Abstract
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder in children/adolescents and occurs frequently with psychiatric/neurologic comorbidities. The objective of this study was to assess the impact of psychiatric/neurologic comorbidities on pharmacotherapy patterns among patients with ADHD in Sweden.
Methods
A retrospective cohort analysis was conducted using medical records from a regional database in Sweden. Patients aged 6–17 years, with ≥1 prescription for ADHD medication between July 1, 2007 and June 30, 2009, and continuously active in the database for ≥12 months before and after their prescription index date were selected. Patients were categorized as ADHD alone (ADHD-only) or with comorbidities (ADHD-comorbid). Between-group differences were analyzed before and after adjusting for potentially confounding variables.
Results
Data on 1794 patients (1083 ADHD-only; 711 ADHD-comorbid) were analyzed. Among newly treated patients, 21.7% augmented their index therapy (ADHD-only, 20.5%; ADHD-comorbid, 24.4%; p = 0.23). After adjustment, ADHD-only patients were less likely (p = 0.002) to augment versus ADHD-comorbid patients [odds ratio = 0.44, 95% confidence interval (CI) 0.27, 0.73]. ADHD-comorbid patients received more prescriptions versus ADHD-only patients (mean 13.1 vs 10.0; p < 0.001), and had more outpatient visits (mean 11.9 vs. 8.1; p < 0.001) and hospitalizations (10.7% vs. 6.0%; p < 0.001). After adjustment, ADHD-only patients had fewer outpatient visits (p < 0.001) and referrals (p < 0.001) versus ADHD-comorbid patients (visits: β = −0.21, 95% CI −0.28, −0.13; referrals: β = −0.25, 95% CI −0.33, −0.18).
Conclusion
Patients with ADHD with comorbidities had more hospitalizations, physician visits, and medication prescriptions during 12 months’ follow-up than did those with ADHD alone. ADHD therapy augmentation was prevalent among children/adolescents with ADHD, even among those without psychiatric/neurologic comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by core symptoms of inattention and/or hyperactivity and impulsiveness [1]. Psychological, social, educational, and/or occupational impairment must be present to meet ADHD diagnostic criteria [1, 2]. ADHD is one of the most commonly occurring mental health disorders among children and adolescents [3]. Indeed, the prevalence of ADHD among children/adolescents in Europe is estimated to be almost 5% [4, 5]. In Sweden, ADHD is reported to affect 4.0–5.6% of school-aged children in population-based studies [6, 7].
ADHD frequently occurs with other health conditions including psychiatric and neurologic disorders [7,8,9,10]. For example, depression occurs approximately five times more frequently among children/adolescents with ADHD than in the general population [11]. Approximately 60% of children with ADHD are also reported to meet diagnostic criteria for oppositional defiant disorder [11, 12]. Other conditions such as developmental coordination disorder, conduct disorder, autism spectrum disorder (ASD), anxiety disorder, tics, and substance abuse disorder also frequently coexist with ADHD [9, 12, 13]. The presence of multiple psychiatric comorbidities is associated with poorer psychological functioning and greater impairment in quality of life among patients with ADHD [8].
European evidence-based management guidelines for ADHD recommend multimodal therapy, comprising both non-pharmacologic interventions and pharmacotherapy [1, 14]. Stimulants have favorable efficacy and tolerability profiles among patients with ADHD. Accordingly, stimulants are generally recommended in Europe as first-line pharmacotherapy for children/adolescents with ADHD [1, 14]. However, approximately 30% of children/adolescents with ADHD are unresponsive to the first prescribed stimulant medication and approximately 10% fail to respond to a second-line stimulant [15, 16]. Two non-stimulant ADHD medications are approved for use among children/adolescents in Europe [17, 18]: atomoxetine was approved in 2004 and guanfacine extended-release in 2015 [19, 20]. Combination pharmacotherapy may be indicated for patients with ADHD and comorbidities, and is sometimes required in clinical practice for patients with ADHD alone [14].
Although it is well established that ADHD often occurs with comorbid conditions, data on the associated burden of disease in Europe are sparse, and no data are available for Sweden. This association is complex as patients may have symptoms potentially attributable to multiple disorders. This study was conducted to assess the impact of comorbidities on pharmacotherapy patterns among children/adolescents with ADHD in Sweden.
