Abstract
Introduction
Recent observational studies have reported the association between ischemic stroke (IS) and cerebral microbleeds (CMBs). Whether this reflects a causal association remains to be established. Herein, we adopted a two-sample bidirectional Mendelian randomization (MR) analysis to comprehensively evaluate the causal association of IS and CMBs.
Methods
The summary-level genome-wide association studies (GWASs) data of IS were obtained from the GIGASTROKE consortium (62,100 European ancestry cases and 1,234,808 European ancestry controls). All IS cases could be further divided into large-vessel atherosclerosis stroke (LVS, n = 6399), cardio-embolic stroke (CES, n = 10,804) and small-vessel occlusion stroke (SVS, n = 6811). Meanwhile, we used publicly available summary statistics from published GWASs of CMBs (3556 of the 25,862 European participants across 2 large initiatives). A bidirectional MR analysis was conducted using inverse-variance weighting (IVW) as the major outcome, whereas MR-Egger and weighted median (WM) were used to complement the IVW estimates as they can provide more robust estimates in a broader set of scenarios but are less efficient (wider CIs). A Bonferroni-corrected threshold of p < 0.0125 was considered significant, and p values between 0.0125 and 0.05 were considered suggestive of evidence for a potential association.
Results
We detected that higher risk of IS [IVW odds ratio (OR) 1.47, 95% confidence interval (CI) 1.04–2.07, p = 0.03] and SVS (IVW OR 1.62, 95% CI 1.07–2.47, p = 0.02) were significantly associated with CMBs. Reverse MR analyses found no significant evidence for a causal effect of CMBs on IS and its subtypes.
Conclusions
Our study provides potential evidence that IS and SVS are causally linked to increased risk of CMBs. Further research is needed to determine the mechanisms of association between IS and CMBs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Recent observational studies have reported the association between ischemic stroke (IS) and cerebral microbleeds (CMBs), but the causal relationship between IS and CMBs has not been established |
In this study, we examined the bidirectional causal relationship between IS and CMBs using a genetically informed method |
What was learned from the study? |
Our study provides potential evidence that IS and SVS are causally linked to the increased risk of CMBs |
Reverse MR analyses found no significant evidence for a causal effect of CMBs on IS |
Introduction
Ischemic stroke (IS) occurs primarily when cerebral blood flow is interrupted, causing severe neural damage [1,2,3]. It is ranked second among the leading causes of death worldwide, causing an estimated 5.9 million deaths and 102 million disability-adjusted life years lost [2]. With the ever-changing development of medical imaging technology, especially the magnetic resonance (MR) imaging system, an increasing number of patients with IS are found to be accompanied by different degrees of cerebral microbleeds (CMBs) [4].
CMBs are typically detected on susceptibility-weighted imaging (SWI) as small (< 10 mm), hypointense (black), ovoid or rounded regions which develop because of red blood cell leakage from arteries and capillaries [5, 6]. The potential increased risk of intracranial hemorrhage associated with CMBs is well known [7]. Evidence, however, suggests CMBs may not only provide information about bleeding propensity but also about future ischemic events. According to Cordonnier et al., approximately one-third of IS patients have one or more CMBs [8]. Additionally, several observational studies have demonstrated that CMBs increase the chances of subsequent intracranial hemorrhage and recurrent IS in those who have suffered from IS [9,10,11,12]. Despite this, observational studies have some limitations, such as reverse causation and unmeasured confounding factors that are difficult to eliminate, which may limit the ability of causal inference. In consequence, the causal association between CMBs with IS has not been examined systematically.
Mendelian randomization (MR), using genetic variants as instrumental variables (IVs) to assess the causal effects of risk factors related to diseases, can overcome confounding biases inherent in observational studies. Previous studies have found the causality relationship between IS and other diseases by using MR analysis [13,14,15]; however, the causal association between IS and CMBs has not been demonstrated yet.
Herein, we adopted a two-sample bidirectional MR analysis to comprehensively evaluate the causal association of CMBs and IS.
