Abstract
Since the introduction of transvenous cardiac pacing leads, pacemaker system design has remained similar for several decades. Progressive miniaturisation of electronic circuitry and batteries has enabled a smaller, single pacing unit comprising the intracardiac electrodes, generator and computer. This review explores the development of leadless pacing, the clinical trials comparing leadless to transvenous pacing in addition to the future developments of multi-chamber leadless pacing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The miniaturisation of the electronic circuitry and battery has facilitated new possibilities for a transcatheter-delivered, single, leadless pacing unit. |
There is an acute learning curve with respect to the implementation of any new technology, including leadless pacing. As experience has grown, acute complications with single-chamber (RV) pacing are now similar to transvenous pacing. Single-chamber (right ventricular) VVI pacing has been successfully used clinically for several years with accumulating data suggesting a reduction in medium- to long-term complications compared with conventional transvenous devices, due to the lack of intravascular leads and a pocket. |
A new single-chamber device (in the right ventricle) can also have the capability to sense signals in the atrium and through VDD pacing, provide atrial sensing and ventricular pacing. This may be suitable for patients with intact sinus node function and AV node conduction disease who require pacing. AV synchrony is not, however, perfect and this can be challenging to maintain especially during patient activity. |
The leadless left ventricular endocardial system (WiSE CRT) is a small electrode which works alongside an existing co-implant or right ventricular pacing system (transvenous or leadless). This enables leadless biventricular pacing or cardiac resynchronisation and may be an excellent alternative to conventional transvenous CRT. |
With the exponential interest in conduction system pacing, a leadless system to deliver physiological pacing is a very attractive concept and early attempts have shown this can be successfully performed. |
Introduction
Since the first implantation of a permanent cardiac pacemaker in 1958, technological iterations have facilitated the introduction of more reliable hardware alongside complex algorithms. Transvenous pacing systems have saved countless lives and improved patient quality of life through the treatment of symptomatic bradycardias. Nevertheless, they come at a cost of potential procedure- and device-related complications, affecting up to 10% of patients [1]. Intravenous leads are considered the Achilles heel of transvenous systems due to the chronic fibrotic and occlusive changes in the venous system, alongside the inevitability of lead dysfunction or damage over time and the clinical consequences of repeated procedures with associated morbidity and mortality [2,3,4,5,6]. Decisions are taken by physicians on a daily basis weighing up the potential benefits of device therapy with the long-term risks of lead-related complications, especially in younger patients. This is particularly pertinent where the device is implanted for a non-bradycardia indication, such as primary prevention defibrillator therapy or cardiac resynchronization therapy (CRT).
The concept of leadless pacing has been considered for several decades. The first self-contained intracardiac pacemakers were conceptualized and implanted in animals over 50 years ago [7, 8]. Since then, the evolution and miniaturisation of technology have led to the development of fully programmable, leadless systems, containing the battery, circuitry and electrodes, all functioning as a single unit.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Leadless Pacemakers Today
The mercury and nuclear batteries in the first experimental devices have been replaced with more patient- and environment-friendly options [9, 10]. According to the European Heart Rhythm Association (EHRA) survey, 86% of EHRA research centres report using leadless pacemakers (LPs) today but most of them implant less than 30 devices per year [11] due to the limitations of single-chamber ventricular pacing when most patients require pacing in both the atria and the ventricle.
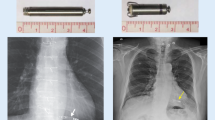
Nanostim™ (St. Jude Medical Inc., Saint Paul, MN, USA; now Abbott Medical Inc., IL, USA) was the first self-contained intracardiac pacemaker implanted in a human. At just over 4 cm long, the device had a helix at the distal tip to ensure stable fixation in the right ventricle (RV) (Fig. 1). Almost 1500 devices were implanted since 2013 and three clinical trials confirmed stable performance and acceptable safety results, including at 1-year follow-up, with a rate of device-related serious adverse events of less than 7% [12,13,14]. As a result of rare but serious battery failures, the Nanostim had to be discontinued in 2016 [15], but the concept of leadless pacing has continued to grow. Indeed, the investigational device exemption (IDE) study of a new iteration of this device, the Aveir Leadless Pacing System (Abbott, Fig. 1 [16]), has recently reported preliminary results. The LEADLESS II – phase 2 IDE study [17] recruited 200 patients in North America and Europe. It reported an implant success rate of 98% and met its primary safety endpoint with a 4% rate of device-related complications at a 6-week follow-up. Ninety-six per cent of successfully implanted patients met the primary effectiveness endpoint based on a composite score of RV capture thresholds and R wave amplitude.

