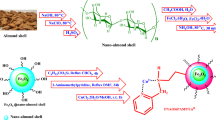
Abstract
In this paper, boron trifluoride supported on coconut shell nano-particles (nano-BF2O-coc) was prepared from nano-crystalline coconut shell through mechanical method using ultrasonication. The resultant was utilized to obtain nano-crystalline BF3 supported on coconut shell nano-particles (nano-BF2O-coc). The novel nanostructure has been characterized using Fourier transform infrared spectroscopy, transmission electron microscopy, field-emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy (EDX) techniques. The catalytic activity of the solid acid catalyst has been successfully examined in a one-pot, three-component condensation reaction of barbituric acid, ethyl cyanoacetate or malononitrile, and aldehydes in refluxing ethanol to furnish pyrano[2,3-d]pyrimidine derivatives.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) are accounted as environmental friendly benign synthetic procedures to gain complex organic compounds with maximum complexity and various levels of structural diversity with an outlook to green chemistry. The power of MCRs is synthesis of desired adducts in a single operation from three or more reactants without exposure of inadvertent intermediates and/or products [1,2,3,4]. Pyrano[2,3-d]pyrimidine derivative synthesis method can be conduct through multicomponent reactions. Pyrimidine derivatives are biologically interesting and attractive compounds with antibacterial, antitumor, cardiotonic, antihypertensive, antibronchitic, antifungal, and analgesic properties [5,6,7]. They are commonly synthesized via a one-pot, three-component reaction of various aromatic aldehydes, barbituric acid, and malononitrile or ethyl cyanoacetate in the presence of some conditions and catalysts such as DABCO [8], ultrasonic irradiation [9], glycerol [10], ionic liquids [11], diammonium hydrogen phosphate [12], l-proline [13], H14[NaP5W30O110] [14], sulfonic acid nano-porous silica (SBA-Pr-SO3H) [15], as well as being promoted by microwave irradiation [16].
Recently, nano-solid-supported reagents such as nano-crystalline TiO2–HClO4 [17], nano-TiCl4·SiO2 [18], nano-SnCl4·SiO2 [19], nano-sawdust-BF3 [20], nano-sawdust-OSO3H [21], BF3–SiO2 nano-particles [22], HClO4–SiO2 nano-particles [23], nano-cellulose-OSO3H [24], nano-silica sulfuric acid [25], SbCl5/SiO2 nano-particles [26], Pt@GO-PVP nano-composite [27], RhPt/TC@GO NPs [28], graphene oxide [29], and monodisperse Pt NPs@AC [30] have developed due to the high activity and selectivity [31].
Boron trifluoride (BF3) is a steaming liquid, and usually, it is utilized in many important industrial’s processing and organic reactions. Due to high specific gravity along with having fumes in air, it reacts with the moisture to form HF; therefore, the supported form is actually suitable and preferable. According to the literatures, it has been acclaimed which the supported BF3 with various materials is known as a solid Lewis superacid. Coconut shell ash due to be highly effective, having a large surface area and it usability for dispersing all of materials over it largely, has claimed and proved for preparation of a supported catalyst in liquid and vapor phase reactions [32]. In this study, we prepared the BF3 supported on coconut shell (BF2O-coc) as an efficient and new nano-catalyst. Not only its efficiency regarding the chemical yield and reaction rate as a nano-catalyst is superior in compare with other previous reported catalysts for the same reactions, but also the catalyst preparation method could obviate the steaming inherent of boron catalysts; therefore, catalyst handling and operation becomes extremely clean and benign.
During the course of our studies and investigations on the synthesis of pyrano[2,3-d]pyrimidine compounds and our interest in MCRs [20,21,22,23,24,25], we intended to study the feasibility of synthesizing their derivatives by the one-pot three-component condensation reactions strategy of aromatic aldehydes (1a–j) with ethyl cyanoacetate or malononitrile 2 and barbituric acid 3 in the presence of boron trifluoride supported on coconut shell (nano-BF2O-coc) as a novel nano-catalyst at reflux ethanol (Scheme 1).