Methods
Study Design
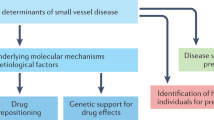
In this study, a two-sample bidirectional MR study was conducted to explore the causal relationship between IS and CMBs. Figure 1 shows the flowchart of the overall study design. MR must be premised on the following three basic criteria: (1) relevance: the genetic variant, usually single nucleotide polymorphism (SNP), should be closely associated with the exposure; (2) independence: genetic variants should not be associated with any potential confounders; (3) exclusion-restriction: genetic variants should not be associated with the outcome except via the way of exposure [16].
Conceptual framework for the Mendelian randomization analysis of the causal association of cerebral micobleeds and ischemic stroke. The design follows the following three basic criteria: (1) relevance: genetic variants, usually single nucleotide polymorphisms, should be close to the exposure; (2) independence: genetic variants should not be associated with any potential confounders; (3) exclusion-restriction: genetic variants should not be associated with the outcome except via the way of exposure
Data Source Description
The summary-level genome-wide association study (GWAS) data of IS were obtained from the GIGASTROKE consortium (62,100 European ancestry cases and 1,234,808 European ancestry controls) [17]. Based on the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification, all IS cases could be further classified as large-vessel atherosclerosis stroke (LVS, n = 6399), small-vessel occlusion stroke (SVS, n = 6811) and cardio-embolic stroke (CES, n = 10,804) [18, 19]. Meanwhile, we used publicly available summary statistics from published GWASs of CMBs (3556 out of 25,862 participants had CMBs), which performed genome-wide association studies in 11 population-based cohort studies and 3 case-control or case-only stroke cohorts [20] (https://cd.hugeamp.org/datasets.html). This study used publicly available de-identified data from participant studies that were approved by an ethical standards committee with respect to human experimentation. No separate ethical approval was required in this study.
Selection of Genetic Instruments
All selected single-nucleotide polymorphisms (SNPs) should be clearly associated with exposures [p < 5 × 10–7, linkage disequilibrium (LD) r2 < 0.01, and F-statistics > 10] [21]. The SNPs (P < 5 × 10–6) in the PhenoScannner database, a comprehensive information platform on the relationship between genotypes and phenotypes, were eliminated with potential confounders and outcomes, such as, drinking, diabetes, smoking, hypertension, treatment with aspirin and treatment with warfarin.
Mendelian Randomization Analyses
A bidirectional MR analysis was conducted using inverse-variance weighting (IVW) as the preferred method, whereas MR-Egger and weighted median (WM) were used to complement the IVW estimates as they could provide more robust estimates in a broader set of scenarios but are less efficient (wider CIs). Furthermore, we adopted MR-Egger intercept and MR-PRESSO as secondary analyses to detect heterogeneity and horizontal pleiotropy [22]. The MR-Egger method relaxes the IVW assumption that the average pleiotropic effect is zero, allowing all genetic variants to have a pleiotropic effect (not via the exposure) [23]. In addition, we performed a leave-one-out analysis to further investigate the impact of outlying and/or pleiotropic genetic variants. If estimates of these approaches in our study were inconsistent, a tighten instrument p value threshold was set, and then the MR analysis was performed again [23]. Furthermore, we calculated the r2 (r2 = beta2/(se2 × (n − 2) + beta2)) of each SNP and summed them up to calculate the overall r2 [r2 × (N − 2)/(1 − r2)] and F statistics using the sample size of exposure GWASs [24].
Statistical analyses
Statistical analyses and data visualization were performed using the R software, version 4.2.0 (http://www.r-project.org). All two-sample MR analyses were performed using the MR-Base R package ("TwoSampleMR") “TwoSampleMR” (https://github.com/MRCIEU/TwoSampleMR) [25]. The mRnd was used to calculate the statistical power for Mendelian randomization (https://cnsgenomics.shinyapps.io/mRnd/) [26].
Strength of Evidence
Bonferroni correction was applied to correct the threshold of statistical significance for multiple comparisons, and a p-value below 0.0125 (where p = 0.05/4) was considered strong evidence of significant association; p values between 0.0125 and 0.05 were considered suggestive of evidence for a potential association [27].