Modified from Ibrahim et al. [16], with permission
Aveir Leadless Pacemaker by Abbott.
In 2015 the Micra™ (Medtronic, Minneapolis, MN, USA, Fig. 2 [18]) demonstrated a similar safety profile to that of a transvenous system while providing low and stable pacing thresholds in over 700 patients [19]. Slightly thicker and shorter than Nanostim, it employed four tines for stable fixation in the RV. Duray et al. reported that over a 1-year follow-up the risk of major complications from Micra™ was 4% (n = 726), 48% lower than the 1-year complication rate from a historical cohort of patients with transvenous devices (n = 2667). The rate of cardiac perforation from Micra™ in this cohort was 1.5% [20].

Modified from Ritter et al. [18], with permission
MICRA transcatheter leadless pacing system by Medtronic.
Leadless Versus Transvenous Pacing Systems
Multiple real-world clinical registries have demonstrated stable performance and reassuring safety results both acutely and longer term [12, 13]. Implantation success rates are above 99% with a relatively low rate of periprocedural major complications at 1.5–4.0% [20,21,22]. In a propensity-matched comparison of leadless pacing with Micra versus transvenous pacemakers, significantly lower complication rates were noted for the leadless system at medium-term follow-up [2, 23] (Table 1).
Similar favourable findings were observed with Nanostim, which included fewer infectious and device-related events (compared with Micra), but more pericardial effusions which were uncommon but some with life-threatening consequences [6]. El-Chami et al. observed a low systemic infection incidence of 2.2% with Micra devices in the 720-patient Micra IDE study [24].
The prevalence of complication rates across all transvenous systems (including ICD/CRT) is approximately 10% with a wide range in some clinical series [1, 5]. Intravascular leads, considered the weakest link of the cardiac pacing system, can potentiate venous obstruction and are prone to insulation breaks, conductor fractures, and infection necessitating extraction [3, 4, 25]. One should acknowledge that whilst the initial acute complication rate of pericardial effusion and tamponade was higher with leadless pacing, successive clinical registries with larger numbers of patients have shown low rates of acute complications [22], similar to contemporary transvenous implants. This likely reflects the early clinical experience with the transcatheter delivery system and the learning curve associated with this [26].
Advantages of Leadless Pacing
Infection Risk
Leadless pacing at its inception was proposed as a solution for patients with pre-existing cardiac device infections [27]. Given most of the medium- to long-term complications are related to wound breakdown, erosion, infection (from the presence of a pocket) and transvenous lead attrition, the absence of these appears to contribute to a lower rate of such complications with a leadless pacing system. Additionally, the Micra has a small surface area, a tendency for encapsulation and a parylene coating with antibacterial properties which all help to resist infection [28]. Moreover, leadless pacing is an attractive route for patients with superior central venous occlusion who cannot undergo conventional transvenous pacing, as well as those in whom preservation of central venous access is critical for chronic catheter placement in haemodialysis for end-stage renal disease.
Extraction
Despite the sturdy fixation mechanism to the ventricular myocardium, the leadless pacemaker can be safely extracted percutaneously [29, 30]. In a high-volume, single-centre retrospective analysis of 302 patients, extraction of an LP was performed mainly for an upgrade to cardiac resynchronisation therapy (CRT) and was associated with no procedural or long-term complications [31] which is noteworthy given that transvenous lead extraction may have periprocedural rates of morbidity and mortality of 5–10% in some cases [25]. However, there is not enough data to comment on the extraction of LPs with longer dwelling times as opposed to transvenous pacing systems which have been studied quite well owing to their long presence in clinical practice.
