activity of cefoperazone-sulbactam against multidrug resistant organisms. J Microbiol Immunol Infect. 2018;51:207–13.
Levin AS. Multiresistant acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Clin Microbiol Infect. 2002;8:144–53.
Xia J, Zhang D, Xu Y, Gong M, Zhou Y, Fang X. A retrospective analysis of carbapenem-resistant Acinetobacter baumannii-mediated nosocomial pneumonia and the in vitro therapeutic benefit of cefoperazone/sulbactam. Int J Infect Dis. 2014;23:90–3.
Guclu E, Kaya G, Ogutlu A, Karabay O. The effect of cefoperazone sulbactam and piperacillin tazobactam on mortality in gram-negative nosocomial infections. J Chemother. 2020;32:118–23.
Chandra A, Dhar P, Dharap S, et al. Cefoperazone-sulbactam for treatment of intra-abdominal infections: results from a randomized, parallel group study in India. Surg Infect (Larchmt). 2008;9:367–76.
Lan SH, Chang SP, Lai CC, Lu LC, Tang HJ. Efficacy and safety of cefoperazone- sulbactam in empiric therapy for febrile neutropenia: a systemic review and meta-analysis. Medicine (Baltimore). 2020;99: e19321.
Huang CT, Chen CH, Chen WC, et al. Clinical effectiveness of cefoperazone-sulbactam vs. piperacillin-tazobactam for the treatment of pneumonia in elderly patients. Int J Antimicrob Agents. 2022;59:106491.
Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing: 29th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
Monstein H-J, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. Multiplex PCR amplification assay for the detection of bla SHV, bla TEM and bla CTX-M genes in Enterobacteriaceae. APMIS. 2007;115:1400–8.
Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–62.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med. 2004;140:26–32.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock. JAMA. 2016;315:801–10.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Cefoperazone-sulbactam package insert. https://www1.ndmctsgh.edu.tw/pharm/pic/medinsert/005BRO06.pdf.
Lai CC, Chen CC, Lu YC, Chuang YC, Tang HJ. In vitro activity of cefoperazone and cefoperazone-sulbactam against carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Infect Drug Resist. 2019;12:25–9.
Jean SS, Liao CH, Sheng WH, Lee WS, Hsueh PR. Comparison of commonly used antimicrobial susceptibility testing methods for evaluating susceptibilities of clinical isolates of Enterobacteriaceae and nonfermentative gram-negative bacilli to cefoperazone-sulbactam. J Microbiol Immunol Infect. 2017;50:454–63.
Kuo HY, Wang FD, Yen YF, Lin ML, Liu CY. In vitro activities of piperacillin or cefoperazone alone and in combination with beta-lactamase inhibitors against gram-negative bacilli. New Microbiol. 2009;32:49–55.
Wang Q, Wang Z, Zhang F, et al. Long-term continuous antimicrobial resistance surveillance among nosocomial gram-negative bacilli in China from 2010 to 2018 (CMSS). Infect Drug Resist. 2020;13:2617–29.
Sader HS, Carvalhaes CG, Streit JM, Castanheira M, Flamm RK. Antimicrobial activity of cefoperazone-sulbactam tested against gram-negative organisms from Europe, Asia-Pacific, and Latin America. Int J Infect Dis. 2020;91:32–7.
Lai CC, Chen CC, Lu YC, Lin TP, Chuang YC, Tang HJ. Appropriate composites of cefoperazone-sulbactam against multidrug-resistant organisms. Infect Drug Resist. 2018;11:1441–5.
Wang JT, Chang SC, Chang FY, et al. Antimicrobial non-susceptibility of Escherichia coli from outpatients and patients visiting emergency rooms in Taiwan. PLoS ONE. 2015;10:e0144103.
Rossolini GM, D'Andrea MM. Mugnaioli C. The spread of CTX-M-type extended- spectrum beta-lactamases. Clin Microbiol Infect. 2008;14(Suppl 1):33–41.
Zhang H, Tong D, Johnson A, et al. Antimicrobial susceptibility changes of Escherichia coli and Klebsiella pneumoniae intra-abdominal infection isolate-derived pathogens from Chinese intra-abdominal infections from 2011 to 2015. Infect Drug Resist. 2019;12:2477–86.
Armand-Lefevre L, Angebault C, Barbier F, et al. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother. 2013;57:1488–95.
Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–50.
Harris PNA, Tambyah PA, Lye DC, et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320:984–94.
Henderson A, Paterson DL, Chatfield MD, et al. Association between minimum inhibitory concentration, beta-lactamase genes and mortality for patients treated with piperacillin/tazobactam or meropenem from the MERINO study. Clin Infect Dis. 2021;73:e3842–50.
Su J, Guo Q, Li Y, et al. Comparison of empirical therapy with cefoperazone/sulbactam or a carbapenem for bloodstream infections due to ESBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73:3176–80.
Acknowledgements
We thank the participants of the study.
Funding
This work was sponsored by TTY Biopharm Company, Taiwan. The sponsors had no role in the study design, data collection and analysis, manuscript preparation, or the decision to submit for publication. We have no conflicts of interest to declare. The journal’s Rapid Service Fee was funded by TTY Biopharm Company.
Medical Writing and/or Editorial Assistance
The authors thank Editage Academic Editing for editing the manuscript. This assistance was funded by TTY Biopharm Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and study design: Shang-Yi Lin, Po-Liang Lu, Yin-Ching Chuang and Hung-Jen Tang. Data acquisition: all authors. Analyses of the data: Shang-Yi Lin, Ting-Shu Wu, Hung-Jen Tang. Statistics: Shian-Sen Shie, Feng-Yee Chang, Ya-Sung Yang and Tsung-Ta Chiang. Supervision: Tsung-Ta Chiang, Fu-Der Wang, Mao-Wang Ho, Chia-Hui Chou, Jien-Wei Liu and Zhi-Yuan Shi. Manuscript preparation: Shang-Yi Lin, Po-Liang Lu and Hung-Jen Tang. Review and approval: all authors.
Disclosures
Shang-Yi Lin, Po-Liang Lu, Ting-Shu Wu, Shian-Sen Shie, Feng-Yee Chang, Ya-Sung Yang, Tsung-Ta Chiang, Fu-Der Wang, Mao-Wang Ho, Chia-Hui Chou, Jien-Wei Liu, Zhi-Yuan Shi, Yin-Ching Chuang and Hung-Jen Tang all have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Institutional Review Boards of KMUH (IRB no. KMUHIRB-E(I)-20210154) and all other participating hospitals reviewed and approved this study. The study was carried out according to the principles expressed in the Declaration of Helsinki of 1964 and its later amendments. The need for informed consent was waived by the ethics committee at each study site.
Data Availability
All data containing relevant information to support the study findings are provided in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lin, SY., Lu, PL., Wu, TS. et al. Correlation Between Cefoperazone/Sulbactam MIC Values and Clinical Outcomes of Escherichia coli Bacteremia. Infect Dis Ther 11, 1853–1867 (2022). https://doi.org/10.1007/s40121-022-00672-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00672-2