Abstract
Introduction
The aim of this study was to evaluate the accuracy of automated software (iStroke) on magnetic resonance (MR) apparent diffusion coefficient (ADC) and perfusion-weighted imaging (PWI) against ground truth in assessing infarct core, and compare the hypoperfusion volume and mismatch volume on iStroke with those on Food and Drug Administration-approved software (RAPID) in patients with acute ischemic stroke.
Methods
We used the single-volume decomposition method to develop the iStroke (iStroke; Beijing Tiantan Hospital, Beijing, China) software. Patients with ischemic stroke were collected from two educational hospitals in China with MR-PWI performed in the emergency department within 24 h of symptom onset. Infarct core volume was defined as ADC < 620 × 10−6 mm2/s and hypoperfusion volume was defined as Tmax > 6 s. We compared the accuracy of infarct core volume using iStroke and RAPID (iSchema View Inc, Menlo Park, CA) software with ground truth.
Results
We included 405 patients with acute ischemic stroke with MR ADC and PWI sequences. The infarct core volume on iStroke (median 2.43 ml, interquartile range [IQR] 0.60–10.32 ml) was not significantly different from the ground truth (median 2.89 ml, IQR 0.77–9.17 ml) (P = 0.07); Bland–Altman curves showed that the core volume of iStroke and RAPID software were comparable with each other on individual agreement with ground truth. The hypoperfusion volume and mismatch volume on iStroke were not statistically different from those on the RAPID software, respectively. In patients with large vessel occlusion (n = 74), the agreement between iStroke and RAPID was substantial (kappa = 0.76) according to DEFUSE 3 criteria (infarct core < 70 ml, mismatch volume ≥ 15 ml, and mismatch ratio ≥ 1.8).
Conclusions
The iStroke automatic processing of ADC and PWI is a reliable software for the identification of diffusion–perfusion mismatch in acute ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Magnetic resonance diffusion-weighted imaging and perfusion-weighted imaging sequences allow more accurate identification of core and hypoperfused tissue than computed tomography perfusion in patients with acute ischemic stroke, but automatic perfusion-weighted imaging data assessment in the Chinese population is lacking. |
We hypothesized that our newly developed automated perfusion-weighted imaging software was comparable with Food and Drug Administration-approved software on infarct core and hypoperfusion volumes. |
What was learned from the study? |
The infarct core volume on iStroke (median 2.43 ml, interquartile range, 0.60–10.32 ml) was not significantly different from the ground truth (median 2.89 ml, interquartile range 0.77–9.17 ml) (P = 0.07), and the hypoperfusion volume on the iStroke (median 7.68 ml, interquartile range, 0–56.65 ml) was not statistically different from that on the RAPID software (median 5, interquartile range, 0–56.00 ml) (P = 0.87) |
The iStroke perfusion-weighted imaging software may be serve as an acceptable automatic evaluation software for infarct core and hypoperfusion volumes in China. |
Introduction
Magnetic resonance perfusion-weighted imaging (MR-PWI) allows noninvasive identification of hypoperfused tissue in patients with acute ischemic stroke (AIS), which may progress to infarction without timely reperfusion. Quick, brief, and accurate assessment of the volume of the hypoperfused brain tissue area on MR-PWI is yielded for optimal management strategy selection for AIS in clinical practice, e.g., endovascular treatment or thrombolysis treatment.
Automatic machine analysis utility in neuroimaging has largely saved time for hemodynamic assessment. Currently, several software packages have been developed and widely used to automatically assess CT perfusion (CTP) to identify the ischemic core [1,2,3], such as the RAPID software (iSchema View Inc, Menlo Park, CA, USA), and the Olea software (Olea Medical Solutions, La Ciotat, France) (https://www.olea-medical.com/en/), but they were challenged by low sensitivity for infarct core [4]. MR diffusion-weighted imaging (DWI) is the gold standard for infarct core evaluation, and the RAPID software has just been approved by the Food and Drug Administration (FDA) for automatic assessment of MR-PWI [5]. Moreover, MRI use in emergency departments is getting increasingly popular in China but there is a lack of reports of automatic MR-PWI data assessment in the Chinese population, and no relevant automatic assessment software has been approved by the National Medical Products Administration for cerebral perfusion assessment in China.