Disadvantages of Leadless Pacing
Atrioventricular Dyssynchrony
Currently available LPs only offer single-chamber ventricular pacing. It is well recognized that VVIR pacing is inferior to atrioventricular (AV) synchronous pacing as a result of patients developing pacemaker syndrome and a significant increase in the risk of atrial fibrillation in patients with intact sinus node function [32]. Furthermore, a sizeable minority of patients with AV nodal block will go on to develop, or indeed already have the presence of sinus node disease, necessitating dual-chamber (atrial and ventricular) pacing. As such, the ESC 2021 pacing guidelines advocate for dual-chamber systems for patients with either sinus and AV node disease [33], precluding them from receiving single-chamber leadless devices. The successful use of an accelerometer in the recent generation of Micra pacemakers to overcome this problem is described in the next section of the article.
Management at End of Battery Life
Although battery life may vary, the estimated average longevity for the Micra pacemaker is about 12 years from implantation based on device settings observed in the Micra clinical trial [19]. Of course, this estimate will depend on the pacing parameters and the burden of RV pacing. Of note, there is also a limit of accessible ventricular endocardium where several leadless units can be safely placed in the right ventricle without interference with each other [34]. Whilst options for the extraction of leadless devices have been demonstrated [30], some physicians and patients may opt for an additional leadless device implant without extraction. Through this approach, a limited number of implants are permissible and they may not be suitable especially for young patients who will require multiple new devices throughout their lives.
Interference with Cardiac Magnetic Resonance Imaging
Leadless pacemakers are approved for scanning in a magnetic field up to 3 T. Cardiac magnetic resonance (CMR) imaging in patients with LP is feasible and offers good image quality allowing an accurate evaluation of LV function, cardiac structures and valves. However, an inevitable area of artefact in proximity to the leadless implant (broadly but not exclusively septal segments) may limit the diagnostic utility of this investigation. The use of scanners with lower magnetic field strength, i.e. 1.5 T, may help improve the image quality in this area [35, 36].
Atrioventricular Synchronous Pacing
In patients with preserved sinus node function, AV synchronous pacing is superior to ventricular pacing alone [37]. Therefore, whilst the overall complication rates appear lower with leadless devices [38] (mostly due to lower medium- to long-term complications in the leadless cohort), the majority of patients who require pacing have AV block, and therefore losing AV synchrony would have deleterious effects.
Using a three-axis accelerometer within the Micra pacemaker enables the sensing of atrial systole and a sequential response with synchronised ventricular pacing. It uses four-point detection of mechanical activity of the atria and ventricles which is translated into signals recognized by the device. There are two programmable intervals (post‐ventricular atrial blanking period or PVAB and passive ventricular filling period) and two programmable thresholds (passive ventricular filling threshold and atrial contraction threshold) to troubleshoot and optimize the pacemaker function.
AV synchrony was demonstrated 87% of the time in the MARVEL study [39]. In further sub-analysis, the MARVEL Evolve substudy showed stable performance of the algorithm over time, including during atrial arrhythmias [40]. MARVEL 2 confirmed good discrimination of atrial contractions in patients with preserved atrial function [41] (Fig. 3). However, AV synchrony achieved using this method is not without limitations; most notably AV synchrony dropped to 70% when patients stood up and began to walk. Clearly filtering out patient motion is an important design feature to enable high percentages of AV synchrony and this may be a limitation of VDD sensing using this method.

Modified from Steinwender et al. [41], with permission
a Atrioventricular synchronous pacing percentage between VVI 50 bpm and VDD mode with Micra AV in 40 patients with complete heart block. b Change in left ventricular outflow tract velocity–time integral (LVOT-VTI) during VDD relative to VVI 50 bpm pacing with a Micra AV device.
Whilst a VDD mode for atrial sensing and ventricular pacing is feasible as already described in the Micra AV, many patients with conduction disease exhibit both features of the sinus node and AV node disease; as such many will require some degree of atrial pacing. Animal data already exists demonstrating the clinical efficacy of two separate leadless devices, one atrial and the other ventricular with wireless communication between the two [42]. Furthermore, LPs implanted in an atrial position have shown excellent chronic pacing performance and easy retrieval at 6 months [43]. However, the current target for fixation in the atrium (the right atrial appendage) has a far thinner myocardial layer as compared with its ventricular counterpart; implantation safety data on perioperative complications, particularly perforation and pericardial effusion and tamponade, will be no doubt closely scrutinised in the clinical data which will follow. Another important issue relates to wireless transmission and programmability between two separate structures; this process inevitably requires energy expenditure and will likely be the next hurdle for device engineers to navigate. A dual-chamber AV synchronous leadless pacing is currently being tested in Aveir DR i2i clinical trial (NCT05252702) and will soon provide further insight into the feasibility of migrating the mature algorithms which exist within current conventional device generators to the miniaturised leadless systems.
