Abstract
Introduction
Intravenous immunoglobulin (IVIG) is recommended as first-line therapy for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), an immune-mediated neuropathy. The clinical profile of patients with CIDP newly initiating IVIG is poorly characterized. This claims-based cohort study describes characteristics of US patients with CIDP initiating IVIG treatment.
Methods
Adult immunoglobulin (IG)-naïve patients with CIDP diagnosed between 2008 and 2018 and a subgroup of patients subsequently initiating IVIG were identified in the Merative MarketScan Research Databases. Demographics, clinical characteristics, and diagnostic procedures were described for patients initiating IVIG.
Results
Of 32,090 patients with CIDP identified, 3975 (mean age 57 years) subsequently initiated IVIG. In the 6 months prior to IVIG initiation, diagnoses of comorbidities including neuropathy (75%), hypertension (62%), and diabetes (33%) were frequent, as were CIDP features/symptoms/markers of functional status including chronic pain (80%), difficulty walking (30%), and weakness (30%). CIDP-related laboratory/diagnostic procedures were performed in approximately 20– > 40% of patients in the 3 months prior to IVIG initiation (63.7% underwent electrodiagnostic/nerve conduction testing in the 6 months prior to IVIG initiation). Patient characteristics by initial IVIG product differed only in IVIG initiation year, US geographic region, and insurance type. Comorbidities, CIDP severity or functional status markers, and other clinical variables were generally well balanced across initial IVIG product groups.
Conclusion
A heavy burden of symptoms, comorbidities, and diagnostic testing exists in patients with CIDP initiating IVIG. Characteristics of patients with CIDP initiating different IVIG products are well balanced, suggesting an absence of clinical or demographic determinants underlying IVIG selection.
Plain Language Summary
Intravenous immunoglobulin, also called IVIG, involves giving antibodies through a drip into a vein. IVIG is recommended as one of the first treatments that patients receive if they have chronic inflammatory demyelinating polyradiculoneuropathy, also called CIDP, which is a rare disease that causes the body’s immune system to attack its nerves. Our study described the characteristics of patients with CIDP who received IVIG in the USA. Information was collected from a large health insurance database and included records of patients aged ≥ 18 years who were diagnosed with CIDP between 2008 and 2018. Overall, 3975 patients with CIDP who received IVIG were included in the study. In the 6 months before starting IVIG, patients frequently had diagnoses of other diseases in addition to their CIDP; these included neuropathy (75% of patients), hypertension (62%), and diabetes (33%). CIDP features and symptoms that affected patients’ daily lives were also frequently reported in these 6 months, including long-lasting pain (80%), difficulty walking (30%), and weakness (30%). In the 3 months before starting IVIG treatment, 20% to > 40% of patients underwent diagnostic procedures related to their CIDP. Different IVIG products were used similarly, but the year of IVIG initiation, geographic region, and insurance type all differed by IVIG product. In conclusion, patients with CIDP who receive IVIG experience a heavy burden caused by their symptoms, other diseases, and CIDP-related procedures. Patient characteristics were generally similar between patients receiving different IVIG products, suggesting that no specific characteristics are factored in when doctors select an IVIG product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The clinical profile of patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) who initiate treatment in a real-world setting with intravenous immunoglobulin (IVIG) is poorly characterized |
This claims-based cohort study aimed to assess the characteristics of US patients initiating IVIG treatment for CIDP |
What was learned from the study? |
Patients initiating IVIG for CIDP in a real-world setting experience a heavy burden of symptoms and comorbidities |
There is a large degree of heterogeneity in diagnostic testing and degree of adherence to guideline criteria in diagnosing patients with CIDP in a real-world setting |
The characteristics of patients initiating different IVIG products for CIDP are well balanced, suggesting an absence of clinical or demographic determinants underlying IVIG selection |
Diagnostic guideline criteria for CIDP may not be consistently followed in a real-world setting |
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare inflammatory disorder in which the myelin sheath of peripheral nerves is gradually destroyed, resulting in significant burden on functional outcomes such as mobility, balance, and social functioning [1,2,3,4]. Clinical presentation of CIDP is diverse; symptoms include severe muscle weakness, numbness, and fatigue, leading to gait disturbances, impaired balance, and difficulty completing routine tasks [4]. Patients with CIDP also experience a substantial comorbidity burden, with the existence of concomitant conditions potentially influencing choice of treatment [5]. The disease demonstrates ‘typical’ and ‘atypical’ or variant subtypes [6, 7]. Typical CIDP manifests with relatively symmetrical distal and proximal weakness as well as impairment of large fiber sensations and sensory dysfunction across all extremities [6, 7]. CIDP variants share the main aspects of demyelination and response to immunotherapy but may differ either by the pattern of involvement or the modality primarily affected [5, 6, 8]. Diagnosis of CIDP may be challenging and represent additional disease burden for patients [9, 10]. Consensus around the most suitable diagnostic workup and the testing to conduct is limited, though diagnosis of the disease is mainly based on clinical manifestation and electrodiagnostic testing [8, 11]. Additional supportive information may be provided by imaging, nerve biopsy, immunologic testing, histologic analysis or investigation of cerebrospinal fluid, as well as response to treatment [8, 11]. Published data estimate that the incidence of CIDP is 0.2–0.7 cases per 100,000 person-years and the prevalence is 0.7–10.3 cases per 100,000 people [12, 13].