Methods
This was a retrospective cohort study of patients with ADHD, with or without psychiatric or neurologic comorbidities, aged 6–17 years initiating or continuing product label-approved ADHD pharmacotherapy in Sweden. Data from July 1, 2006 to June 30, 2010 were evaluated using the Swedish Clinical Evidence Based Prescription Analysis (CEBRxA) medical records database.
CEBRxA covers the Västra Götaland region of southwest Sweden and includes approximately 1.5 million patients (approximately 15% of Sweden’s total population). The age distribution of patients in the database is very close to the Swedish national average (40.6 years), and data cover Greater Gothenburg and rural areas, including a high percentage of immigrants from southeast Europe, the Middle East, and Africa. For patients within the database, over 99% of all healthcare contacts, including inpatient, specialist, primary care, and pharmacy prescriptions (approximately nine million contacts per year), are included.
For inclusion in the study, patients must have received an ADHD medication prescription between July 1, 2007 and June 30, 2009 (“index period”); the first prescription date was assigned as the patient’s index date. Only short- and long-acting stimulants and non-stimulant ADHD medications were considered, and patients could be either new to or continuing treatment. Patients were required to have a minimum of 12 months of available data prior to the first prescription to determine initial treatment for ADHD (“baseline period”) and a minimum of 12 months of available data following the first prescription for measurement of outcomes (“follow-up period”; Fig. 1). Further details on inclusion criteria are provided in Supplemental File 1.
Patient Characteristics
Demographic and clinical characteristics during the baseline period were collected. Characteristics included sex, age, psychiatric comorbidities, new or continuing treatment, index dose of ADHD medication, total pre-index healthcare costs, and healthcare utilization (all-cause).
Comorbidity-Based Cohort Formation
Patients were categorized into one of two mutually exclusive cohorts for comparison: (1) ADHD alone (ADHD-only) or (2) ADHD with psychiatric or neurologic comorbidities (ADHD-comorbid). Multiple comorbid disorders were included, and a full list of conditions and associated International Classification of Diseases, Tenth Revision (ICD-10) codes is listed in Supplemental Table 1. Patient records were assessed for relevant comorbidities listed during the baseline period and at index; those with no evidence of any such diagnoses were categorized as ADHD-only.
Initial Treatment for ADHD
Patient records during the index period were assessed for presence of initial ADHD therapy, defined as the first prescription for one of the primary treatments (stimulants: methylphenidate and dextroamphetamine; non-stimulant: atomoxetine). Further information is in Supplemental File 1.
The sample was stratified as either receiving index monotherapy (a single ADHD-indicated medication class filled at index date and no other classes filled within 60 days prior to or following index) or index combination/adjunctive therapy (first ADHD-indicated medication class filled at index, with other classes filled within 60 days prior to or following index).
Outcomes Measures
Primary ADHD medication outcomes included: average daily dose, switching, augmentation, persistence, time to first therapy modification, and healthcare utilization and associated costs (as defined in Supplemental Table 2). The occurrence of specific psychiatric/neurologic conditions as listed in Supplemental Table 1 were also evaluated.
Data on ADHD medication use during the follow-up period were collected from the subset of patients new to ADHD treatment and mental health medications, and who had either switched or augmented their index ADHD medication during the follow-up period. Patients were categorized as receiving European Medicines Agency (EMA) product label-indicated (on-label) or non-indicated (off-label) treatment (based on approval status at that time). Frequency distributions for both on-label and off-label modifications were provided for other mental health medications (antipsychotics, tranquilizers/benzodiazepines, antidepressants and mood stabilizers, psycholeptic–psychoanaleptic combinations, anti-epileptics, hypnotics/sedatives, and clonidine). For ADHD-only patients, augmentation was considered off-label as adjunctive/combination therapy is not currently approved by the EMA for ADHD treatment. In the ADHD-comorbid cohort, if patients were switched to a medication approved for the treatment of ADHD, they were considered to have received on-label treatment. The number and percentage of patients in the ADHD-comorbid cohort who augmented their therapy in the follow-up period were also reported, although no categorization of on- or off-label use was applied, as this use pertains to treatment of conditions other than ADHD.
Post-index assessments (number and percentage) were performed for patients who received ADHD-indicated medication (long- and short-acting stimulants, and non-stimulants) and other mental health medications.
Statistical Methods
For evaluation of differences in unadjusted measures between the cohorts, Chi square tests were used for categorical variables, and t test (means) or Wilcoxon rank-sum test (medians) were used for continuous variables. All statistical tests were two-tailed, and alpha levels were set at 0.05 for determination of significance. All outcome measures were evaluated in unadjusted reporting prior to decisions on the most appropriate multivariate approaches.