Experimental
Chemical and materials
All chemicals utilized in this work were purchased from Merck or Fluka and used as received.
Melting points were determined with an Electrothermal 9100 apparatus. Fourier transform infrared spectroscopy (FT-IR) was recorded using FT-IR spectrometer (Vector 22- Bruker). X-ray diffraction (XRD) measurements were achieved by Bruker D8 diffractometer using Cu-Kα radiation (λ = 1.5418 Å) in a range of Bragg’s angle (0°–100°) at room temperature. The morphologies of the nano-catalyst were observed using field-emission scanning electron microscopy (FESEM) of an MIRA3 TESCAN microscope with an accelerating voltage of 15 kV. The EDX analysis was done using a SAMx analyser. Transmission electron microscopy (TEM) experiments were conducted by a Philips EM 208 electron microscope. 1H and 13C NMR spectra were recorded on Bruker DRX-300 Avance spectrometer at solution in DMSO-d6 using TMS as an internal standard.
Preparation of BF3 supported on coconut shell (BF2O-coc) as nano-catalyst
At first, outer brown shell of coconut was separated and washed several times with deionized water at room temperature. The mixture put into ice/water bath and set in the ultrasonicator for 15 min at output power of 1200 W. The obtained colloidal suspension was centrifuged and freeze dried, and the powder was stored at 5 °C. To a suspension of nano-coconut powder (1 g) in n-hexane (10 ml), BF3 (0.7 ml) was added dropwise at 0 °C during 20 min. Then, the mixture was stirred for 2 h at room temperature to remove HF from the reaction vessel. The mixture was filtered, and the collected solid was washed with n-hexane (10 ml) and dried at room temperature. Subsequently, for obtaining a fine and homogenized dried coconut shell powder, a mortar and pestle was used for grinding. Obtained powder was BF3 supported on coconut shell (BF2O-coc) (Scheme 2).
General procedure for the preparation of compounds 4a–n
To a mixture of barbituric acid (1 mmol), aromatic aldehyde (1 mmol) and ethyl cyanoacetate or malononitrile (1 mmol) in EtOH (5 mL) was added 15 mg BF3 supported on coconut shell (nano-BF2O-coc) as nano-catalyst at reflux condition for 15 min. Having completed the reaction (as monitored by TLC), the mixture was filtered to separate the heterogeneous catalyst. Then, the mixture vial was cooled at room temperature and solid was filtered. Then, obtained crude product was recrystallized from ethanol/water to give the desired product a powder. Spectral data of products are listed below:
Selected spectral data
Ethyl 7-amino-2,4-dioxo-5-phenyl-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4a)
IR (KBr, cm−1): 3381, 3168, 1664; 1H NMR (300 MHz, DMSO-d6): δ 3.6 (s, 3H, CH3), 4.19 (s, 1H), 4.31 (s, 2H, CH2), 6.07 (s, 1H, ArH), 7.10 (br s, 2H, NH2), 6.51–8.13 (m, 5H, ArH), 11.12 (br s, 1H, NH), 12.14 (br s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 30.0, 60.2, 69.2, 128.6, 129.8, 130.9, 135.4, 146.4, 148.6, 152.8, 156.3, 159.2, 160.7, 161.0 ppm.
Ethyl 7-amino-5-(3-chlorophenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4b)
IR (KBr, cm−1): 3376, 3343, 3192, 1687; 1H NMR (300 MHz, DMSO-d6): δ 2.17 (s, 3H, CH3), 3.94 (s, 2H, CH2), 4.73 (s, 1H), 6.99 (s, 2H, ArH), 7.11–7.25 (m, 2H, ArH), 7.21 (s, 2H, NH2), 9.10 (br s, 1H, NH), 11.17 (br s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 35.7, 52.8, 76.9, 78.8, 124.8, 125.2, 127.9, 129.6, 133.5, 137.4, 143.0, 150.2, 160.1, 160.3, 163.2, 165.4 ppm.