Joint guidelines from the European Academy of Neurology and the Peripheral Nerve Society (EAN/PNS) continue to recommend intravenous immunoglobulin (IVIG) or systemic corticosteroids as first-line treatment for CIDP with disabling symptoms, for both induction treatment and maintenance of response. The usual dosing regimen of IVIG used for CIDP is a total induction dose of 2 g/kg, divided over 2–5 days, followed by maintenance dosing (most commonly 1 g/kg every 3 weeks) [8]. In the most recent (2021) update to these CIDP guidelines from the EAN/PNS, immunoglobulin (IG) treatment recommendations have been further extended to allow for use of subcutaneous IG (SCIG) as an alternative maintenance treatment in IVIG-responsive patients with active disease [8]. Early detection and initiation of appropriate therapy may prevent loss of nerve function [14]. However, misdiagnosis of CIDP is common because many of the early clinical features mimic other more common neuropathies [15, 16]. This may result in up to 5-month delays in diagnosis and initiation of treatment, potentially causing considerable patient burden [15, 16]. IVIG itself may be associated with substantial treatment burden, and supply may be limited in some global regions [17]. Some patients may experience issues with the cost of therapy, depending on their location and medical insurance [18], which may represent an important source of patient concerns [19]. Treatment may also place considerable economic burden on healthcare systems, with mean annual costs for IVIG therapy in the USA reported at > $136,000 (2018 US dollars) per patient [20, 21]. Infusion frequency, access to an infusion center, and the associated patient time missed from work or school are other treatment burdens associated with IVIG, along with the number of needlesticks required and the potential for local infusion site reactions [18, 19]. In light of this, and the disease burden of CIDP, real-world analyses of patients with CIDP are needed to improve understanding of the characteristics of this population, their diagnostic and treatment journey, and their experiences of initiating IVIG; to our knowledge, no such study has been undertaken.
The aims of this study were to (1) describe the demographics, clinical characteristics, and diagnostic procedures performed among patients initiating IVIG in the USA for treatment of CIDP and (2) understand patterns of IVIG initiation by using existing US healthcare databases.
Methods
Data Source and Study Period
In this claims-based cohort study, de-identified patient data from 2003 to 2018 were collected from three distinct databases (Commercial Claims and Encounters Database, Medicare Supplementary and Coordination of Benefit Database, and Multi-State Medicaid Database) within the Merative MarketScan Research Databases. These insurance claims databases contain information on insurance plan enrollment, outpatient pharmacy dispensing information, and inpatient and outpatient diagnoses and procedures recorded on adjudicated, paid insurance claims. This study did not involve the collection, use, or transmittal of individually identifiable data. The databases used contained fully de-identified data and were compliant with the Health Insurance Portability and Accountability Act (HIPAA); this study was, therefore, exempt from institutional review board approval.
Study Population
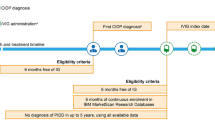
Adult IG-naïve patients with one or more recorded diagnosis codes for CIDP (International Classification of Diseases, 9th edition, clinical modification [ICD-9-CM] code 357.81; International Classification of Diseases, 10th edition, clinical modification [ICD-10-CM] code G61.81; diagnosis codes could be included in any coding position) from January 1, 2008 to September 30, 2018 were identified and are designated in this study as the “Full CIDP Cohort.” All patients from the commercial, Medicare, and Medicaid databases were combined into one analytic cohort. Patients were included if they were ≥ 18 years of age on the CIDP diagnosis date and had no recorded use of IG therapy in the 6 months before diagnosis (patients could receive other non-IG therapy for CIDP during this period). No minimum enrollment time was required before CIDP diagnosis. The first CIDP diagnosis meeting these eligibility criteria in the study period was assigned as the CIDP eligibility date (Fig. 1).