In this study, we aimed to introduce newly developed automated software that could assess MR-PWI automatically and investigate its accuracy in assessing infarct core volume in AIS by comparing it with manually assessed infarct core volume and comparing hypoperfusion and mismatch volume between the new software and FDA-approved software.
Methods
Study Design and Subjects
This was a multicenter, retrospective, and observational study. We recruited patients admitted to Beijing Tiantan hospital and Shanghai Fourth People's Hospital between January 2016 and August 2020 with the following criteria: (1) adult patients with AIS admitted within 24 h of symptom onset; (2) the patient underwent MR-PWI within 24 h of symptom onset; (3) imaging quality that allowed successful assessment of infarct core volume. We excluded patients being diagnosed as stroke mimics, chronic heart failure, low-quality imaging data (e.g., significant artifacts, etc.), and incomplete/low quality medical records. For eligible patients, we collected the demographics (age and sex), cardiovascular risk factors (hypertension, hyperlipidemia, diabetes mellitus, etc.), systolic and diastolic blood pressure at admission, NIHSS at admission, large vessel occlusion (LVO) site by magnetic resonance angiography, prior modified Rankin scale, treatment strategies (intravenous thrombolysis, endovascular treatment) and modified Rankin scale score at 90 days.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the principles of the Declaration of Helsinki. The study was approved by the institutional review board and ethics committee at Beijing Tiantan hospital (KY2022-029-01) and Shanghai Fourth People’s Hospital (2020066-001) without requirement of patients’ written informed consents.
MRI Protocol
At Beijing Tiantan Hospital, MRI was performed on a single 3.0 Tesla clinical scanner (SIEMENS TrioTim, Siemens Healthineers). DWI sequences were acquired at baseline with two b values of 0 and 1000 s/mm2. Scanner parameters for DWI were slice thickness = 5 mm, repetition time = 3000 ms, echo time = 75 ms, number of averages = 2, flip angle = 90°, voxel spacing = 1.80 × 1.80 × 6.5 mm3, matrix = 128 × 128, FOV = 230 × 230 mm2. Dynamic susceptibility contrast (DSC) MR-PWI was acquired in the axial direction. Scanner parameters for the DSC-MR-PWI were slice thickness = 5 mm, repetition time = 1400 ms, echo time = 32 ms, flip angle = 90°, voxel spacing = 1.80 × 1.80 × 6.5 mm3, axial slices = 19 slices, temporal coverage = 50 time points, matrix = 128 × 128, FOV = 230 × 230 mm2.
At Shanghai Fourth People’s Hospital, MRI was performed on a single 1.5-Tesla clinical scanner (SIEMENS Avanto Magnetic Field Strength, Siemens Healthineers). DWI sequences were acquired at baseline with two b values of 0 and 1000 s/mm2. Scanner parameters for DWI were slice thickness = 5 mm, repetition time = 3600 ms, echo time = 102 ms, number of averages = 4, flip angle = 90°, voxel spacing = 1.20 × 1.20 × 6.5 mm3, matrix = 192 × 192, FOV = 229 × 229 mm2. DSC-MR-PWI were acquired in the axial direction. Scanner parameters for DSC-MR-PWI were slice thickness = 5 mm, repetition time = 1590 ms, echo time = 32 ms, flip angle = 90°, voxel spacing = 0.90 × 0.90 × 6.5 mm3, axial slices = 20 slices, temporal coverage = 50 time points, matrix = 256 × 256, FOV = 230 × 230 mm2.
Processing Pipeline
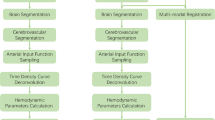
Image analysis software (iStroke; Beijing Tiantan Hospital, Beijing, China) is used for the measurement of ischemic lesion volumes and volumes of hypoperfused tissue from the DWI and DSC-MR-PWI sequences. The specific module is divided into four parts: (1) image pre-processing; (2) apparent diffusion coefficient (ADC) lesion segmentation; (3) hypoperfused tissue evaluation; and (4) calculation of perfusion–diffusion mismatch. The main theory methodology is similar as a previously published study [6]. The selection of arterial input and venous output functions was in a fully automatic, operator-free manner according to a detection algorithm [6].