A series of multivariate models were constructed to adjust for potential confounding of the relationship between the two primary cohorts and outcomes of interest. All baseline demographic and clinical characteristics deemed necessary for adjustment based on clinical study team review of unadjusted results, and not identified as having a high likelihood of correlation to the two cohorts, were included in the same models. Other variables were selected from significant baseline characteristics and considered for inclusion in the models using a stepwise approach (in the order provided below) with a p ≤ 0.10 threshold for inclusion and retention. All clinically relevant variables were retained. Model variables entered into the stepwise models included: sex, age group, age (continuous), new to all ADHD medications (yes/no), new to index ADHD medication (yes/no), sick leave visit (yes/no), ADHD medications [index and non-index: mean, standard deviation (SD)], other medications (non-ADHD or mental health: mean, SD), index and pre-index medications of interest (long- and short-acting stimulants, non-stimulants), and index dose for the subset for which it was available.
Regarding differences in treatment patterns, Cox proportional hazard models, adjusting for the aforementioned potential differences in known baseline covariates between the two cohorts, were constructed for the following outcomes (dependent variables): time to first treatment modification, time to discontinuation, and time to first switch in ADHD therapy. Additionally, differences in treatment patterns were evaluated using logistic regression models, which included previously listed demographic and clinical characteristics, comparing the two cohorts. The following outcomes were dependent variables in logistic regression models: augmentation of (1) ADHD therapy with ADHD-indicated medications, (2) ADHD therapy with ADHD-indicated or non-indicated mental health medications, and (3) mental health therapy with ADHD-indicated or non-indicated mental health medications.
Differences in healthcare costs between the two cohorts were compared using generalized linear models (GLMs). The following outcomes were dependent variables in GLMs: (1) total outpatient visits, (2) total outpatient referrals, (3) total hospitalizations (all-cause), and (4) total healthcare costs (all-cause).
All analyses were performed using SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA).
Compliance with Ethics Guidelines
This article does not contain any studies with human participants performed by any of the authors.
Results
A total of 5938 patients who received at least one ADHD-indicated prescription during the index period were initially identified (Fig. 2). After applying all inclusion and exclusion criteria, 1794 patients were selected for analysis (ADHD-only cohort n = 1083; ADHD-comorbid cohort n = 711). In the ADHD-only cohort, 1045 (96.5%) received index monotherapy and 38 (3.5%) received index combination/adjunctive therapy. In the ADHD-comorbid cohort, 534 (75.1%) received index monotherapy and 177 (24.9%) received index combination/adjunctive therapy.
Study disposition. aOnly a single ADHD-indicated medication class filled at index date and no other classes filled within 60 days prior to or following index. bFirst ADHD-indicated medication class filled at index, with the other classes filled within 60 days prior to and following index. ADHD attention-deficit/hyperactivity disorder
Demographic Characteristics
Patients had a mean (SD) age of 12.3 (3.0) years. The ADHD-comorbid cohort had significantly greater proportions of 13- to 17-year-olds (54.1% vs 47.0%; p = 0.003) and girls (28.3% vs 20.2%; p < 0.001) (Table 1) than the ADHD-only cohort.
Pre-Index Comorbidities and Resource Use
In the ADHD-comorbid cohort, the most common pre-index psychiatric comorbidities were pervasive developmental disorder (40.1%), conduct disorder (19.3%), and anxiety and other neurotic disorders (19.0%) (Table 2).
Compared with the ADHD-only cohort, the ADHD-comorbid cohort had more mean annual physician visits (9.6 vs. 15.8; p < 0.001), received a greater mean number of medications (5.7 vs. 8.7; p < 0.001, especially other mental health medications: 2.4 vs. 5.5; p < 0.001), and had a greater proportion of patients experiencing ≥1 hospitalization (all-cause; 5.6 vs. 10.4%; p < 0.001) in the baseline period (Table 3). Consequently, the ADHD-comorbid cohort incurred higher median pre-index total costs than did the ADHD-only cohort [2011 Krona (SEK) 33,096.7 vs. SEK 18,482.4; p < 0.001].
The proportion of patients new to all ADHD-indicated medications was similar in the two cohorts (Table 4). However, the ADHD-only cohort had significantly greater proportions of patients new to all ADHD-indicated and other mental health medications, and of patients receiving index long-acting stimulants (Table 4). Compared with the ADHD-only cohort, a greater proportion of the ADHD-comorbid cohort received index short-acting stimulants and index adjunctive/combination therapy (Table 4).