Patient inclusion and treatment timeline. CIDP chronic inflammatory demyelinating polyradiculoneuropathy, IG immunoglobulin, IVIG intravenous immunoglobulin, PIDD primary immunodeficiency disease, SCIG subcutaneous immunoglobulin. aPatients were ≥ 18 years of age on the CIDP eligibility date. bEligible patients could not have received any immunoglobulin therapy (IVIG or SCIG) for 6 months prior to diagnosis of CIDP to be included. To confirm, patients could have received other non-immunoglobulin therapies during this period. cIVIG treatments were identified through procedural (inpatient and outpatient Healthcare Common Procedure Coding System) and pharmacy dispensing (National Drug Code) codes
Compliance with Ethics Guidelines
This study did not involve the collection, use, or transmittal of individually identifiable data. The databases used contained fully de-identified/anonymized data and were compliant with the Health Insurance Portability and Accountability Act (HIPAA); this study was, therefore, exempt from institutional review board approval and administrative permission from individuals was not required.
IVIG Initiation
Within the Full CIDP Cohort, a sub-cohort of patients (the “IVIG Cohort”) who initiated IVIG after the CIDP eligibility date was identified. Initiation was defined as the first claim for an IVIG product preceded by at least 6 months of continuous enrollment with no IG therapy. The first IVIG administration date after the CIDP eligibility date was identified and assigned as the IVIG index date (Fig. 1). The IVIG product initiated for each patient was defined as the index IVIG, and the first dose was defined as the index IVIG dose. Patients with a diagnosis of a primary immunodeficiency disease occurring up to 5 years before the IVIG index date were excluded.
IVIG use was determined from outpatient or inpatient Healthcare Common Procedure Coding System (HCPCS) codes for the administration of an IVIG or from pharmacy dispensing codes (National Drug Codes) of an IVIG product. IVIG products were grouped by brand (Gammagard Liquid [Baxalta US Inc., Lexington, MA, USA], Gamunex-C [Grifols Therapeutics LLC, Research Triangle Park, NC, USA], Gammaked [Grifols Therapeutics LLC], and Privigen [CSL Behring AG, Bern, Switzerland]; all human intravenous immunoglobulin 10% infusions) and were assumed to be administered on the date of the outpatient procedure claim or the pharmacy dispensing claim. Gamunex-C and Gammaked have the same HCPCS code and were considered a composite IVIG product because they could not be differentiated in outpatient procedure coding.
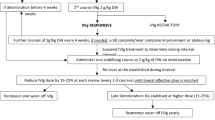
Evaluation of IVIG dosing in the 14 days after the IVIG index date (14-day loading period) to account for variability in dosing (e.g., loading dosing, titration to tolerance) immediately after initiation was also conducted. The steady-state dose was defined as the first administration of the index IVIG after the 14-day loading period. Patients were presumed to have discontinued the IVIG if they had not received a subsequent dose of IVIG by 12 weeks after the previous dose.
Patient and Treatment Characteristics
Patient and treatment characteristics were ascertained from enrollment information and from recorded diagnosis, procedure, or pharmacy-dispensing claims. Demographic characteristics, including age, sex, US geographic region, and insurance type, were determined at the CIDP eligibility date for the Full CIDP Cohort and at the IVIG index date for the IVIG Cohort. Comorbidities were identified up to 5 years before the CIDP eligibility date for the Full CIDP Cohort and up to 5 years before the IVIG index date for the IVIG Cohort, except when noted. Other characteristics, including markers of CIDP severity or functional status, prior CIDP treatments, and imaging or laboratory tests ordered, were assessed in the 6 months before the CIDP eligibility date for the Full CIDP Cohort and in the 6 months before the IVIG index date for the IVIG Cohort. High-dose steroids were defined as > 10 mg/day prednisolone or equivalent (oral or injectable). The immunosuppressant or immunomodulatory medications assessed were mycophenolate mofetil, interferon (2-α), etanercept, methotrexate, cyclophosphamide, cyclosporine A, rituximab, alemtuzumab, natalizumab, and eculizumab.