Image Pre-processing
Due to the number of slices of MR-PWI and DWI may be inconsistent; furthermore, the application of the strong motion-probing gradients, the effect of minute motion can lead to erroneous image encoding and substantial artifacts, so a pre-processing step was utilized to enable the consistent analysis of cohorts. Nonlinear image registration, a complimentary approach to rigid body registration, can accommodate inter-subject differences in brain morphology and can also offer the opportunity to track tissue outcomes more accurately in the same subject where the structure of the brain varies across timepoints. Therefore, we adopted nonlinear image registration to solve slice inconsistency, motion correction, and time point correction. Skull stripping is a crucial pre-processing step incorporated in brain magnetic resonance imaging processing applications. It deals with the removal of non-brain tissues from brain. Although manual segmentation has the highest accuracy, it is a time-consuming task. Therefore, we were composed of adaptive iterative thresholding to analyze and remove of connected components. Finally, morphological operations were carried out to obtain the brain mask [7].
ADC Lesion Segmentation
DWI lesion volumes are assessed on the affected sections with hyperintense areas visible from the b = 1000 mm/s2 images. Because a brain tumor and edema also show high signals on the b1000 images, the physician paid particular attention to the typical locations of bilateral artifact and produced ADC maps as necessary to identify positive DWI lesions. In the iStroke, infarct regions are automatically segmented on the ADC maps by applying normalized absolute thresholding. A quantile curve of ADC intensities within the brain mask is constructed for each ADC map of each subject, and an intersection point between two tangent lines with maximum and minimum differential coefficients is identified on each quantile-intensity curve. The ADC maps are normalized by dividing the intensities on each by the intensity at the intersection point. This normalization process results in the intensities of normal white matter tissues converging into one. In this way, white matter can be prevented from being misjudged as an infarct lesion.
Hypoperfused Tissue Evaluation
After the iStroke read all MR-PWI datasets, it automatically gave the first pass curve of the bolus injection contrast agent to determine the first pass time [8]. When confirming the input time, this program can automatically calculate brain perfusion parameter maps with circulant singular value decomposition (cSVD) deconvolution methods to eliminate the effect of tracer-arrival delay [9], including relative cerebral blood flow (rCBF) map, relative cerebral blood volume map, time-to-peak map, and mean transit time map, and time-to-peak impulse response (Tmax) map, which reflects the delay of the bolus agent reaching the ischemic area.
Calculation of Perfusion–Diffusion Mismatch
Perfusion–diffusion mismatch (penumbra) is defined as the difference in the volume of segmented hypoperfused tissue volume and the segmented volume of an ADC lesion. This mismatch may help identify patients for thrombolysis [10]. iStroke quantified the location of hypoperfused tissue and ADC lesion and automatically calculated the volume of perfusion–diffusion mismatch. A delay of 6 s (Tmax > 6 s) of the time to the maximum of the residue function was chosen as a threshold for hypoperfused tissue and ADC (620 × 10−6 mm2/s) volume estimation was also chosen as a threshold for infarct focus.
RAPID Software on MR-PWI
The RAPID software (iSchema View Inc, Menlo Park, CA) was approved by the FDA and was used to automatically measure the infarct core volume and hypoperfusion volume. ADC < 620 × 10−6 mm2/s was considered as infarct core, and Tmax > 6 s as hypoperfusion volume.
Ground Truth for Infarct Core
A 15-year experienced neurologist (X.Y.) and a 10-year experienced neuroradiologist (W.M.) measured the infarct core volume manually on DWI with corresponding of ADC images using 3D Slicer (https://www.slicer.org/). Twenty cases were randomly selected to test inter-rater reliability and intra-rater reliability 1 month later, which yielded intraclass correlation coefficients 0.956 (95% confidence interval 0.889–0.983) and 0.985 (95% confidence interval 0.964–0.994), respectively.
Statistical Analyses
Chi-square tests and Fisher’s exact tests were used to compare the categorical variables. Inter-rater reliability and intra-rater reliability were evaluated by intraclass correlation tests. Mann–Whitney U tests were used to assess the differences of infarct core ground truth and MR-PWI infarct core volume assessed by the iStroke software and by the RAPID software. Dice value was used to assess the morphological agreement between ground truth of infarct core and iStroke infarct core. In addition, Bland–Altman plots were utilized to assess the agreement of ground truth and MR-PWI infarct core volumes by the iStroke software and by the RAPID software. Statistical analysis was performed using the SPSS version 22 software package (SPSS Inc, Chicago, IL, USA).