Results
The Causal Effects of IS and its subtypes on CMBs
Causal Effect of IS on CMBs
An overall analysis found 25 SNPs associated with IS both significantly and independently (Table 1). The IVW method showed that IS was causally associated with an increase in risk of CMBs significantly (OR 1.47, 95% CI: 1.04–2.07, p = 0.029) (Fig. 2).
Mendelian randomization estimates from ischemic stroke and its subtypes on genetically predicted cerebral micobleeds. CMBs cerebral microbleeds; IVW inverse-variance weighted; WM weighted median; IS ischemic stroke; LVS large-vessel atherosclerosis stroke; SVS small-vessel occlusion stroke; CES cardio-embolic stroke
No heterogeneity was observed with a Cochran Q derived in MR study between IS with CMBs (p = 0.657). Furthermore, Egger intercepts did not detect any pleiotropy (intercept = − 0.02; SE = 0.04. p = 0.66) (Supplementary Fig. 1A). No single SNP was strongly violating the overall effect of IS on CMBs in the leave-one-out sensitivity analysis (Supplementary Fig. 2A). The funnel plot was symmetrical, indicating no heterogeneity (Supplementary Fig. 1B). Similarly, the MR-PRESSO global test showed the absence of pleiotropy (p = 0.10). The power and F-statistics of MR analysis are displayed in Table S1.
Causal Effect of LVS on CMBs
Using the seven LVS-related SNPs (Table 1), no evidence suggested a potential causal effect of LVS on the risk of CMBs (IVW OR 1.15, 95% CI 0.83–1.57, p = 0.40). However, opposing results were observed using the MR-Egger approach (OR 0.50, 95% CI 0.19–1.31, p = 0.22). Since the MR estimates of MR-Egger and IVW were inconsistent, we tightened the instrument p value threshold to 1.2 × 10−7, and two SNPs (rs142395500 and rs476762) were used as instrument tools. The MR estimates still indicated that LVS had no causality on CMBs (OR 1.04, 95% CI: 0.27–3.93, p = 0.96) (Fig. 2). However, heterogeneity was observed with a Cochran Q-derived p value < 0.05 in MR study between LVS with CMBs (p = 0.002). As we used the random effects IVW as main result, heterogeneity was acceptable [22]. MR-Egger and MR-PRESSO global tests could not be performed because only two SNPs were included in the analysis.
Causal Effect of SVS on CMBs
Five SNPs were taken as IVs for SVS (Table 1). We found that SVS increased the risk for CMBs significantly (IVW OR 1.39, 95% CI: 1.05–1.85, p = 0.022), while opposing results were observed using the MR-Egger approach (OR 0.86, 95% CI: 0.35–2.14, p = 0.77). Then, we tightened the instrument p value threshold to 3 × 10−7 and two SNPs were identified, which were significantly and independently associated with SVS (rs12445022 and rs7766042). The result of IVW indicated that SVS had causality on CMBs (IVW OR 1.62, 95% CI: 1.07–2.47, p = 0.02) (Fig. 2). No obvious heterogeneity was observed (the Cochran Q-test derived p value was 0.35). However, MR-Egger and MR-PRESSO global tests could not be performed.
Causal Effect of CES on CMBs
Using the six CES-related SNPs (Table 1), we found that CES had no obvious causal effect on CMBs (OR 0.98, 95% CI: 0.64–1.50, p = 0.93) (Fig. 2), while opposing results were observed using the MR-Egger approach (OR 1.11, 95% CI: 0.27–4.52, p = 0.89). Then, we tightened the instrument p value threshold to 3 × 10−7, and five SNPs were identified. The result of IVW still indicated that CES had no causality on CMBs (OR 1.07, 95% CI: 0.71–1.60, p = 0.75). The p value for MR-Egger intercept is > 0.05. No outliers were identified in the leave-one-out plot (Supplementary Fig. 2B). No pleiotropy and heterogeneity were observed (Supplementary Fig. 1C and D). No pleiotropy was found in the MR-PRESSO global test (p = 0.179).
