Abstract
In this research a high yield, homogenous and fast bottom-up wet-chemical method has been carried out for the synthesis of platinum nanocrystal (Pt-NC) and up to micron size (Pt-M). After synthesis of the particles, surface plasmon resonance (SPR), ultra violet (UV) spectrum, size, shape, and composition were measured for each set. Platinum nanocrystal attained by preventing particles to grow directly after reduction of Pt+4 to Pt0. Pt-NC constructed by statistic repulsion of ionic surfactant surrounded nanocrystals. The final size of the Pt-NC found to be 3.8 ± 0.72 nm. Before sonication treatment, particle size of 705.2 ± 80.3 nm was achieved. After sonication, particle size increased to 1046.1 ± 199 nm. Particles were formed in a controllable way, homogenous and mono-disperse in size and shape. It is confirmed that sonication except for the sharpness of the spectrum did not alter the peak wavelengths. The suggested synthesis method enabled cost-effective concrete control over size, shape, concentration, and time of the synthesis.
Graphic abstract

Similar content being viewed by others
Introduction
Bulk platinum metals are well-known for it’s highly catalytic and photocatalytic properties [1, 2]. Moreover, it has novel applications including microelectronics, magnetic materials, catalysis, photocatalysis, and plasmonic-based electrochemical imaging (SPR based) [3,4,5]. Nevertheless the use of platinum micro and nanoparticles, especially in catalysis is particularly interesting due to the reduced cost and the greater surface area with respect to the bulk platinum. Thanks to its excellent properties, its application could be observed as cathode in low-temperature solid oxide fuel cells ( < 500 °C), as an electrocatalyst in pure hydrogen production, as anode in direct ethanol fuel cells, and as electro-oxidizer in biosensors [6]. Recent applications of platinum required it to be used in the form of nano thin films, micro-particles and nanoparticles. Beside platinum nanocrystals (Pt-NC), platinum microparticles (Pt-M) size are suitable alternatives for developing new generations of fuel cells because they are commercially available and suggest alternative energy sources, and electrochemical activity, while functioning under wide operating temperatures [6, 7]. Surface plasmon resonance (SPR) is the dipolar excitation of entire particle between free electron and its positively charged lattice induced by incoming electromagnetic wave (EM). Although the plasmonic peak for platinum is not highly active due to one empty space in the d-band and one empty space in the s-band [8], investigation of plasmonic peak for Pt-NC and Pt-M to obtain a spectrum might be useful mainly because of its anisotropic nature [9]. The first form of platinum particles, i.e., platinum nanocrystal (Pt-NC) offers quantum size effects and confined size in the range of 1–10 nm that enables it to be applied as catalyst in quantum physics and chemistry [8,9,10]. The second one is platinum micro-particles (Pt-M) that four advantages for its synthesis has been suggested: first, it is mono-dispersed, even though the particles are larger than the previous set of Pt-NC; second, the size barrier, from nanometer to micrometer, is overcome; third, Pt-M is synthesized with a one-step fast reduction, rather than a multiple step process which would be more complex and cost time; fourth, Pt-M characterizations has not been extensively reported in literature so it could introduce new opportunities in relevant research areas [3, 11,12,13,14,15,16,17,18,19]. A fundamental challenge is to improve the reproducibility of the synthesis process so a consistent particle size can be obtained. Studying the details of Pt-M synthesis seems to be more useful than that of the other types of platinum nanoparticles, e.g., Pt-NC in terms of vast for opportunities to investigate the formation mechanism. In the current study we aim to propose a straightforward, single-stage chemistry using homogeneous nucleation method to synthesize platinum particles in nano and micron size.