Post-Index Persistence and Dosing among Newly Treated Patients
Therapy duration for ADHD-indicated index medication classes (mean 178.9 days for ADHD-only, 175.1 days for ADHD-comorbid) and average daily dose while persistent on ADHD-indicated index medications were similar for newly treated patients across the two cohorts. Mean (SD) average daily dose for long-acting stimulants in the ADHD-only cohort versus the ADHD-comorbid cohort was 34.3 (15.7) mg versus 34.2 (17.4) mg (p = 0.925). Mean (SD) average daily dose for short-acting stimulants was 9.9 (3.7) mg and 16.5 (10.6) mg (p = 0.331) in the ADHD-only and ADHD-comorbid cohorts, respectively. For ADHD-indicated and other mental health index medication classes, the newly treated ADHD-only cohort had longer persistence to index medication than the newly treated ADHD-comorbid cohort (165.6 days vs. 143.8 days; p = 0.015).
Post-Index Switching and Augmentation among Newly Treated Patients
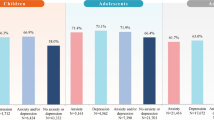
Of 750 patients new to all ADHD-indicated medications and other mental health medications, 163 (21.7%) patients augmented their initial medication during the follow-up period, and the unadjusted proportion of patients between the two cohorts was similar (ADHD-only: 20.5% [105/512]; ADHD-comorbid: 24.4% [58/238]; p = 0.233) (Fig. 3). Similar proportions of patients from the ADHD-only and ADHD-comorbid cohorts augmented with ADHD label-indicated medications [13.9% (71/512) vs. 10.9% (26/238); p = 0.264], while augmentation with medications that do not have an approved indication was significantly lower for ADHD-only versus ADHD-comorbid patients [6.6% (34/512) vs. 13.9% (33/238); p = 0.001].
Therapy augmentation among the newly treated, by cohort, during the follow-up period. a p = 0.001 (Chi square test) for the comparison between the ADHD-only and ADHD-comorbid cohorts. bOne patient augmented with both an ADHD-indicated medication and another mental health medication (non-ADHD indicated) on the same day. ADHD attention-deficit/hyperactivity disorder
Index medication was switched by 15.9% (119/750) of patients during the follow-up period. Unadjusted proportions of switch patients were similar between the two cohorts [15.0% (77/512) ADHD-only vs. 17.6% (42/238) ADHD-comorbid; p = 0.363]. Switching frequency for ADHD-only patients versus ADHD-comorbid patients was comparable with ADHD label-indicated medication [13.1% (67/512) vs. 13.9% (33/238); p = 0.77], and with medications without an approved ADHD indication [2.1% (11/512) vs. 3.8% (9/238); p = 0.196].
During the follow-up period, a significantly greater proportion of the ADHD-comorbid cohort switched/augmented to receive on-label treatment for ADHD [52.4% (22/42) vs. 8.9% (4/45); p < 0.001]. In contrast, a significantly greater proportion of the ADHD-only cohort switched/augmented to receive off-label ADHD treatment [91.1% (41/45) vs. 47.6% (20/42); p < 0.001].
After adjusting for discrepant baseline variables via logistic regression, the ADHD-only cohort was significantly less likely to augment index therapy with either ADHD label-indicated or other mental health medications (odds ratio 0.44; 95% confidence interval 0.27, 0.73; p = 0.002).
Kaplan–Meier survival analysis and Cox proportional hazard models revealed no significant differences in time to first treatment modification among the newly treated ADHD-indicated index medication patients across the two cohorts. Similarly, no significant differences were observed for time to discontinue/switch or in switching probabilities for ADHD-indicated index medication classes.
Post-Index All-Cause Healthcare Resource Utilization and Costs
The ADHD-comorbid cohort received a significantly higher mean number of medications than did the ADHD-only cohort (p < 0.001), had a significantly higher mean number of total outpatient care visits (p < 0.001), and had a greater incidence of inpatient admissions (p < 0.001) in the follow-up period (Table 5). Similarly, median total healthcare, pharmacy, and outpatient visit costs were higher in the ADHD-comorbid cohort than the ADHD-only cohort (Table 5).