Results
Patients’ Characteristics
We enrolled 405 patients with AIS with MR-PWI examination from Beijing Tiantan Hospital and Shanghai Fourth People's Hospital. Among 405 patients, 74 were diagnosed as LVO patients by magnetic resonance angiography. The occlusion sites were 38 middle cerebral artery, 24 internal cerebral artery, six basilar artery, five vertebral artery, one posterior cerebral artery. Table 1 shows the clinical characteristics of the included subjects and patients with LVO.
Infarct Core Volume on Automated Software and Ground Truth
The infarct core volume on iStroke (median 2.43 ml, IQR 0.60–10.32 ml) was not significantly different from the core infarct ground truth (median 2.89 ml, IQR 0.77–9.17 ml) (P = 0.07), whereas the infarct core volume on the RAPID (median 0 ml, IQR 0–11.50 ml) was significantly smaller than the core infarct ground truth (median 2.67 ml, IQR 0.73–7.25 ml) (P < 0.001). In LVO patients, the infarct core volume on iStroke and that on RAPID were not significantly different from the ground truth either (Table 2).
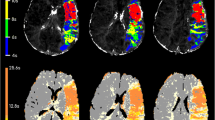
Bland–Altman curves showed that infarct core volume of iStroke and RAPID software were comparable with each other on individual agreement with ground truth in all patients (Fig. 1) and in patients with LVO (Fig. 2). Figure 3 shows one case with similar infarct core volumes on the iStroke, RAPID, and ground truth.
A case’s penumbral evaluation on iStroke, RAPID, and DWI source image. A iStroke summary result: infarct core volume 17.3 ml, hypoperfusion volume 137.6 ml. B iStroke artery input function: left middle cerebral artery M1 segment; artery input function and venous output function curves were acceptable. C, D magnetic resonance-diffusion-weighted imaging and apparent diffusion coefficient showed. Centrum semiovale and corona radiata infarct with ground truth 15.8 ml. E RAPID summary result: infarct core volume 13 ml, hypoperfusion volume 141 ml. F RAPID artery input function: left middle cerebral artery M2 segment. G RAPID artery input function and venous output function curves were acceptable as well
Hypoperfusion and Mismatch Volume on Automated Software
In all subjects, the hypoperfusion volume on the iStroke (median 7.68 ml, interquartile range [0–56.65 ml]) was not statistically different from that on the RAPID software (median 5 ml, interquartile range [0–56.00 ml]) (P = 0.87). The mismatch volume on the iStroke (median 1.32 ml, interquartile range [0–34.4 ml) was not significantly different than that on the RAPID (median 0 ml, interquartile range [0–40.5 ml]) (P = 0.15).
In LVO patients, the hypoperfusion volume on the iStroke (median 68.09 ml, interquartile range [15.67–128.11 ml]) was numerically larger than that on the RAPID software (median 58 ml, interquartile range [20–149.50 ml]) (P = 0.07). The mismatch volume on the iStroke (median 16.51 ml, interquartile range [0–80.16 ml]) was numerically smaller than that on the RAPID (median 39.00 ml, interquartile range [0–282.25 ml]) (P = 0.10).
The intraclass correlation between iStroke core and RAPID core was 0.968 (0.961–0.974), and the intraclass correlation between iStroke hypoperfusion and RAPID hypoperfusion was 0.916 (0.898–0.931).
Agreement of Detection Large Infarct Core
Among all patients, 23 of 405 patients had large infarct core (ground truth core volume > 70 ml). The iStroke and RAPID had high agreement with the infarct core ground truth (kappa = 0.82 and kappa = 0.79, respectively). In LVO patients, 14 of 74 patients had large infarct core (ground truth core volume > 70 ml), and the iStroke and RAPID had high agreement with the infarct core ground truth (kappa = 0.86 and kappa = 0.80, respectively) as well.