Causal Effects of CMBs on IS and Its Subtypes
We further performed bidirectional MR analysis to estimate the causal effects of CMBs on IS and its subtypes. At the global level, two SNPs were taken as IVs for CMBs (Table 1). We found that CMBs had no causal relationship with IS (IVW OR 1.01, 95% CI 0.94–1.08, p = 0.818), LVS (IVW OR 1.10, 95%CI 0.92–1.33, p = 0.30), SVS (IVW OR 1.07, 95% CI 0.88–1.31, p = 0.49) and CES (IVW OR 0.93, 95% CI 0.79–1.11, p = 0.43). No obvious heterogeneity was observed. MR-Egger and MR-PRESSO global tests could not be performed. Details are presented in Table S2.
Discussion
To our knowledge, this is the first two-sample bidirectional MR study to comprehensively evaluate the causal relation between IS and CMBs. In the present MR study, we found that IS and SVS causally increased the risk of CMBs. However, no significant causal effect of CES and LVS on CMBs was observed, and no evidence to support the causal links of CMBs on IS and its subtypes.
Previous studies reported that the incidence of CMBs in the early onset of acute IS patients was about 12–39% [28], and 12.7% of IS patients developed new CMBs within 1 week after the onset [29]. In addition, it was found that IS patients with CMBs have a more than threefold increased risk of stroke recurrence compared with those without CMBs [30, 31], including hemorrhagic transformation or recurrent IS [32]. As of yet, the relationship between IS and CMBs has remained unclear. In all previous observational studies, it was difficult to avoid violations from confounding risk factors, while this present study, by applying MR methods, enabled us to confidently prove causality without bias because of a better study design. The primary MR analyses in our study were performed by IVW method, which provides the most precise estimates. If the IVW method result is significant (p < 0.05), even if the results of other methods are not, and no pleiotropy and heterogeneity were identified, it can be regarded as a positive result, provided that the beta values of the other methods are in the same direction. Therefore, through this MR study, we have sufficient reason to show that CMBs occur more frequently in patients with any IS and SVS. However, when exploring the causal effects of SVS on CMBs, because of the presence of only two significant and independent SNPs (rs12445022 and rs7766042) associated with SVS, sensitivity studies such as MR-Egger and MR-PREESO cannot be conducted. Furthermore, to explore whether CMBs have causal effects on IS and its subtypes, we also performed reverse MR analysis and found that CMBs are not causally connected with IS. A meta-analysis of patients with IS and transient ischemic attacks (TIA) found that CMBs increased the risk of recurrent IS [33]. A European study also discovered that CMBs led to a ninefold increased risk of recurrent IS [11]. Accordingly, CMBs may not contribute to IS, but they may increase the risk of stroke recurrence.
Although we found that IS increased the occurrence of CMBs, the pathophysiologic mechanisms and histopathologic basis of the relationship between CMBs and IS remain poorly understood and highly debated [10]. It was hypothesized that small vessels could be damaged by CMBs, leading to thrombosis in situ and reduced arterial circulation distal to CMBs, which not only reflect vessel fragility and endothelial instability, but also increase the risk of IS and hemorrhagic stroke [10, 11]. Additionaly, intracranial microvessels are particularly sensitive to excessive pressure and hemodynamic pulsatility. For patients with IS, the intracranial microvascular resistance decreases, and the excessive pressure in the carotid artery will be directly transmitted to the intracranial microcirculation, increasing cerebral blood perfusion, thereby playing a compensatory role [34]. However, high-pulse blood flow may cause microvascular damage to the brain, which may lead to damage to smooth muscle cells and vascular endothelial cells, thereby promoting the development of CMBs [35]. A study has also shown new CMBs are most likely to appear in the early stage of acute IS patients, and early control of blood pressure will count for much to prevent the occurrence and development of CMBs [29].
Several strengths of this study include the use of summarized statistics derived from very large genetic association studies. Additionally, several conservative MR methods were used to assess the consistency of the results. Due to the fact that genetic variation is allocated at the time of conception, MR analysis can also prevent reverse causation. There are, however, several limitations to our study. First, although there is no cohort overlap between IS (n = 1,296,908) and CMBs (n = 55,280), both population-based studies were obtained from mostly European research and might therefore have some sample overlap, resulting in inflation of test results. However, to avoid biases in causal estimates introduced by sample overlap, we chose the maximal sample sizes in GWAS while minimizing sample overlap between exposures and outcomes [36]. Second, the enrolled patients were all European; hence, there is no evidence that IS and CMBs are causally linked in other populations. Another factor to consider is that potential violations of instrumental variable assumptions could bias the MR analysis. Causing effect estimates to be biased may be the result of directional pleiotropy, which is difficult to completely eliminate. It has been observed, however, that pleiotropic effects are not evident in MR-Egger regression analysis or sensitivity analyses with other instruments, and other robust models show mostly similar results.