After adjusting for discrepant baseline variables between cohorts, the ADHD-only cohort had significantly fewer total outpatient visits (p < 0.001; β = −0.21; 95% confidence interval −0.28, −0.13) and outpatient referrals (p < 0.001; β = −0.25; 95% confidence interval −0.33, −0.18). No significant differences were observed between the two cohorts for inpatient hospitalizations and total costs incurred.
Mental Health-Related Resource Utilization and Costs (Post-Index)
The ADHD-comorbid cohort received a significantly higher mean number of medications than the ADHD-only cohort, and had a significantly higher mean number of total outpatient care visits and greater incidence of inpatient admissions, in the follow-up period (Table 5). Similarly, median total healthcare, pharmacy, and outpatient visit costs were higher for the ADHD-comorbid cohort (Table 5).
ADHD-Related Resource Utilization and Costs (Post-Index)
The ADHD-comorbid cohort experienced higher mean total outpatient care visits and a greater incidence of inpatient admissions in the follow-up period than the ADHD-only cohort (Table 5). There were no significant differences in median ADHD-related total healthcare costs incurred during the follow-up period for the two cohorts (Table 5).
Discussion
The findings of this retrospective cohort study add to our understanding of treatment patterns, including rates of combination pharmacotherapy, among children/adolescents with ADHD in Sweden. Over a 12-month follow-up period, patients with ADHD and comorbidities required more physician visits (all-cause, mental health-related, and ADHD-related), medication prescriptions (all-cause and mental health-related), and hospitalizations (all-cause, mental health-related, and ADHD-related) than did those with ADHD alone. Augmentation of newly initiated ADHD therapy was prevalent among children/adolescents with ADHD (21.7%), even among those without documented psychiatric/neurologic comorbidities (20.5%). Differences in augmentation, outpatient referrals, and outpatient visits between patient cohorts remained after controlling for differences in baseline characteristics.
Our data on the prevalence of ADHD comorbidities showed similarities and differences with findings of previous studies in Sweden [9, 21, 22]. These differences are likely due to differences in study design, data sources, “diagnostic” definitions, and available baseline and follow-up periods. For example, one Swedish study that used school-based screening instruments, including the six-question ADHD Self-Report Scale and the Depression Self-Rating Scale, reported that the prevalence of co-occurring symptoms of ADHD and depression was 2.4% (boys 1.0%, girls 4.0%; p < 0.001) [22]. The current study, which was based on a clinical diagnosis of depressive episode or recurrent depressive disorder, reported a higher prevalence of depression among patients with ADHD (13.1%).
It is important to note that the two cohorts in the current study had inherent differences, as evidenced by baseline demographic and clinical characteristics. Before adjustment, measured differences in treatment patterns and healthcare costs and utilization indicated that these inherent differences persisted from baseline to throughout the follow-up period. There were also some similarities between the two cohorts, such as the high prevalence of augmentation and switching. It is worth noting that a significantly greater proportion of the ADHD-comorbid cohort switched/augmented to receive on-label treatment for ADHD during the follow-up period (52.4% vs. 8.9%; p < 0.001), while a significantly greater proportion of the ADHD-only cohort switched/augmented to receive off-label ADHD treatment (91.1% vs. 47.6%; p < 0.001).
Clinical Implications
There is increasing evidence that ADHD often exists with one or more comorbid conditions, and each condition modifies the overall clinical presentation and treatment response. Indeed, the results of the current study support previous assertions that children with neurodevelopmental problems commonly experience symptoms that span various disorders and lead to impairment in multiple functional domains [23]. This finding has important clinical implications as it speaks against the development of subspecialized services targeting, for example, children/adolescents with only a single condition. As such, we believe that ADHD and comorbid conditions should be considered collectively to optimize patient outcomes, ideally by specialist multidisciplinary clinics that are designed to identify and manage ADHD and comorbidities [24].
Effective treatment of ADHD may improve some symptoms of comorbid conditions. However, combination therapy may be required in the presence of complex comorbidity. Examples include the use of stimulant medication plus adjunctive sodium valproate [25] for children/adolescents with ADHD and oppositional defiant or conduct disorder, and antipsychotic plus adjunctive methylphenidate or atomoxetine for ADHD and comorbid bipolar spectrum disorders [26, 27], although it must be noted that such medications are not specifically indicated by regulatory agencies for use in these conditions. Moreover, our findings suggest that some patients with ADHD alone receive concomitant medications without an observed indication.