Ethyl 7-amino-5-(4-chlorophenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4c)
IR (KBr, cm−1): 3311, 3188, 3091, 1648; 1H NMR (300 MHz, DMSO-d6): δ 2.17 (s, 3H, CH3), 4.8 (s, 2H, CH2), 5.28 (s, 1H), 7.25–7.31 (m, 2H, ArH), 7.35–7.41 (m, 2H, ArH), 7.75 (br s, 2H, NH2), 10.99 (s, 1H, NH), 11.55 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 88.3, 98.5, 114.8, 126.9, 128.8, 129.0, 129.9, 130.5, 135.9, 150.1, 155.4, 155.8, 159.7, 160.9 ppm.
Ethyl 7-amino-5-(3-nitrophenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4d)
IR (KBr, cm−1): 3380, 3321, 1640; 1H NMR (300 MHz, DMSO-d6): δ 3.63 (s, 3H, CH3), 4.1 (s, 2H, CH2), 4.82 (s, 1H), 7.26 (s, 2H, NH2), 7.52 (m, 2H, ArH), 8.14 (m, 2H, ArH), 11.12 (s, 1H, NH), 12.17 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 35.7, 57.5, 87.5, 119.0, 124.4, 130.7, 130.9, 146.4, 149.6, 151.9, 152.7, 157.8, 159.2, 161.7, 162.6 ppm.
Ethyl 7-amino-5-(4-nitrophenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4e)
IR (KBr, cm−1): 3422, 3367, 3106, 1604; 1H NMR (300 MHz, DMSO-d6): δ 3.09 (s, 3H, CH3), 3.92 (s, 1H), 4.12 (s, 2H, CH2), 7.26 (s, 2H, NH2), 7.32 (m, 2H, ArH), 8.09 (m, 2H, ArH), 9.67 (s, 1H, NH), 10.15 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 37.2, 61.7, 79.5, 121.0, 128.0, 130.0, 131.2, 145.4, 148.3, 150.5, 160.3, 162.3, 163.8, 167.2 ppm.
Ethyl 7-amino-5-(4-methylphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4f)
IR (KBr, cm−1): 3395, 3367, 3103, 1662; 1H NMR (300 MHz, DMSO-d6): δ 2.36 (s, 3H, CH3), 2.60 (s, 3H, CH3), 4.13 (s, 1H), 5.21 (s, 2H, CH2), 7.12 (m, 2H, ArH), 7.20 (m, 2H, ArH), 7.60 (br s, 2H, NH2), 10.89 (s, 1H, NH), 11.43 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 20.9, 88.7, 98.3, 115.5, 127.5, 128.1, 133.7,137.4, 150.1, 155.5, 155.9, 159.1, 159.9, 160.8 ppm.
Ethyl 7-amino-5-(4-methoxyphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4g)
IR (KBr, cm−1): 3413, 3389, 3106, 1667; 1H NMR (300 MHz, DMSO-d6): δ 2.49 (s, 3H, CH3), 3.32 (s, 3H, OCH3), 3.71 (s, 2H, CH2), 4.41 (s, 1H), 6.93 (m, 2H, ArH), 7.65 (m, 2H, ArH), 9.07 (br s, 2H, NH2), 10.03 (s, 1H, NH), 11.09 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 33.0, 37.2, 55.8, 75.6, 114.2, 126.0, 128.4, 130.1, 134.2, 143.9, 150.5, 157.2, 162.4, 167.3 ppm.
Ethyl 7-amino-5-(3,4-dimethoxy phenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4h)
IR (KBr, cm−1): 3495, 3303, 3123, 1662; 1H NMR (300 MHz, DMSO-d6): δ 3.12 (s, 3H, CH3), 3.50 (s, 2H, CH2), 3.60 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.2 (s, 1H), 7.1 (s, 2H, NH2), 8.27 (m, 1H, ArH), 8.47 (m, 2H, ArH), 11.1 (s, 1H, NH), 11.4 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 37.9, 56.3, 57.4, 79.7, 114.2, 124.3, 127.5, 128.7, 135.8, 138.0, 143.8, 146.9, 149.4, 150.1, 157.3, 157.9, 163.9 ppm.