Agreement Between iStroke and RAPID According to DEFUSE 3 Criteria
In patients with LVO (n = 74), the agreement between iStroke and RAPID was substantial (kappa = 0.76) according to DEFUSE 3 criteria (infarct core < 70 ml, mismatch volume ≥ 15 ml, and mismatch ratio ≥ 1.8).
Morphological Agreement Between Core Infarct on iStroke and Ground Truth
In all patients, the morphological agreement between iStroke core and ground truth was moderate with a mean dice value of 0.65, while in patients with LVO and in patients with large infarct core (ground truth > 70 ml), the agreement between iStroke infarct core and ground truth was both good with mean dice value 0.77 and 0.79, respectively.
Discussion
We have developed a novel real-time, fully automated software to identify diffusion–perfusion mismatch in patients with AIS in China. With a large sample of datasets, the iStroke core volume was closely correlated with the ground truth, and it was comparable with the RAPID software on agreement with the ground truth infarct core. In patients with LVO, morphological agreement between iStroke and the ground truth infarct core was good, and mismatch selection agreement between iStroke and RAPID was substantial according to DEFUSE 3 criteria.
The current study demonstrated that the core volume on iStroke was closely correlated with the ground truth and had a comparable capacity to assess infarct core volume on MR-PWI as the RAPID software. The iStroke was proved to be able to assess the infarct core volume on MR-PWI. Positron emission tomography (PET) was the most widely used method to measure microcirculation in the past. However, its disadvantages were poor spatial and temporal resolution and patient exposure to ionizing radiation and functional MRI may be a more optimal imaging method [11]. Tissue blood volume, which represents core volume, is easier to compute compared with tissue blood flow [12], especially through MR scan [13].
The development of iStroke provided an option for stroke centers and neurologists to quantify ischemic penumbra rapidly and automatically in China. The mismatch volumes on the iStroke software were numerically smaller than the RAPID software, due to limited sample in patients with LVO, it did not reach statistically significance, however, the relatively smaller mismatch volume may induce restrict patient selection for thrombectomy in China, only large mismatch volume patients on the RAPID software will be candidates for thrombectomy, which may be safer for Chinese patients considering the rate of symptomatic rate intracranial hemorrhage and mortality rate were higher in China than that in the USA [14]. In addition, one of the most important functions for RAPID was case selection for thrombectomy in LVO patients according to the DEFUSE 3 trial criteria, and the iStroke reached high agreement with the RAPID software on case selection based on such criteria.
The patients we included mostly had small infarcts. Although the perfusion mismatch is targeting for case selection for reperfusion therapy, patients with LVO are a perfect group for software comparison, while whether small lesions also have important view of the accuracy of the software from a clinical point of view. Local doctors will challenge the accuracy of the software result if the DWI clearly shows a small lesion but with 0 ml core on the software. The motion, spatial resolution, and machine model affects the result. However, we excluded severe motion, and adjusted the algorithm for different machine model, this factors were adjusted through repeated improvement of the algorithm. We particularly focused on the infarct core detection and improved the model sensitivity to small infarcts, so we did not exclude small lesions in the analyses.
The development of the RAPID software was based on the imaging data from Caucasian people, and iStroke was developed based on the imaging data from Eastern-Asian people. Many regions in East-Asia have a great burden of ischemic stroke and the resources of the health care system are limited, which limits the generalization of reperfusion therapy. Hence, the development and release of iStroke would contribute to the generalization of reperfusion therapy in the late window and reduce the burden of ischemic stroke in East-Asian regions and other developing countries.
The current study has the advantage of a large sample size to test the model in the training set and validation set. Patients with MR-PWI before reperfusion therapy are scarce in clinical practice because an MRI scan is more time-consuming than CT, and prolonged the door-to-needle time and door-to-puncture time. Fortunately, the current study enrolled sufficient patients and had a large sample size to prove the accuracy of iStroke infarct core against the ground truth. In addition, we used circulant singular value decomposition (cSVD), a single global threshold value for truncating the number of small singular values before decomposition, to develop our software. To avoid the leakage of the contrast agent, which leads to CBV underestimation, we increase the step of leakage correction to improve the accuracy of the program.