Leadless Pacing and ICD Options
The subcutaneous ICD (S-ICD, Boston Scientific) is a well-established fully extravascular alternative to the transvenous ICD with increasing data on the safety, efficacy of shocks and similar inappropriate therapy deliveries compared with their transvenous counterparts [44]. Given higher attrition rates for transvenous ICD leads than those of pacing leads and the morbidity/mortality associated with percutaneous lead extraction, the argument for an extravascular ICD option is increasingly attractive. A significant drawback of the S-ICD, however, is the inability to deliver AV synchronous bradycardia pacing, which a sizeable proportion of patients would require. In addition, the lack of ventricular stimulation (anti-tachycardia pacing, ATP) to overdrive arrhythmias is also a disadvantage and an area currently being addressed by the industry. A combination of an S-ICD with a leadless unit (with VVI pacing and ATP as an option) was tested in both animal and human models with the Nanostim LP [45, 46]. Whilst this initially provided some promise with excellent communication between devices, the discontinuation of the Nanostim LP derailed this development for some time. A similar concept has been successfully tested by a new device, the EMPOWER/EMBLEM unit (Boston Scientific, Marlborough, MA, USA), which uses their S-ICD in addition to an LP implanted in the right ventricle which can deliver both VVI pacing and ATP and demonstrated excellent performance up to 18 months in a preclinical model [46, 47]. Furthermore, a case report of a Micra AV pacemaker implanted along with an S-ICD showed the feasibility of AV synchronous pacing alongside an extravascular ICD [48]; however, the lack of cross-communication between the two devices (made by different companies) highlights the complexity and the need for further clinical research. An alternative extravascular ICD (Medtronic) is currently being tested in a clinical study, the EV ICD study (NCT04060680); this device comprises a substernal, extravascular lead which, in addition to defibrillation, can enable epicardial ATP and pacing owing to the proximity to the heart [49].
Wireless Left Ventricular Pacing
A combination of a transvenous pacemaker/ICD along with a leadless ultrasound-based technology for endocardial LV resynchronization has been proposed as an alternative to a coronary sinus lead in cardiac resynchronisation therapy (CRT) in the form of the WiSE-CRT device (EBR Systems Inc., Sunnyvale CA, Fig. 4) [50]. The system consists of three components: a submuscular transmitter under the left breast connected to a subcutaneous battery on the left side of the chest wall and an endocardial left ventricular receiver electrode. The system needs a co-implant that can pace the right ventricle.

Reproduced from Wijesuriya et al. [50], with permission
The WiSE CRT system.
An early feasibility study has shown good acute outcomes in patients who could not receive a conventional CRT device [51]. Subsequently, the SELECT-LV study showed that 84.8% of patients had improvement in the clinical composite score at 6 months, and 66% demonstrated a positive echocardiographic CRT response (> 5% absolute increase in LV ejection fraction) with the WiSE-CRT system [52]. Imaging-guided endocardial implants were associated with a higher degree of chronic LV reverse remodelling [53]. A multicentre registry comprising 90 patients with implanted WiSE-CRT system confirmed technical feasibility and a high success rate [54]. A recent meta-analysis of the current data for WiSE-CRT (n = 181) reported a procedural success rate of over 90%, and a mean increase in LV ejection fraction of 6.3%, in a high-risk population including a significant number of non-responders to conventional CRT [55]. Initial complication rates were reasonably high, including vascular injury (5%), cardiac tamponade (3%) and procedure-related death (3%). However, whilst the learning curve for operators undertaking these procedures was initially quite steep, cumulative clinical experience has shown a significant reduction in complication rates [54]. This may be in part due to the development of a transseptal delivery method for the LV endocardial electrode (very common clinical practice for most electrophysiologists) as compared with large bore femoral arterial access and a retrograde aortic approach [56]. In addition, after initially high rates of pericardial effusions in the WiSE-CRT study [51], the delivery sheath was modified to subvert this complication. The most recently published prospective series reported no pericardial effusions in a cohort of 31 patients [57].