Statistical Analysis
For reporting distributions of patient and treatment characteristics of the Full CIDP Cohort and the IVIG Cohort, continuous variables were described in terms of means and standard deviations (SDs) and categorical and binary variables were described as counts (n) and percentages (%). Distributions of characteristics in the IVIG Cohort were stratified by IVIG product.
The cumulative proportions of patients in the IVIG Cohort with claims for laboratory tests and diagnostic procedures occurring before and after the IVIG index date were described using a population profile summary plot [22]. The aim of this evaluation was to inform whether patients initiating IVIG treatment were receiving the expected diagnostic workup at the time of treatment initiation. Additionally, population profile summary plots of the 30 most frequent ICD-9-CM diagnoses, ICD-10-CM diagnoses, and Current Procedural Terminology (CPT) procedure codes occurring in the 30-day periods before and after the IVIG index date were constructed for the IVIG Cohort.
To evaluate whether there were differences in the users of the three IVIG product categories, propensity scores (i.e., the predicted probability of receiving one treatment versus comparator, based on patient demographic and clinical characteristics) were estimated for each patient using logistic regression, with patient characteristics identified as predictor variables a priori. The distribution of the propensity scores by treatment group was plotted to visualize the extent of overlap [23]. Distributions were compared as follows.
-
Gammagard Liquid versus other IVIGs (Gamunex-C + Gammaked, Privigen).
-
Gamunex-C + Gammaked versus other IVIGs (Gammagard Liquid, Privigen).
-
Privigen versus other IVIGs (Gammagard Liquid, Gamunex-C + Gammaked).
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
A glossary of the study terms defined in the methods is shown in Table S1 in the electronic Supplementary Material.
Results
Study Population
A total of 32,090 eligible adults from the USA from January 2008 to September 2018 were identified from the Merative MarketScan Research Databases and included in the Full CIDP Cohort (Table S2 in the electronic Supplementary Material). In total, 357 patients were excluded owing to a diagnosis of primary immunodeficiency disease. Overall, 3975 patients subsequently initiated treatment with IVIG and were included in the IVIG Cohort.
IVIG Initiation
The mean age of the IVIG Cohort was 57.1 (SD 14.1) years, and 59.3% were men (Table 1). Almost all new IVIG use was started in an ambulatory setting (88.1% of index IVIG doses were identified in outpatient procedure coding; 11.2% from pharmacy dispensing for IVIG products). Diagnosis of comorbidities and disease features and markers of CIDP severity or functional status were reported frequently in the 6 months before the IVIG index date in the IVIG Cohort; comorbidities included neuropathy (75.4%), hypertension (62.1%), diabetes (33.1%), and disease features and markers of CIDP severity or functional status including neuropathic or chronic pain (80.2%), difficulty walking (30.2%), and weakness (30.9%). Few patients had previous non-IG CIDP therapy (e.g., plasma exchange, immunomodulation therapy), except for high-dose systemic corticosteroids (34.8%).
Patient characteristics on the IVIG index date generally were similar when grouped by IVIG product, except in terms of US geographic region, insurance type (Table 1), and index year (Table S2 in the electronic Supplementary Material). Other clinical variables, including comorbidities, use of other CIDP treatments, markers of CIDP severity or functional status, and healthcare utilization, were all generally balanced between treatment groups. Compared with patients in the Full CIDP Cohort, patients in the IVIG Cohort tended to have higher prevalence of comorbidities, more disease features, markers of CIDP severity or functional status, more imaging or laboratory testing, and more prior high-dose corticosteroid treatment (Table 1, Fig. 2, Table S2).
Comorbidity, CIDP features, and markers of disease severity or functional status in patients with CIDPa. CIDP chronic inflammatory demyelinating polyradiculoneuropathy, IVIG intravenous immunoglobulin. aThe evaluated comorbidities, CIDP features, and markers of disease severity or functional status were selected based on clinical input and include CIDP signs and symptoms, disease features, markers of symptom severity and closely related conditions that may have resulted in misclassification of disease status. Items were assessed in the 5 years before the CIDP eligibility date for the Full CIDP Cohort and in the 5 years before the IVIG index date for the IVIG Cohort, except where noted. bNeuropathies include hereditary motor and sensory neuropathy, hereditary and idiopathic neuropathy, systemic lupus erythematosus, multifocal motor neuropathy, and drug-induced polyneuropathy. cStroke category includes ischemic, traumatic, and sequelae of strokes. dAssessed in the 6 months before the CIDP eligibility date for the Full CIDP Cohort and in the 6 months before the IVIG index date for the IVIG Cohort
IVIG Initiation Patterns
During the 14-day loading period, 44.3% of patients received at least one additional dose of the index IVIG, and 44.7% of patients received at least one additional dose of any IVIG. Most patients received only one dose of index IVIG during the 14-day loading period (median [quartile 1, quartile 3] = 1 [1, 3]).