Conclusions
This is the first two-sample bidirectional MR study to comprehensively evaluate the potential causal relation between between IS and its subtypes with CMBs. Accordingly, our study provides evidence that IS and SVS are causally linked to an increased risk of CMBs. Further research is needed to determine the mechanisms of association between IS and CMBs.
References
Li Y, Tang Y, Yang G-Y. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc Neurol. 2021;6:483–95. https://doi.org/10.1136/svn-2020-000419.
Qin C, et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct Target Therapy. 2022. https://doi.org/10.1038/s41392-022-01064-1.
Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97:S6–16. https://doi.org/10.1212/wnl.0000000000012781.
Schlemm L, et al. Cerebral microbleeds and treatment effect of intravenous thrombolysis in acute stroke: an analysis of the WAKE-UP randomized clinical trial. Neurology. 2022;98:e302–14. https://doi.org/10.1212/wnl.0000000000013055.
Wilson D, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. 2019;18:653–65. https://doi.org/10.1016/s1474-4422(19)30197-8.
Greenberg SM, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74. https://doi.org/10.1016/s1474-4422(09)70013-4.
Tsai H-H, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds A PiB-PET study. Neurology. 2019;92:E774–81. https://doi.org/10.1212/wnl.0000000000006953.
Cordonnier C, Salman RA-S, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. https://doi.org/10.1093/brain/awl387.
Wilson D, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA a meta-analysis. Neurology. 2016;87:1501–10. https://doi.org/10.1212/wnl.0000000000003183.
Lim J-S, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack results from the Korean transient ischemic attack expression registry. JAMA Neurol. 2015;72:301–8. https://doi.org/10.1001/jamaneurol.2014.3958.
Fluri F, et al. Significance of microbleeds in patients with transient ischaemic attack. Eur J Neurol. 2012;19:522–4. https://doi.org/10.1111/j.1468-1331.2011.03522.x.
Jeon S-B, et al. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007;254:508–12. https://doi.org/10.1007/s00415-006-0406-6.
Li H-Q, et al. Causal relations between exposome and stroke: a Mendelian randomization study. J Stroke. 2022;24:236. https://doi.org/10.5853/jos.2021.01340.
Georgakis MK, et al. Genetic architecture of stroke of undetermined source: overlap with known stroke etiologies and associations with modifiable risk factors. Ann Neurol. 2022;91:640–51. https://doi.org/10.1002/ana.26332.
Fang S, et al. Parkinson’s disease and ischemic stroke: a bidirectional Mendelian randomization study. Transl Stroke Res. 2022;13:528–32. https://doi.org/10.1007/s12975-021-00974-6.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–6. https://doi.org/10.1001/jama.2017.17219.
Mishra A, et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. 2022;611:115–23. https://doi.org/10.1038/s41586-022-05165-3.
Malik R, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes (vol 50, pg 524, 2018). Nat Genet. 2019;51:1192–3. https://doi.org/10.1038/s41588-019-0449-0.
Adams HP Jr, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41.
Knol MJ, et al. Association of common genetic variants with brain microbleeds A genome-wide association study. Neurology. 2020;95:E3331–43. https://doi.org/10.1212/wnl.0000000000010852.
Pan A. Causal effect of Lp(a) Lipoprotein(a) level on ischemic stroke and alzheimer disease: a Mendelian randomization study (vol 50, pg 3532, 2019). Stroke. 2019;50:439–439. https://doi.org/10.1161/str.0000000000000214.
Hone L, et al. Age-specific effects of childhood body mass index on multiple sclerosis risk. J Neurol. 2022;269:5052–60. https://doi.org/10.1007/s00415-022-11161-4.