SOLVE-CRT is an international multicentre trial currently underway that aims to implant the WiSE-CRT system in 300 patients who have previously failed an attempt at conventional LV lead implant, or those considered high risk for implant [58].
Completely leadless biventricular pacing in patients with chronic atrial fibrillation can be achieved with the WiSE-CRT system in combination with a Micra leadless pacemaker in the right ventricle [59]. The use of the Micra AV leadless pacemaker may allow atrioventricular synchrony to extend use to patients in sinus rhythm. The addition of an S-ICD has been reported, achieving a completely leadless CRT-defibrillator system [60]. At present, this can only be delivered using devices from three separate vendors, which are not designed to function as a single unit. This leads to complex programming requirements, with the potential for complications related to communication between the devices [61].
Conduction System Pacing
His bundle pacing was described many decades ago, with the knowledge that maintenance of intrinsic conduction of the myocardium leads to a more physiological ventricular activation, as compared with right ventricular pacing which inevitably leads to left ventricular electromechanical delay (recognisable on the surface ECG as left bundle branch block-like QRS morphology). However, the tools to deliver and implant pacing leads to the His bundle region have taken longer to develop and in the last 5 years there has been an exponential rise in their development. Whilst initially, many studies reported improved electrical parameters and more physiological activation of the ventricles as compared with conventional RV pacing, recent analyses of medium-term data has suggested up to one-third of patients with a His bundle pacing lead have high thresholds at follow-up, with 17% demonstrating loss of His bundle capture [62]. Alongside this, data has accumulated on the ability to directly capture the conduction system through left bundle branch pacing. This involves directing (and screwing) a pacing lead through the right ventricular septum to capture the deep septal conduction tissue thus directly activating the myocardium through rapid conducting tissue. More consistent capture, lower thresholds and a shorter learning curve appear to be the main headline features of the clinical studies which describe this method [63]. However, medium- to long-term data will be needed to provide confidence to the pacing community that this route is a reasonable strategy for patients going forward [64]. Of note, transvenous pacing leads are made for attachment to the ventricular endocardium; however, the capture of the left bundle requires a direct screw into the myocardial tissue which may create high torque forces within a lead which is not designed for this purpose. Conduction system pacing certainly presents an attractive option for physiological pacing; coupled with the leadless technology described above, this may expand the options for the pacing physician. Recently, implantation of the WiSE-CRT electrode on the LV aspect of the interventricular septum was reported, demonstrating the feasibility of delivering leadless conduction system pacing [65].
Future Directions and Summary
Demonstration of the feasibility and reliability of leadless RV pacing devices was the first important step in a long journey for leadless technology. There have been ongoing efforts towards achieving AV synchrony using leadless pacing, and it will be exciting to follow the trajectory of this over the next decade. Advances in S-ICD technology, leadless LV endocardial pacing, and the possibility of a leadless device providing ATP would enable a completely leadless system with a full array of CRTD functionality. Whilst this is an extremely attractive concept, especially in younger patients who are currently exposed to a lifetime of lead-related complications, several challenges must be overcome for this to be translatable in a real-world setting: these include miniaturisation of technology to allow complex programming within leadless units; enabling communication between devices from different manufacturers; and how to manage devices at the end of battery life.
Indeed, battery drain and finite capacity is one limitation which surrounds both transvenous and leadless devices. Wireless charging which now exists with smartphones may be possible with pacemaker batteries in the future. Other concepts include complete disruption of the battery concept; harvesting of the kinetic energy generated by the cardiac movement to drive electrical current and pace the heart; feasibility models have been tested and will inevitably undergo numerous further iterations over the coming years [66, 67].
With the indications for device therapy increasing in recent years, leadless pacing has the potential to provide safe long-term treatment options for a large cohort of patients with cardiovascular disease. If rapid innovation and development continue at their current trajectory, it is plausible that leadless technology may become a first-line treatment option in decades to come.
References
Udo EO, Zuithoff NPA, Van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9:728–35.
Wang Y, Hou W, Zhou C, et al. Meta-analysis of the incidence of lead dislodgement with conventional and leadless pacemaker systems. PACE Pacing Clin Electrophysiol. 2018;41:1365–71.
Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. 2007;9:328–32.
Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol. 2000;85:774–6.