Experimental
Preparation of platinum nanoparticles was done in two stages base on reagents as follows: acid hexachloroplatinic (IV) (H2PtCl6∙6H2O; Merck KGaA), polyvinylpyrrolidone (PVP; Sigma–Aldrich), sodium borohydride (NaBH4, Fluka), and cetyltrimethylammonium bromide (CTAB; TCI Europe N.V.). All the chemicals were used as received from the suppliers, without any additional purification. Water was purified with a Mili pore MiliQ system (MQ water 18.1 MΩ). Glassware used in the syntheses was cleaned with the mixture of hydrochloric acid and nitric acid (HCl: HNO3, 3:1). Sonication was carried out with an ultrasonic bath (Branson Ultrasonic Cleaner 2520E-DTH). Centrifugation was performed with a Scan Speed 1730R centrifuge. In the first stage, for the formation of platinum nanocrystal (Pt-NC), 1 ml of 20 mM of platinum solution was added to 1 ml of 1 mM of PVP. Then 500 μl of 0.05 mM of pre-cooled sodium borohydrate was added and stirred. While stirring, 750 μl of 0.1 M of CTAB was added drop-wise. Subsequently, the mixture was kept sufficiently long at 26.5 °C to prevent crystallization of CTAB. To purify the obtained product centrifugation was done twice at 1300 rpm. The reaction mixture was stirred vigorously for a short period of time. In the second stage, the formation of platinum micron size (Pt-M) occurred by transporting of 1 ml of 20 mM of platinum solution to a falcon tube containing a stirrer bar. Next, 1 ml of 1 mM of PVP solution was added as described earlier. Then the mixture was stirred and incubated. After stirring, pre-cooled sodium borohydrate 500 μl was added. Then the mixture was stirred gently. The purification stage lasted a few seconds before performing sonication. Transmission electron microscopy (JEOL 1010 TEM) was used to investigate the 2D morphology and size of the particles. A UV–visible spectrophotometer (Varian-Cary-50 Conc.) has been used as well for measuring the nanoparticles absorption spectra. We used nanoparticle size and concentration analyzer (NANO sight-NS500) to analyze the presence, size distribution, and concentration of nanoparticles in liquid. However, as consistent data for Pt-M size cannot be provided (setup restriction in measuring larger particles > 700 nm), we excluded this set of data.
Results and discussion
Figure 1 shows a schematic mechanism for the possible formation of the CTAB micelles around Pt-NC. The reduction of platinum starts with the combination of a platinum metallic salt precursor, a reducing agent and a stabilizer. There are different types of platinum salt precursors that have been used for the reduction of platinum such as potassium tetrachloroplatinate-II (K2PtCl4) and chloroplatinic acid hexahydrate (H2PtCl6·6H2O). The main reduction reaction based on potassium tetra chloroplatinate-II is presented by the reaction of formula (1) [20]:
H2PtCl6 salt precursor was freshly used in our research instead of K2PtCl4 and better results were obtained, however, the main working mechanisms were the same. The former salt precursor (K2PtCl4) needed aging time, therefore, in the case of any failure plenty of time could be lost. Nevertheless, the final product was pure platinum, using different salts as precursor would have led to different types of particles [21]. The inside reaction in terms of electron donation can be interpreted as the formula (2) [22]:
As it has been shown in the reaction (2), sodium borohydride directly reduces the platinum complex and turns Pt+4 into Pto. This stage was obviously visible because of the clear change of the solution color from pale yellow to very dark brown.
Later, cetyltrimethylammonium bromide (CTAB) was introduced as an ionic surfactant to control the size and shape of platinum nanoparticles as shown in Fig. 1. The presence of non-ionic surfactant, polyvinylpyrrolidone (PVP) prevented aggregation of the particles after the reduction of platinum [23]. In fact, since they were characterized by an electrostatic charge, these compounds enabled electrostatic repulsion between particles and inhibit their further growth [24, 25].
Figure 2a shows the final color of the solution that was dark brown. A transmission electron microscopy (TEM) image of the Pt-NC has been shown in Fig. 2b. To avoid a thick layer of excess surfactant on the TEM grid surface, centrifugation of the solution was necessary prior to further investigation of each type of synthesized particles in TEM. Thorough statistical investigation was necessary due to the limitations of the TEM resolution; statistical analysis was performed manually and analyzed by exploring eight different regions of a TEM grid to get a clear idea of the size range that was shown in Fig. 3a. The average measured diameter of the particles was found to be 3.85 ± 0.72 nm as shown in Fig. 3a. The surface plasmon resonance (SPR) of Pt-NC was 230 nm as shown in Fig. 3b.
The reduction mechanism of platinum micro-sized particles was based on the presence of platinum salt (H2PtCl6·6H2O), the strong reducing agent, sodium borohydrate (NaBH4), polyvinylpyrrolidone (PVP) and the capping agent. The distinction in this case was that there were no other types of reagents as for Pt-NC; consequently, particle size became greater. Although there is not much literature available about the formation mechanism of Pt-M particles, it can be inferred from the previously described formation mechanisms of the Pt-NC particles by reactions (1) and (2). After the introduction of the reducing agent to the reaction solution, the platinum complex was directly reduced resulting in a spherical particle shape. In the second stage, the particle solution underwent a sonication treatment. Thanks to the extreme heat and pressure of the ultrasound waves (T = 5000 K, P = 1000 atm), the nanosize particles grew in size up to 1 μm in diameter, most likely due to an ultrasound-induced Ostwald-ripening phenomenon [26], which re-dissolves the smallest unstable particles in favor of the biggest ones, possibly contributing to their growth. As previously mentioned, the final color of the solution was very dark brown as shown in Fig. 2a.