Chen X, et al. Kidney damage causally affects the brain cortical structure: a Mendelian randomization study. EBioMedicine. 2021. https://doi.org/10.1016/j.ebiom.2021.103592.
Zhang Q, et al. Causal relationship between lung function and atrial fibrillation: a two sample univariable and multivariable, bidirectional Mendelian randomization study. Front Cardiovasc Med. 2021;8:769198. https://doi.org/10.3389/fcvm.2021.769198.
Hemani G, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018. https://doi.org/10.7554/eLife.34408.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501. https://doi.org/10.1093/ije/dyt179.
Karhunen V, Bakker MK, Ruigrok YM, Gill D, Larsson SC. Modifiable risk factors for intracranial aneurysm and aneurysmal subarachnoid hemorrhage: a Mendelian randomization study. J Am Heart Assoc. 2021;10:e022277. https://doi.org/10.1161/jaha.121.022277.
Dannenberg S, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2014;45:2900–5. https://doi.org/10.1161/strokeaha.114.006448.
Jeon SB, et al. Rapid appearance of new cerebral microbleeds after acute ischemic stroke. Neurology. 2009;73:1638–44. https://doi.org/10.1212/WNL.0b013e3181bd110f.
Yakushiji Y, Yokota C, Yamada N, Kuroda Y, Minematsu K. Clinical characteristics by topographical distribution of brain microbleeds, with a particular emphasis on diffuse microbleeds. J Stroke Cerebrovasc Dis. 2011;20:214–21. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.12.001.
Boulanger JM, et al. Cerebral microhemorrhages predict new disabling or fatal strokes in patients presenting with acute ischemic stroke or transient ischemic attack. Stroke. 2006;37:643–643.
Thijs V, et al. Microbleeds and the risk of recurrent stroke. Stroke. 2010;41:2005–9. https://doi.org/10.1161/strokeaha.110.588020.
Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995. https://doi.org/10.1161/strokeaha.111.000038.
Schrag M, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119:291–302. https://doi.org/10.1007/s00401-009-0615-z.
Shan Y, et al. Association of aortic compliance and brachial endothelial function with cerebral small vessel disease in type 2 diabetes mellitus patients: assessment with high-resolution MRI. Biomed Res Int. 2016;2016:1609317. https://doi.org/10.1155/2016/1609317.
Huang Y, et al. Associations of visceral adipose tissue, circulating protein biomarkers, and risk of cardiovascular diseases: a Mendelian randomization analysis. Front Cell Dev Biol. 2022;10:840866. https://doi.org/10.3389/fcell.2022.840866.
Acknowledgements
We sincerely thank the original GWASs and the related consortiums for sharing and managing the summary statistics.
Funding
There was no funding for this study. The rapid service fee was funded by the authors.
Author Contributions
Renjie Liu proposed the idea and wrote the draft of the manuscript. Haoyuan Yin, Jiahui Feng and Yuhao Zhao contributed to the data analysis and manuscript revision. Jianmin Piao, Zhongxi Yang and Xin Shi prepared the figures and tables. Xuan Chen supervised the whole research and is responsible for the integrity of the study. All authors read and approved the final manuscript.
Disclosures
Renjie Liu, Xin shi, Jiahui Feng, Jianmin Piao, Zhongxi Yang,Yuhao Zhao, Haoyuan Yin and Xuan Chen have nothing to disclose.
Compliance with Ethics Guidelines
This study used publicly available de-identified data from participant studies that were approved by an ethical standards committee with respect to human experimentation. No separate ethical approval was required in this study.
Data Availability
All data generated or analyzed during this study are included in this article and its additional materials. Summary statistics generated by the GIGASTROKE consortium across ancestries and stroke subtypes are available in the GWAS Catalog (GCST90104540–GCST90104543). Summary statistics from published GWASs of CMBs are available in the Cerebrovascular Disease KP genetic association datasets (https://cd.hugeamp.org/datasets.html).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, R., Shi, X., Feng, J. et al. Ischemic Stroke and Cerebral Microbleeds: A Two-Sample Bidirectional Mendelian Randomization Study. Neurol Ther 12, 1299–1308 (2023). https://doi.org/10.1007/s40120-023-00500-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00500-w