Cantillon DJ, Exner DV, Badie N, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. 2017;3:1296–305.
Cantillon DJ, Dukkipati SR, Ip JH, et al. Comparative study of acute and mid-term complications with leadless and transvenous cardiac pacemakers. Heart Rhythm. 2018;15:1023–30.
Spickler JW, Rasor NS, Kezdi P, Misra SN, Robins KE, LeBoeuf C. Totally self-contained intracardiac pacemaker. J Electrocardiol. 1970;3:325–31.
Vardas P, Politopoulos C, Manios E, Parthenakis F, Tsagarkis C. A miniature pacemaker introduced intravenously and implanted endocardially: preliminary findings from an experimental study. Eur J Card Pacing Electrophysiol. 1991;1:27–33.
Takeuchi ES, Quattrini PJ, Greatbatch W. Lithium/silver vanadium oxide batteries for implantable defibrillators. Pacing Clin Electrophysiol. 1988;11:2035–9.
Bock DC, Marschilok AC, Takeuchi KJ, Takeuchi ES. Batteries used to power implantable biomedical devices. Electrochim Acta. 2012;84:155–64.
Boveda S, Lenarczyk R, Haugaa KH, et al. Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey. Europace. 2018;20:555–9.
Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129:1466–71.
Knops RE, Tjong FVY, Neuzil P, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol. 2015;65:1497–504.
Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–35.
Lakkireddy D, Knops R, Atwater B, et al. A worldwide experience of the management of battery failures and chronic device retrieval of the Nanostim leadless pacemaker. Heart Rhythm. 2017;14:1756–63.
Ibrahim R, Khoury A, El-Chami MF. Leadless pacing: where we currently stand and what the future holds. Curr Cardiol Rep. 2022;1:1–8. https://doi.org/10.1007/s11886-022-01752-y.
Reddy VY, Exner DV, Doshi R, et al. Primary results on safety and efficacy from the LEADLESS II—phase 2 worldwide clinical trial. Clin Electrophysiol. 2022;8:115–7. https://doi.org/10.1016/j.jacep.2021.11.002.
Ritter P, Group MTPS, Duray GZ, et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J. 2015;36:2510–9. https://academic.oup.com/eurheartj/article/36/37/2510/2465991. Accessed 2 Sep 2022.
Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–41.
Duray GZ, Ritter P, El-Chami M, et al. Long-term performance of a transcatheter pacing system: 12-month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017;14:702–9.
Roberts PR, Clementy N, Al Samadi F, et al. A leadless pacemaker in the real-world setting: the Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm. 2017;14:1375–9.
El-Chami MF, Al-Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15:1800–7.
Tjong FVY, Knops RE, Udo EO, et al. Leadless pacemaker versus transvenous single-chamber pacemaker therapy: a propensity score-matched analysis. Heart Rhythm. 2018;15:1387–93.
El-Chami MF, Soejima K, Piccini JP, et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. PACE Pacing Clin Electrophysiol. 2019;42:1105–10.
Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm. 2007;4:154–60.
Vamos M, Erath JW, Benz AP, Bari Z, Duray GZ, Hohnloser SH. Incidence of cardiac perforation with conventional and with leadless pacemaker systems: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017;28:336–46.
El-Chami MF, Johansen JB, Zaidi A, et al. Leadless pacemaker implant in patients with pre-existing infections: results from the Micra postapproval registry. J Cardiovasc Electrophysiol. 2019;30:569–74.
El-Chami MF, Bonner M, Holbrook R, et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm. 2020;17:1393–7.
Dar T, Akella K, Murtaza G, et al. Comparison of the safety and efficacy of Nanostim and Micra transcatheter leadless pacemaker (LP) extractions: a multicenter experience. J Interv Card Electrophysiol. 2020;57:133–40.
Li J, Hou WB, Cao MK, et al. Safety and efficacy of leadless pacemaker retrieval. J Cardiovasc Electrophysiol. 2019;30:1671–8.
Bhatia NK, Kiani S, Merchant FM, et al. Life cycle management of Micra transcatheter pacing system: data from a high-volume center. J Cardiovasc Electrophysiol. 2021;32:484–90.
Loring Z, North R, Hellkamp AS, et al. VVI pacing with normal QRS duration and ventricular function: MOST trial findings relevant to leadless pacemakers. PACE Pacing Clin Electrophysiol. 2020;43:1461–6.