Co-existing psychiatric symptoms may cause considerable health impairment, but it may be difficult to place them within traditional diagnostic categories. For example, it can be difficult to determine whether affect regulation in the context of ADHD or irritability in the context of ASD are true comorbidities or features of the primary diagnosis. Further research on categorizing psychiatric symptoms in the context of co-occurring ADHD and ASD is needed [28]. In addition, the primary presenting complaint/symptom may relate to a co-existing psychiatric condition, with ADHD missed. The reality of clinical practice is such that the presenting symptom(s) is (are) treated and, as a result, ADHD may be underdiagnosed and undertreated.
Policy Implications
Our study data corroborate previous reports that ADHD often occurs with co-existing conditions [7,8,9,10]. Our findings support recommendations for a multidisciplinary, patient-centered approach to ADHD care [1, 24]. We believe that future policy recommendations should include the need for greater awareness of the full range of potential comorbid conditions among patients presenting with ADHD, and improvements in the diagnostic process. Estimated annual healthcare costs per child/adolescent with ADHD across Europe range from €798 to €2191 (adjusted to 2012 Dutch euros) [29]. Appropriate understanding of ADHD and optimal patient management could potentially improve outcomes for patients who have ADHD and comorbidities and, thus, reduce associated healthcare costs.
In the past decade, there have been notable advances in the awareness and treatment of ADHD, as evidenced by the progression of expert recommendations on policy changes, which were first formally addressed by the “Knowing Me, Knowing You” initiative from 2000 to 2002 [30]. This project found that there was an overall lack of data on the prevalence, complexity, and diversity of ADHD and how it affects the social exclusion of children, adults, and families across Europe and the negative impact that this can have on communities and societies as a whole [31]. This exercise showed that the lack of facts, research, and awareness concerning ADHD was a significant problem for children, adults, and families living with ADHD, and for healthcare professionals working with ADHD.
Our current study highlights some key issues for clinicians and policy-makers alike. Children/adolescents with ADHD and comorbidities are a distinct and yet diverse population. These patients may require combination pharmacotherapy; however, they often receive medications that are not indicated for the treatment of ADHD. As our understanding of the complexity of ADHD and associated comorbidities improves, there are opportunities to improve the economic and humanistic burden of disease for patients and wider society.
We acknowledge that this study has a number of limitations. First, the study is subject to the common limitations of retrospective research, such as (but not limited to) lack of randomization or blinding. In addition, even after adjusting for differences between cohorts to limit potential confounding, there may be residual confounding by additional unadjusted variables. Patients were classified as ADHD-only or ADHD-comorbid based on the presence and recording of psychiatric/neurologic comorbidities only during the 12-month pre-index period. Patients in the ADHD-only cohort could have developed comorbidities in the follow-up period or before the 12-month baseline period, but may not have been assessed/treated for those conditions in the 24 months of our study window. In such cases, treatment with other mental health (i.e. non-ADHD indicated) medications may have been appropriate. While a judgment on the appropriateness of treating newly developed comorbidities with specific medications in the follow-up period was not made, differences in frequencies of comorbidities at baseline and during follow-up were compared between the two cohorts and reported in a descriptive manner. In addition, the reliance on ICD-10 diagnosis codes within the Swedish database may have insufficiently captured symptoms (e.g. aggression, defiance) or other disorders that may have been the intended target of therapy (e.g. schizoaffective disorder). As in all studies using clinical diagnoses, adherence to diagnostic criteria cannot be guaranteed, and thus the rigor of comorbid diagnoses could vary throughout the database. It should also be acknowledged that the ADHD and comorbidities group included patients with very different comorbid conditions and, as such, was highly heterogeneous.
Among patients who were prescribed non-indicated ADHD medications, the data did not adequately differentiate between appropriate and inappropriate use for the treatment of ADHD or comorbidities. Specific medications and medical conditions are not found within the same record line item in this database. Thus, for cases in which multiple diagnoses and multiple medications are evident in the patient files, no determination can be made regarding which medication is linked to which specific condition. Finally, the study is based on approved ADHD medications that were available between July 1, 2007 and June 30, 2009. Despite these limitations, it should be noted that, even among the ADHD-only cohort, 17.5% were found to have received adjunctive therapy, and, among patients who switched/augmented, 91.1% switched/augmented to receive off-label ADHD treatment. Moreover, we did not include children/adolescents who did not have an ADHD diagnosis, but were receiving ADHD medication(s), so rates of inappropriate ADHD medication use may in fact be even higher than estimated in our study.