Thirty-two percent of patients did not receive a steady-state dosage after the 14-day loading period; 6% of patients were lost to follow-up (e.g., patient disenrolled from insurance or follow-up ended) during the loading period, 4% switched to another IG product (3% to another IVIG, 1% to a non-IV IG product) before the loading period elapsed, and 22% never received a subsequent IVIG dose after the loading period.
Laboratory Tests and Procedures Before and After IVIG Index Date
For approximately 20– > 40% of patients in the IVIG Cohort, laboratory tests and diagnostic procedures typically used for CIDP diagnosis were performed before the IVIG index date, with 63.7% of patients undergoing electrodiagnostic/nerve conduction testing within the 6 months prior to IVIG initiation (Fig. 3). The tests and procedures conducted were consistent with the diagnostic workup for CIDP. For example, analyses of cerebrospinal fluid and nerve biopsies were performed in 45.4% and 20.5% of patients, respectively, in the 6 months before the IVIG index date. Electrodiagnostic/nerve conduction tests and complete blood counts were performed in a high proportion of patients (> 40%) in the 60–84 days before the IVIG index date. Far fewer electrodiagnostic/nerve conduction tests were performed in patients in the 84 days after the IVIG index date (10.4%), compared with the 84 days before the IVIG index date (46.6%). Magnetic resonance imaging (13.8% vs. 3.8%), hemoglobin A1c assays (20.4% vs. 12.2%), and serum IG tests (24.2% vs. 6.4%) also were more common in the 84 days before the IVIG index date than after, consistent with a diagnostic workup for CIDP before IVIG treatment initiation. Complete blood count laboratory tests occurred frequently in the 84 days both before (41.7%) and after (41.0%) the IVIG index date. Approximately 85% of patients had either an ICD-9-CM or ICD-10-CM diagnosis code for inflammatory and toxic neuropathies on the IVIG index date (Fig S1 in the electronic Supplementary Material).
IVIG cohort claims for laboratory tests and diagnostic procedures occurring relative to index date. ALT alanine aminotransferase, BUN blood urea nitrogen, CBC complete blood count, CMP comprehensive metabolic panel, CT computed tomography, electro electrodiagnostic/nerve conduction, HbA1c hemoglobin A1c, IG immunoglobulin, IVIG intravenous immunoglobulin, MRI magnetic resonance imaging. The cumulative percentage of claims for each test as days deviated away from index date was compared, while keeping the denominator (i.e., number of patients with index) constant. Patient drop-out may affect or offset the percentage after the index date, but not before
IVIG Product User Differences
On visual inspection, there was substantial overlap in propensity score distributions between individual IVIG products (Fig. 4) with only minor differences in the modes and minimal nonoverlapping regions at the extremes. Overall, the distributions suggested reasonably high exchangeability between users of specific IVIG products.
Distribution of propensity scores by treatment group among patients in the IVIG cohort. a Gammagard Liquid n = 1507) versus other IVIGs (Gamunex-C + Gammaked, Privigen, n = 2468). b Gamunex-C + Gammaked n = 1780) versus other IVIGs (Gammagard Liquid, Privigen; n = 2195). c Privigen n = 688) versus other IVIGs (Gammagard Liquid, Gamunex-C + Gammaked; n = 3287). Comp composite IVIG comparator, IVIG intravenous immunoglobulin. Propensity scores, or the predicted probability of receiving 1 treatment versus comparator, were estimated for each patient and plotted by treatment groups to visualize the degree of overlap between the groups. Overlap between groups suggests that the probabilities of receiving the treatments were similar
Discussion
The findings of this US claims-based cohort study provide insight into the clinical and demographic characteristics of patients with CIDP who initiate IVIG products as well as the patterns of IVIG initiation in real-world practice. Patients with CIDP initiating IVIG were found to have a heavy burden of disease. Diagnoses of comorbidities and CIDP features and symptoms occurred frequently among patients with CIDP who initiated IVIG. In particular, neuropathies and neuropathic or chronic pain were recorded in the claims data of > 75% of patients initiating IVIG, representing significant disability and loss of functional status. The disease burden was compounded by a complex diagnostic pathway for some patients, with frequent laboratory tests and procedures ordered around the time of IVIG treatment initiation and diagnoses of other neuropathies and related disorders. Up to 40% of patients had European Federation of Neurological Societies (EFNS)/Peripheral Nerve Society (PNS) criteria procedures performed to aid in CIDP diagnosis [24], which is lower than might be expected given that the guidelines at the time of the study described electrodiagnostic testing as “mandatory” for CIDP diagnosis, with additional supportive tests if electrodiagnostic criteria are not met. This raises considerations around the feasibility and potential associated burden of consistent adherence to diagnostic guidelines in clinical practice across a range of real-world settings, as these findings reflect previous studies showing that adherence to such diagnostic criteria is not often followed in real-world settings [25, 26].