Brignole M, Auricchio A, Baron-Esquivias G, et al. ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–329.
Razeghi O, Strocchi M, Lee A, et al. Tracking the motion of intracardiac structures aids the development of future leadless pacing systems. J Cardiovasc Electrophysiol. 2020;31:2431–9.
Kiblboeck D, Reiter C, Kammler J, et al. Artefacts in 1.5 Tesla and 3 Tesla cardiovascular magnetic resonance imaging in patients with leadless cardiac pacemakers. J Cardiovasc Magn Reson. 2018;20:47.
Hála P, Neužil P, Keller J, et al. Quantification of artifacts during cardiac magnetic resonance in patients with leadless Micra pacemakers. J Cardiovasc Electrophysiol. 2021;32:1367–75.
Kruse I, Arnman K, Conradson TB, Ryden L. A comparison of the acute and long-term hemodynamic effects of ventricular inhibited and atrial synchronous ventricular inhibited pacing. Circulation. 1982;65:846–55.
El-Chami MF, Bockstedt L, Longacre C, et al. Leadless vs. transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur Heart J. 2022;43:1207–15. https://academic.oup.com/eurheartj/article/43/12/1207/6425620. Accessed 3 May 2022.
Chinitz L, Ritter P, Khelae SK, et al. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: results from the Micra atrioventricular feasibility studies. Heart Rhythm. 2018;15:1363–71.
Garweg C, Splett V, Sheldon TJ, et al. Behavior of leadless AV synchronous pacing during atrial arrhythmias and stability of the atrial signals over time—results of the MARVEL Evolve subanalysis. PACE Pacing Clin Electrophysiol. 2019;42:381–7.
Steinwender C, Khelae SK, Garweg C, et al. Atrioventricular synchronous pacing using a leadless ventricular pacemaker: results from the MARVEL 2 study. JACC Clin Electrophysiol. 2020;6:94–106.
Bereuter L, Gysin M, Kueffer T, et al. Leadless dual-chamber pacing: a novel communication method for wireless pacemaker synchronization. JACC Basic Transl Sci. 2018;3:813–23.
Vatterott PJ, Eggen MD, Hilpisch KE, et al. Implant, performance, and retrieval of an atrial leadless pacemaker in sheep. Heart Rhythm. 2021;18:288–96.
Knops RE, Olde Nordkamp LRA, Delnoy P-PHM, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383:526–36.
Tjong FVY, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016;18:1740–7.
Tjong FVY, Brouwer TF, Koop B, et al. Acute and 3-month performance of a communicating leadless antitachycardia pacemaker and subcutaneous implantable defibrillator. JACC Clin Electrophysiol. 2017;3:1487–98.
Breeman KTN, Swackhamer B, Brisben AJ, et al. Long-term performance of a novel communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable cardioverter-defibrillator system: a comprehensive preclinical study. Heart Rhythm. 2022;19:837–46.
Fernández-Palacios G, García-Morán E, Sandín-Fuentes M, García-Granja P, Rubio J, San Román A. The utility of a combined synchronous atrioventricular leadless pacemaker and subcutaneous implantable cardiac defibrillator system in bilateral upper limb venous occlusion. Europace. 2020;23:814.
Crozier I, O’Donnell D, et al. The extravascular implantable cardioverter-defibrillator: the pivotal study plan. J Cardiovasc Electrophysiol. 2021;32:2371–8.
Wijesuriya N, Elliott MK, Mehta V, et al. Leadless left bundle branch area pacing in cardiac resynchronisation therapy: advances, challenges and future directions. Front Physiol. 2022;13: 898866.
Auricchio A, Delnoy PP, Butter C, et al. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study. Europace. 2014;16:681–8.
Reddy VY, Miller MA, Neuzil P, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT-LV study. J Am Coll Cardiol. 2017;69:2119–29.
Sieniewicz BJ, Behar JM, Gould J, et al. Guidance for optimal site selection of a leadless left ventricular endocardial electrode improves acute hemodynamic response and chronic remodeling. JACC Clin Electrophysiol. 2018;4:860–8.