Large medical record databases can provide unique insights into such aspects over time, yet maintain geographic representativeness. However, data used in this study relate only to the southwest region of Sweden and, although highly representative of that region, may not be generalizable to the full Swedish population. As such, we suggest that future studies compare rates of ADHD and comorbidities across the entire country. In addition, future studies should aim to probe the factors (such as life events, family history, physiological variables), associated with or independent of comorbid disorders, which influence ADHD outcomes.
Conclusion
This study provides valuable data on the prevalence of ADHD with and without psychiatric/neurologic comorbidities among children/adolescents in Sweden, as well as treatment patterns and associated costs; indeed, we believe that this is the first study to report on comorbid conditions and the corresponding resource use associated with ADHD. Our data indicate that children/adolescents with ADHD and comorbidities have greater medical care needs, higher resource utilization, and resulting costs than do those with ADHD alone. Further investigation of subgroups of children/adolescents with greater homogeneity of comorbidities is needed, to identify how specific factors influence poor outcomes. We have also found that augmentation of pharmacotherapy occurs often in patients with ADHD with and without comorbidities. Further evaluation is needed to better understand the impact of combination pharmacotherapy on health and economic outcomes. We believe that early recognition of, and appropriate intervention for, children/adolescents with ADHD comorbidities are warranted, as is a continued focus on supportive multidisciplinary policies.
References
NICE. Attention deficit hyperactivity disorder: diagnosis and management. National Clinical Practice Guideline Number 72 (2008; includes 2016 updates). 2016. https://www.nice.org.uk/guidance/cg72. Accessed 28 Feb 2017.
World Health Organization. International statistical classification of diseases and related health problems 10th revision. 2010. http://apps.who.int/classifications/icd10/browse/2010/en. Accessed 28 Feb 2017.
Polanczyk G, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56:345–65.
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8.
Huss M, Holling H, Kurth BM, Schlack R. How often are German children and adolescents diagnosed with ADHD? Prevalence based on the judgment of health care professionals: results of the German health and examination survey (KiGGS). Eur Child Adolesc Psychiatry. 2008;17(Suppl 1):52–8.
Landgren M, Pettersson R, Kjellman B, Gillberg C. ADHD, DAMP and other neurodevelopmental/psychiatric disorders in 6-year-old children: epidemiology and co-morbidity. Dev Med Child Neurol. 1996;38:891–906.
Holmberg K, Hjern A. Health complaints in children with attention-deficit/hyperactivity disorder. Acta Paediatr. 2006;95:664–70.
Steinhausen HC, Novik TS, Baldursson G, Curatolo P, Lorenzo MJ, Rodrigues PR, et al. Co-existing psychiatric problems in ADHD in the ADORE cohort. Eur Child Adolesc Psychiatry. 2006;15(Suppl 1):I25–9.
Anckarsater H, Lundstrom S, Kollberg L, Kerekes N, Palm C, Carlstrom E, et al. The child and adolescent twin study in Sweden (CATSS). Twin Res Hum Genet. 2011;14:495–508.
Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry. 2005;57:1442–51.
Kadesjo C, Hagglof B, Kadesjo B, Gillberg C. Attention-deficit-hyperactivity disorder with and without oppositional defiant disorder in 3- to 7-year-old children. Dev Med Child Neurol. 2003;45:693–9.
Kadesjo B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school-age children. J Child Psychol Psychiatry. 2001;42:487–92.
Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. Conduct problems, gender and adult psychiatric outcome of children with attention-deficit hyperactivity disorder. Br J Psychiatry. 2002;181:416–21.
Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European clinical guidelines for hyperkinetic disorder—first upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):i7–30.
Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–32.
Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3:200–11.
Shire. Intuniv summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003759/WC500195130.pdf. Accessed 28 Feb 2017.
Eli Lilly and Company. Strattera summary of product characteristics. 2015. http://www.mhra.gov.uk/spc-pil/?subsName=ATOMOXETINEHYDROCHLORIDE&pageID=SecondLevel. Accessed 28 Feb 2017.
Eli Lilly and Company Limited. Strattera 10 mg, 18 mg, 25 mg, 40 mg, 60 mg, 80 mg or 100 mg hard capsules. 2015. http://www.medicines.org.uk/emc/medicine/14482. Accessed 28 Feb 2017.