TEM image of the Pt-M particles before and after sonication have been shown in Fig. 4a, b that confirmed the growth of platinum particles to micron size. Distribution of Pt-M sizes before and after sonication was shown in Fig. 4c, d, respectively. The mean diameter value of the particles before the sonication was 700 nm while after sonication it grew up to micron ( ~ 1000 nm). Additionally, as previously reported for Pt-NC, the plasmonic peak of the platinum nanoparticles does not shift enormously with respect to different sizes and morphologies.
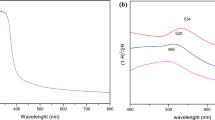
The surface plasmon resonance (SPR) of Pt-M before and after sonication has been shown in Fig. 5a, b, respectively. Before sonication treatment, the absorption spectra of Pt-M showed two major peaks at 230 nm and 240 nm, respectively, and one minor peak at 250 nm. After the sonication treatment, the particles were kept at room temperature for a while, and then the spectrum was measured. The peaks at 230 nm and 240 nm decreased in their y axis (Abs). Although there was a clear shift in the size of particles, the spectrum of Pt-M did not shift significantly after sonication. This was also verified with our final experimental results of the Pt-M where the particles also grew from nano to micron size. It is worth to mention that the sharpness of the plasmonic spectrum after sonication treatment decreased significantly. This can be due to the consumption of smaller particles during formation of large particles.
Conclusions
In this work, the synthesis of micron size platinum particles was done in two steps. Platinum nanocrystals (Pt-NC) fabricated with platinum salt, strong reducing agent NaBH4 and capping agents such as PVP and CTAB as ionic surfactant were used. The repulsion force caused by similar ions around the nanoparticles did not let the particles grow larger and they stayed in a crystal form. The exact control of the particle size and morphology of Pt-NCs by the addition of ionic surfactant was the key factor of their fabrication. A main advantage to the synthesis of Pt-NC was the high yield and high surface to volume ratio for catalytic activities. The reduction mechanism of the platinum micro-sized (Pt-M) particles was formed using platinum salt (H2PtCl6·6H2O), sodium borohydrate (NaBH4), and PVP-as the capping agent. The reduction process occurred immediately after introduction of the strong reducing agent and formed spherical particles. After reduction, sonication was performed on the particles; it was believed that Ostwald-ripening occurred, where energetically favorable for smaller particles to dissolve and adsorb onto larger particles. This work demonstrated the close match of theoretical and practical data to former literature regarding Pt nanocrystals. Investigation of the explicit mechanism of the Pt-M formation and determination of the reproducibility of the particle synthesis can be done in the near future.
References
Attilio, S., Karen, R.W., Oleg, S.A., Michael, D.A.: Synthesis and characterization of Pt clusters in aqueous solutions. J. Catal. 257, 5–15 (2008)
Yoo, E., Okata, T., Kohyama, M., Nakamura, J., Honma, I.: Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 9(6), 2255–2259 (2009)
Liang, W., Yusuke, Y.: Facile synthesis of three-dimensional dendritic platinum nanoelectrocatalyst. Chem. Mater. 21, 3562–3569 (2009)
Liz-Marzan, L.M.: Nanometals: formation and color. Mater. Today 7(2), 26–31 (2004)
Herbert W., Rocha, T.C.R., Wallace C.N., Leandro M.S., Marcelo K., Daniel Z.: Chemical synthesis and structural characterization of highly disordered Ni colloidal nanoparticles. ACS Nano. 2, 1313–1319 (2008)
Ji, S., Chang, I., Cho, G.Y., Lee, Y.H., Shim, J.H., Cha, S.W.: Application of dense nano-thin platinum films for low-temperature solid oxide fuel cells by atomic layer deposition. Int. J. Hydrog. Energy 39, 12402–12408 (2014)