In this study, the disease burden observed in patients with CIDP initiating IVIG is consistent with other data on patients with CIDP. A systematic literature review by Querol et al. [10] assessed a range of functional outcome scales and health-related quality of life measures (e.g., Rankin Scale, physician assessment, Numeric Pain Rating Scale, Fatigue Severity Scale) in CIDP patient populations from the UK, Netherlands, Germany, and multinational studies and concluded that CIDP has a substantial physical effect on patients [10]. Similarly, the present study shows the prevalence and burdens of a wide range of systemic comorbidities, markers of CIDP severity or functional status, and long diagnostic pathways in a large US patient population. In the EAN/PNS guidelines for CIDP, initiation of IVIG treatment in patients with high disease burden is recommended; thus, the IVIG initiation patterns observed herein recommend initial treatment with IVIG, evaluating for response at 3-week intervals or based on clinical experience, then discontinuing as necessary if ineffective [8]. Patient characteristics appeared similar when stratified by IVIG product initiated in this study, indicating that IVIG product selection is not based on patient demographics and that IVIG products are used interchangeably in patients with CIDP; this was further illustrated in the analysis of propensity score distributions. Although product differences were found by year and US geographic region, these probably reflect fluctuations in IG product availability or evolving practice patterns rather than underlying clinical disparities.
Approximately one-third of patients discontinued IVIG treatment before receiving steady-state dosage. Misclassification of CIDP status may have led to inclusion of short-term IVIG use for some purpose other than chronic CIDP treatment; however, these data indicate that initiation of IVIG treatment in patients with CIDP may be challenging and that further education around CIDP diagnosis and IVIG initiation should be made available to clinicians. Contraindications, tolerance to treatment, lack of drug availability, and limited resources and insurance coverage factors probably play a role in treatment discontinuation. Approaches for improving treatment persistence or tolerance should be investigated, including administering IG treatment subcutaneously, because SCIG is reported to be preferable to IVIG in patients with CIDP [27, 28]. Further evaluation is needed of real-world characteristics of patients with CIDP receiving SCIG treatment to understand differences between IVIG and SCIG treatment utilization.
Limitations
This study describes a population of patients presumed to have CIDP in a real-world setting and involved claims data from commercial, Medicare, and Medicaid databases, though most of the patients had commercial insurance. Although the findings in this study represent a large and diverse population of patients with CIDP in the USA, they may not be generalizable to patients without insurance or with insurance types with restrictive formularies or reimbursement requirements.
As with any claims-based database analysis, the current study was limited by data availability and granularity. For example, insurance claims data indicate that a laboratory or diagnostic test was performed but do not include test results. These databases do not allow access to original patient charts or physician notes and, therefore, have less granularity than patient electronic health records. Records of diagnoses and tests prior to patient enrollment in the database are not available. These results also may be affected by misclassification of disease status or variations in coding of different events in claims data; CIDP symptoms may be similar to those of other diseases, and patients frequently receive diagnoses of multiple neuropathy types. Therefore, both the presence of at least one diagnosis code for CIDP and evidence of IVIG use were required when identifying patients eligible for analysis for the IVIG initiator cohort. However, it should be noted that while patients had one or more recorded diagnosis codes for CIDP, not all received laboratory or electrodiagnostic/nerve conduction testing to provide a definitive diagnosis, meaning some patients may have received IVIG ahead of certain diagnostic tests being performed. Finally, Gamunex-C and Gammaked share a billing code and therefore could not be investigated separately.