Shire Pharmaceuticals Limited. Intuniv 1 mg, 2 mg, 3 mg, 4 mg prolonged-release tablets. 2015. Last update: December 2016. http://www.medicines.org.uk/emc/medicine/31294. Accessed 28 Feb 2017.
Kopp S, Kelly KB, Gillberg C. Girls with social and/or attention deficits: a descriptive study of 100 clinic attenders. J Atten Disord. 2010;14:167–81.
Sonnby K, Aslund C, Leppert J, Nilsson KW. Symptoms of ADHD and depression in a large adolescent population: co-occurring symptoms and associations to experiences of sexual abuse. Nord J Psychiatry. 2011;65:315–22.
Gillberg C. Perceptual, motor and attentional deficits in Swedish primary school children. Some child psychiatric aspects. J Child Psychol Psychiatry. 1983;24:377–403.
Coghill D, Seth S. Effective management of attention-deficit/hyperactivity disorder (ADHD) through structured re-assessment: the Dundee ADHD Clinical Care Pathway. Child Adolesc Psychiatry Ment Health. 2015;9:52.
Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry. 2009;166:1392–401.
Chang K, Nayar D, Howe M, Rana M. Atomoxetine as an adjunct therapy in the treatment of co-morbid attention-deficit/hyperactivity disorder in children and adolescents with bipolar I or II disorder. J Child Adolesc Psychopharmacol. 2009;19:547–51.
Zeni CP, Tramontina S, Ketzer CR, Pheula GF, Rohde LA. Methylphenidate combined with aripiprazole in children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder: a randomized crossover trial. J Child Adolesc Psychopharmacol. 2009;19:553–61.
Davis NO, Kollins SH. Treatment for co-occurring attention deficit/hyperactivity disorder and autism spectrum disorder. Neurotherapeutics. 2012;9:518–30.
Le HH, Hodgkins P, Postma MJ, Kahle J, Sikirica V, Setyawan J, et al. Economic impact of childhood/adolescent ADHD in a European setting: the Netherlands as a reference case. Eur Child Adolesc Psychiatry. 2013;23:587–98.
European Commission. Knowing me, knowing you. 2002. http://www.adhdeurope.eu/information/adhd-projects/123-mapping-adhd-across-europe-en.html. Accessed 28 Feb 2017.
European Commission. Mapping ADHD across Europe. 2002. http://www.adhdeurope.eu/downloads/MappingGB.pdf. Accessed 28 Feb 2017.
Acknowledgements
This research was funded by Shire Development LLC. Funding for the article processing charges associated with this manuscript was provided by Shire International GmbH.
Under the direction of the authors, Karen Joy, employee of IMS Health Development, LLC, provided writing assistance for this publication. Editorial assistance in formatting, proofreading, copy editing, and fact checking the manuscript, and coordination and collation of comments was provided by Jackie Marchington and Katie Lay of Caudex (Oxford, UK). Shire Development LLC provided funding to IMS Health Development and Shire International GmbH provided funding to Caudex for support in writing and editing this manuscript.
The authors would like to acknowledge Malcolm Rucker (IMS Health at the time of the study) for his contribution to the data analysis, and Jason Yeaw (IMS Health at the time of the study) for his contribution to the data analysis and critical review of the manuscript.
Although employees of Shire were involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this manuscript, and the interpretation of the data, the decision to submit the manuscript for publication in Neurology and Therapy was made by the authors independently.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Vanja Sikirica was an employee of, and owned stock/stock options in, Shire at the time of this study. Vanja Sikirica’s current affiliation is GlaxoSmithKline, Collegeville, PA, USA. Per A. Gustafsson has received financial support from Shire Development LLC. Charles Makin was an employee of IMS Health Development, LLC at the time of this study, which received funding from Shire Development LLC for this research. Charles Makin’s current affiliation is ICON plc, North Wales, PA, USA.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants performed by any of the authors.
Data Availability
The data analyzed during the current study are not publicly available because they were licensed with restrictions from a third party source.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vanja Sikirica and Charles Makin: Affiliation at the time of this study.
Enhanced content To view enhanced content for this article go to www.medengine.com/Redeem/A408F06002DCB1BB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sikirica, V., Gustafsson, P.A. & Makin, C. Treatment Patterns among Children and Adolescents with Attention-Deficit/Hyperactivity Disorder with or without Psychiatric or Neurologic Comorbidities in Sweden: A Retrospective Cohort Study. Neurol Ther 6, 115–130 (2017). https://doi.org/10.1007/s40120-017-0066-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-017-0066-8