Feng, Y., Bu, L., Guo, S., Guo, J., Huang, X.: 3-D platinum-lead nanowire networks as highly efficient ethylene glycol oxidation electrocatalysts. SMALL 12(33), 4464–4470 (2016)
Svetlana V.B., Ghasemi H., Chen, G.: Plasmonic materials for energy: from physics to applications. Mater. Today 16(10), 375–386 (2013)
Xu, J., Fu, G., Tang, Y., Zhou, Y., Chen, Y., Lu, T.: One-pot synthesis of three-dimensional platinum nanochain networks as stable and active electrocatalysts for oxygen reduction reactions. J. Mater. Chem. 22, 13585–13590 (2012)
Christoph, L., Zhe, Y., Igor, Z., Bengt, K.: Plasmonic properties of supported Pt and Pd nanostructures. Nano Lett. 6(4), 833–838 (2006)
Xiong, Y., Xia, Y.: Shape-controlled synthesis of metal nanostructures: the case of palladium. Adv. Mater. 19, 3385–3391 (2007)
Sara, E.S., Younan, X.: Pushing nanocrystal synthesis toward nonomanufacturing. ACS Nano 3(1), 10–15 (2009)
Byungkwon, L., Majiong, J., Jing, T., Pedro, H.C., Yimei, Z., Younan, X.: Shape-controlled synthesis of pd nanocrystals in aqueous solutions. Adv. Funct. Mater. 19, 189–200 (2009)
Peng, Z., Yang, H.: Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 4(2), 143–164 (2009)
Cheong, S., Watt, J., Ingham, B., Tony, M.F., Tilley R.D.: In situ and ex situ studies of platinum nanocrystals: growth and evolution in solution. J. Am. Chem. Soc. 131(40), 14590–14595 (2009)
Wiley, B.J., Chen, Y., McLellan, J.M., Xiong, Y., Li, Z., Ginger, D., Xia, Y.: Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 7(4), 1032–1036 (2007)
Qu, W.L., Wang, ZhB, Gao, Y., Deng, Ch., Wang, R.H., Zhao, L., Sui, X.L.: WO3/C supported Pd catalysts for formic acid electro-oxidation activity. Int. J. Hydrog. Energy 43, 407–416 (2018)
Qu, W.L., Gu, D.M., Wang, ZhB, Zhang, J.J.: High stability and high activity Pd/ITO-CNTs electrocatalyst for direct formic acid fuel cell. Electrochem. Acta. 137, 676–684 (2014)
Qu, W.L., Wang, ZhB, Jiang, ZhZh, Gu, D.M., Yin, G.P.: Investigation on performance of Pd/Al2O3-C catalyst synthesized by microwave assisted polyol process for electrooxidation of formic acid. RSC Adv. 2(1), 344–350 (2012)
Liang, W., Chunping, H., Yoshihiro, N., Yoshitaka, T., Yusuke, Y.: On the role of ascorbic acid in the synthesis of single-crystal hyperbranched platinum nanostructures. Cryst. Growth Des. 10, 3454–3460 (2010)
Wilson, D.A., Nolte, R.J., van Hest, J.C.: Autonomous movement of platinum-loaded stomatocytes. Nat Chem. 4(4), 268–274 (2012)
Minh, D.P., Oudart, Y., Baubet, B., Verdon, C., Thomazeau, C.: Nanostructured heterogeneous catalysts: well defined platinum nanoparticles supported on alumina. preparation, characterization, and application to the selective hydrogenation of buta-1, 3-diene. Oil Gas Sci. Technol. 64, 697–706 (2009)
Teranishi, T., Hosoe, M., Tanaka, T., Miyake, M.: Size control of monodispersed Pt nanoparticles and their 2D organization by electrophoretic deposition. J. Phys. Chem. B 103(19), 3818–3827 (1999)
Berhault, G., Bausach, M., Bisson, L., Becerra, L., Thomazeau, C., Uzio, D.: Seed-mediated synthesis of pd nanocrystals: factors influencing a kinetic- or thermodynamic- controlled growth regime. J. Phys. Chem. C 111(16), 5915–5925 (2007)
Konsolakis, M., Yentekakis, I.V., Palermo A., Lambert, R.M.: Optimal promotion by rubidium of the CO + NO reaction over Pt/γ-Al2O3 catalysts. Appl. Catal. B Environ. 33(4), 293–302 (2002)
Nguyen, N.T.K. Maclean, N., Mahiddine, S.: Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114(15), 7610–7630 (2014)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tajerian, T., Monsefi, M. & Rowan, A. Simple chemistry drives controlled synthesis of platinum nanocrystal to micron size. J Nanostruct Chem 9, 197–202 (2019). https://doi.org/10.1007/s40097-019-0310-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-019-0310-0