Conclusion
In this claims-based US cohort study, patients with CIDP initiating treatment with IVIG were found to have a heavy burden of CIDP symptoms and diagnostic testing. These findings suggest that IVIG treatment initiation is consistent with clinical guidelines; moreover, characteristics of patients initiating different IVIG products are well balanced, suggesting an absence of clinical or demographic determinants underlying IVIG selection. In addition, some patients received IVIG therapy in the absence of electrodiagnostic or nerve conduction tests. The feasibility and associated burden of consistent adherence to guideline criteria in real-world clinical practice should be considered. Additionally, this finding indicates an unmet need for provision of further clinician education around the diagnostic and therapeutic algorithms supporting diagnosis of CIDP to ensure optimal disease management and appropriate use of IVIG. More work is needed to elucidate the validity of CIDP diagnoses and the patterns and effectiveness of IVIG treatment regimens further.
References
Boukhris S, Magy L, Gallouedec G, Khalil M, Couratier P, Gil J, et al. Fatigue as the main presenting symptom of chronic inflammatory demyelinating polyradiculoneuropathy: a study of 11 cases. J Peripher Nerv Syst. 2005;10(3):329–37. https://doi.org/10.1111/j.1085-9489.2005.10311.x.
Guptill JT, Bromberg MB, Zhu L, Sharma BK, Thompson AR, Krueger A, et al. Patient demographics and health plan paid costs in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2014;50(1):47–51. https://doi.org/10.1002/mus.24109.
Merkies IS, Kieseier BC. Fatigue, pain, anxiety and depression in Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Eur Neurol. 2016;75(3–4):199–206. https://doi.org/10.1159/000445347.
Westblad ME, Forsberg A, Press R. Disability and health status in patients with chronic inflammatory demyelinating polyneuropathy. Disabil Rehabil. 2009;31(9):720–5. https://doi.org/10.1080/09638280802306497.
Doneddu PE, Cocito D, Manganelli F, Fazio R, Briani C, Filosto M, et al. Frequency of diabetes and other comorbidities in chronic inflammatory demyelinating polyradiculoneuropathy and their impact on clinical presentation and response to therapy. J Neurol Neurosurg Psychiatry. 2020;91(10):1092–9.
Allen JA. The misdiagnosis of CIDP: a review. Neurol Ther. 2020;9:43–54.
Khadilkar SV, Yadav RS, Patel BA, Khadilkar SV, Yadav RS, Patel BA. Chronic inflammatory demyelinating polyradiculoneuropathy. Neuromusc Disord. 2018. https://doi.org/10.1007/978-981-10-5361-0_38.
Van den Bergh PYK, van Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force—second revision. J Peripher Nerv Syst. 2021;26(3):242–68. https://doi.org/10.1111/jns.12455.
Eftimov F, Lucke IM, Querol LA, Rajabally YA, Verhamme C. Diagnostic challenges in chronic inflammatory demyelinating polyradiculoneuropathy. Brain. 2020;143(11):3214–24.
Querol L, Crabtree M, Herepath M, Priedane E, Viejo Viejo I, Agush S, et al. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol. 2020;268(10):3706–16. https://doi.org/10.1007/s00415-020-09998-8.
Reynolds J, George Sachs M, Stavros K. Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): clinical features, diagnosis, and current treatment strategies. R I Med J. 2016;99(12):32.
Broers MC, Bunschoten C, Nieboer D, Lingsma HF, Jacobs BC. Incidence and prevalence of chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta-analysis. Neuroepidemiology. 2019;52(3–4):161–72. https://doi.org/10.1159/000494291.
Laughlin RS, Dyck PJ, Melton LJ 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73(1):39–45. https://doi.org/10.1212/WNL.0b013e3181aaea47.
Chan YC, Wilder-Smith E. Predicting treatment response in chronic, acquired demyelinating neuropathies. Expert Rev Neurother. 2006;6(10):1545–53. https://doi.org/10.1586/14737175.6.10.1545.
Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85(6):498–504. https://doi.org/10.1212/WNL.0000000000001833.
Bunschoten C, Blomkwist-Markens PH, Horemans A, van Doorn PA, Jacobs BC. Clinical factors, diagnostic delay, and residual deficits in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2019;24(3):253–9. https://doi.org/10.1111/jns.12344.
Prevot J, Jolles S. Global immunoglobulin supply: steaming towards the iceberg? Curr Opin Allergy Clin Immunol. 2020;20(6):557.
Anderson JT, Cowan J, Condino-Neto A, Levy D, Prusty S. Health-related quality of life in primary immunodeficiencies: impact of delayed diagnosis and treatment burden. Clin Immunol. 2022;236: 108931.