Sieniewicz BJ, Betts TR, James S, et al. Real-world experience of leadless left ventricular endocardial cardiac resynchronization therapy: a multicenter international registry of the WiSE-CRT pacing system. Heart Rhythm. 2020;17:1291–7.
Wijesuriya N, Elliott MK, Mehta V, et al. Leadless left ventricular endocardial pacing for cardiac resynchronization therapy: a systematic review and meta-analysis. Heart Rhythm. 2022;19:1176–83.
Sieniewicz BJ, Gould JS, Rimington HM, Ioannou N, Rinaldi CA. Transseptal delivery of a leadless left ventricular endocardial pacing electrode. JACC Clin Electrophysiol. 2017;3:1333–5. https://doi.org/10.1016/j.jacep.2017.04.020.
Okabe T, Hummel JD, Bank AJ, et al. Leadless left ventricular stimulation with WiSE-CRT System—initial experience and results from phase I of SOLVE-CRT Study (nonrandomized, roll-in phase). Heart Rhythm. 2022;19:22–9.
Singh JP, Walsh MN, Kubo SH, et al. Modified design of stimulation of the left ventricular endocardium for cardiac resynchronization therapy in nonresponders, previously untreatable and high-risk upgrade patients (SOLVE-CRT) trial. Am Heart J. 2021;235:158–62. https://pubmed.ncbi.nlm.nih.gov/33596412/. Accessed 3 May 2022.
Carabelli A, Jabeur M, Jacon P, et al. European experience with a first totally leadless cardiac resynchronization therapy pacemaker system. Europace. 2021;23:740–7.
Sidhu BS, Gould J, Porter B, et al. Completely leadless cardiac resynchronization defibrillator system. JACC Clin Electrophysiol. 2020;6:588–9.
Elliott MK, Sidhu BS, Mehta VS, Gould J, Martic D, Rinaldi CA. The importance of leadless pacemaker positioning in relation to subcutaneous implantable cardioverter-defibrillator sensing in completely leadless cardiac resynchronization and defibrillation systems. Heart Rhythm Case Rep. 2021;7:628–632. https://pubmed.ncbi.nlm.nih.gov/34552857/. Accessed 3 May 2022.
Teigeler T, Kolominsky J, Vo C, et al. Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Heart Rhythm. 2021;18:743–9.
Padala SK, Ellenbogen KA. Left bundle branch pacing is the best approach to physiological pacing. Heart Rhythm O2. 2020;1:59–67. http://www.heartrhythmopen.com/article/S266650182030009X/fulltext. Accessed 17 Jan 2022.
Zhang W, Huang J, Qi Y, et al. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019;16:1783–90.
Elliott MK, Jacon P, Sidhu BS, et al. Technical feasibility of leadless left bundle branch area pacing for cardiac resynchronization: a case series. Eur Heart J Case Rep. 2021;5. https://academic.oup.com/ehjcr/article/5/11/ytab379/6374927. Accessed 17 Jan 2022.
Franzina N, Zurbuchen A, Zumbrunnen A, et al. A miniaturized endocardial electromagnetic energy harvester for leadless cardiac pacemakers. PLoS One. 2020;15:e0239667.
Zurbuchen A, Haeberlin A, Bereuter L, et al. The Swiss approach for a heartbeat-driven lead- and batteryless pacemaker. Heart Rhythm. 2017;14:294–9.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Concept and design—KMR, JB. Drafting manuscript—KMR, ME, NW, VM, TW, CAR, JB. Reviewing and Editing—KMR, ME, NW, VM, TW, CAR, JB.
Disclosures
The authors are supported by the Wellcome/EPSRC Centre for Medical Engineering. [WT203148/Z/16/Z]. Mark Elliot and Vishal Mehta have received fellowship funding from Abbott. Jonathan M Behar receives research funding and/or consultation fees from Abbott, Siemens Healthcare, EBR Systems, Biosense Webster outside of the submitted work. Christopher Aldo Rinaldi receives research funding and/or consultation fees from Abbott, Medtronic, Boston Scientific, Spectranetics and MicroPort outside of the submitted work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Malaczynska-Rajpold, K., Elliot, M., Wijesuriya, N. et al. Leadless Cardiac Pacing: New Horizons. Cardiol Ther 12, 21–33 (2023). https://doi.org/10.1007/s40119-022-00288-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00288-0