There are several limitations to our study. First, the current study only included two sites to enroll study participants and collect clinical and imaging data. Few stroke centers could perform MRI scans in the emergency room in China. Both of the sites in our study provided imaging data of high quality to conduct a qualified dataset. Second, the retrospective design of our study limited the generalization of the study conclusion. However, the demonstration of similar results between the iStroke infarct core and ground truth in our study laid a solid foundation for penumbral evaluation between the two software programs. Thirdly, advanced artificial intelligence algorithms including deep learning or machine learning may increase the correlation between perfusion assessment software and ground truth, which warrants further investigations. Finally, we did not analyze the variability of assessment of penumbra. Further studies could determine the intraobserver and interobserver variability in the assessment of penumbra. In addition, further studies could determine the accuracy of the software to determine the presence of hemorrhage, vascular occlusion, and collateral circulation in the acute stroke setting.
Conclusions
The iStroke automatic processing of ADC and MR-PWI is reliable software for the identification of diffusion–perfusion mismatch in acute ischemic stroke.
References
Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21.
Wheeler HM, Mlynash M, Inoue M, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44:681–5.
Xiong Y, Huang CC, Fisher M, Hackney DB, Bhadelia RA, Selim MH. Comparison of automated CT perfusion softwares in evaluation of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28: 104392.
Wolman DN, Iv M, Wintermark M, et al. Can diffusion- and perfusion-weighted imaging alone accurately triage anterior circulation acute ischemic stroke patients to endovascular therapy? J Neurointerv Surg. 2018;10:1132–6.
Straka M, Albers GW, Bammer R. Real-time diffusion–perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–37.
Jiang C-F, Huang C-H, Yang S-T. Using maximal cross-section detection for the registration of 3D image data of the head. J Med Biol Eng. 2011;31(3):217–26.
Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol. 2011;32:1004–10.
Harris RJ, Cloughesy TF, Hardy AJ, et al. MRI perfusion measurements calculated using advanced deconvolution techniques predict survival in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2015;122:497–505.
Deutschmann H, Hinteregger N, Wiesspeiner U, et al. Automated MRI perfusion–diffusion mismatch estimation may be significantly different in individual patients when using different software packages. Eur Radiol. 2021;31:658–65.
Rempp KA, Brix G, Wenz F, Becker CR, Gückel F, Lorenz WJ. Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology. 1994;193:637–41.
Weisskoff RM, Chesler D, Boxerman JL, Rosen BR. Pitfalls in MR measurement of tissue blood flow with intravascular tracers: which mean transit time? Magn Reson Med. 1993;29:553–8.
Vonken EJ, van Osch MJ, Bakker CJ, Viergever MA. Measurement of cerebral perfusion with dual-echo multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging. 1999;10:109–17.
Zi W, Wang H, Yang D, et al. Clinical effectiveness and safety outcomes of endovascular treatment for acute anterior circulation ischemic stroke in China. Cerebrovasc Dis. 2017;44:248–58.
Acknowledgements
Funding
This study was supported by the Beijing Municipal Science & Technology Commission (Z211100003521019), which funded the journal’s Rapid Service Fee.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have approved the final manuscript for publication.
Author contributions
Study concept and design: Yongjun Wang and Yunyun Xiong. Acquisition of data: Yu Luo, Mingming Wang, Guangshuo Li. Analysis or interpretation of data: all authors. Yunyun Xiong wrote the first draft of the manuscript. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Kaixuan Yang.
Disclosures
Yunyun Xiong, Yu Luo, Mingming Wang, Shih-Ting Yang, Ruiqiong Shi, Wanxing Ye, Guangshuo Li, Kaixuan Yang, Shang Wang, Zixiao Li, and Yongjun Wang have nothing to disclose.
Compliance with ethics guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the principles of the Declaration of Helsinki. The study was approved by the institutional review board and ethics committee at Beijing Tiantan hospital without requirement of patients’ written informed consents (KY2022-029-01).
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xiong, Y., Luo, Y., Wang, M. et al. Evaluation of Diffusion–Perfusion Mismatch in Acute Ischemic Stroke with a New Automated Perfusion-Weighted Imaging Software: A Retrospective Study. Neurol Ther 11, 1777–1788 (2022). https://doi.org/10.1007/s40120-022-00409-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00409-w