Allen JA, Butler L, Levine T, Haudrich A. A global survey of disease burden in patients who carry a diagnosis of chronic inflammatory demyelinating polyneuropathy. Adv Ther. 2021;38:316–28.
Divino V, Mallick R, DeKoven M, Krishnarajah G. The economic burden of CIDP in the United States: a case-control study. PLoS One. 2018;13(10): e0206205.
Burt RK, Tappenden P, Balabanov R, Han X, Quigley K, Snowden JA, et al. The cost effectiveness of immunoglobulin vs. hematopoietic stem cell transplantation for CIDP. Front Neurol. 2021;12: 645263.
Thomas S, Chirila C, Ritchey M. Visualization of patient electronic records to support exploratory analysis and variable derivation of categorical data. In: 25th Annual Southeast SASI Users Group (SESUG) Conference. Cary, NC; 2017.
Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circulation. 2013;6(5):604–11.
Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol. 2010;17(3):356–63. https://doi.org/10.1111/j.1468-1331.2009.02930.x.
Gelinas D, Katz J, Nisbet P, England JD. Current practice patterns in CIDP: a cross-sectional survey of neurologists in the United States. J Neurol Sci. 2019;397:84–91. https://doi.org/10.1016/j.jns.2018.11.031.
Allen JA, Gorson KC, Gelinas D. Challenges in the diagnosis of chronic inflammatory demyelinating polyneuropathy. Brain Behav. 2018;8(3): e00932. https://doi.org/10.1002/brb3.932.
Gentile L, Mazzeo A, Russo M, Arimatea I, Vita G, Toscano A. Long-term treatment with subcutaneous immunoglobulin in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a follow-up period up to 7 years. Sci Rep. 2020;10(1):7910. https://doi.org/10.1038/s41598-020-64699-6.
Hadden RD, Marreno F. Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: improved tolerability and patient satisfaction. Ther Adv Neurol Disord. 2015;8(1):14–9. https://doi.org/10.1177/1756285614563056.
Acknowledgments
Funding
This work was conducted by researchers from RTI Health Solutions and Takeda Development Center Americas, Inc., and was funded by Takeda Development Center Americas, Inc. The rapid service fee was funded by Takeda Pharmaceuticals International AG.
Medical Writing and/or Editorial Assistance
Medical writing services provided by Katherine Chu, PharmD, of Oxford PharmaGenesis, Inc., were funded by Takeda Development Center Americas, Inc., and Takeda Pharmaceuticals International AG.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
J. Bradley Layton, Mary E. Ritchey, and Colin Anderson-Smits contributed to the conception and design of the study, analysis of data, and interpretation of the data. Zhongwen Huang contributed to the analysis of data and interpretation of the data. Shailesh Chavan, Hakan Ay, and Nizar Souayah contributed to the conception and design of the study and interpretation of the data. All authors have read and approved the final manuscript for submission.
Disclosures
J Bradley Layton is an employee of RTI Health Solutions, an organization funded by Takeda to conduct this research. Mary E. Ritchey is a former employee of RTI Health Solutions and is currently affiliated with Med Tech Epi, LLC, Philadelphia, PA, USA, and Center for Pharmacoepidemiology and Treatment Sciences, Rutgers University, New Brunswick, NJ, USA. Zhongwen Huang, Colin Anderson-Smits, and Hakan Ay are employees of Takeda Development Center Americas, Inc., and are Takeda shareholders. Shailesh Chavan was an employee of Takeda Development Center Americas, Inc. at the time of the study and is currently affiliated with Veloxis Pharmaceuticals, Cambridge, MA, USA. Mary E. Ritchey and Nizar Souayah have acted as consultants to Takeda for work outside of this manuscript.
Compliance with Ethics Guidelines
This study did not involve the collection, use, or transmittal of individually identifiable data. The databases used contained fully de-identified/anonymized data and were compliant with the Health Insurance Portability and Accountability Act (HIPAA); this study was, therefore, exempt from institutional review board approval and administrative permission from individuals was not required.
Data Availability
The data that support the findings of this study are available from Merative MarketScan®. Restrictions apply to the availability of these data; these data were used under license for this study. Data are available from the authors with the permission of Merative MarketScan®.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Layton, J.B., Ritchey, M.E., Huang, Z. et al. Intravenous Immunoglobulin Initiation in Patients with Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A US Claims-based Cohort Study. Neurol Ther 12, 1171–1186 (2023). https://doi.org/10.1007/s40120-023-00479-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00479-4