Ethyl 7-amino-5-(3-hydroxyphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4i)
IR (KBr, cm−1): 3439, 3337, 3106, 1677; 1H NMR (300 MHz, DMSO-d6): δ 3.6 (s, 3H, CH3), 3.91 (s, 2H, CH2), 4.10 (s, 1H), 6.56 (s, 2H, NH2), 6.59 (m, 1H, ArH), 7.04–7.10 (m, 3H, ArH), 9.33 (br s, 1H, OH), 11.1 (br s, 1H, NH), 12.1 (br s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 35.6, 59.9, 89.5, 114.7, 114.9, 118.8, 120.1, 127.4, 128.0, 130.1, 146.5, 150.4, 153.1, 158.1, 158.5, 163.3 ppm.
Ethyl 7-amino-5-(4-hydroxyphenyl)-2,4-dioxo-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carboxylate (4j)
IR (KBr, cm−1): 3343, 3191, 1785; 1H NMR (300 MHz, DMSO-d6): δ 2.43 (s, 3H, CH3), 3.17 (s, 2H, CH2), 3.69 (s, 1H), 6.07 (br s, 1H, OH), 6.67 (m, 2H, ArH), 6.74 (m, 2H, ArH), 7.31 (s, 2H, NH2), 10.47 (s, 1H, NH), 11.03 (s, 1H, NH); 13C NMR (75 MHz, DMSO-d6): δ 29.03, 37.2, 61.5, 75.2, 79.9, 115.3, 123.7, 129.0, 134.4, 142.3, 150.4, 155.5, 160.1, 163.5 ppm.
7-Amino-6-cyano-5-(4-bromophenyl)-5H-pyrano[2,3-d]pyrimidinone (4k)
IR (KBr, cm−1): 3391, 3302, 3072, 2197, 1674; 1H NMR (300 MHz, DMSO-d6): δ 4.26 (s, 1H), 7.20 (br s, 2H, NH2), 7.22 (d, 2H, J = 8.2 Hz, Ar), 6.51 (d, 2H, J = 8.2 Hz, Ar), 11.12 (br s, 1H, NH), 12.14 (br s, 1H, NH); 13CNMR (75 MHz, DMSO-d6): δ 35.3, 58.4, 88.0, 119.0, 129.6, 131.1, 143.5, 149.4, 152.3, 157.6, 162.4 ppm.
7-Amino-6-cyano-5-(3-chlorophenyl)-5H-pyrano[2,3-d]pyrimidinone (4l)
IR (KBr, cm−1): 3419, 3324, 3196, 2192, 1690; 1HNMR (300 MHz, DMSO-d6): δ 4.25 (s,1H), 7.18–7.26 (m, 6H, Ar, NH2), 11.08 (br s, 1H, NH), 12.10 (br s, 1H, NH); 13CNMR (75 MHz, DMSO-d6): δ 35.5, 58.2, 87.8, 119.1, 126.3, 127.3, 130.2, 132.9, 146.7, 149.6, 152.5, 157.7, 162.6 ppm.
7-Amino-6-cyano-5-(3-nitrophenyl)-5H-pyrano[2,3-d]pyrimidinone (4m)
IR (KBr, cm−1): 3415, 3315, 3020, 2192, 1688, 1529, 1348; 1HNMR (300 MHz, DMSO-d6): δ 4.47 (s, 1H), 7.28 (br s, 2H, NH2), 7.60 (t, 1H, 3J = 7.8 Hz, Ar), 7.74 (d, 1H, 3J = 7.8 Hz, Ar), 8.06–8.10 (m, 2H, Ar), 11.11 (br s, 1H, NH), 12.17 (br s, 1H, NH); 13CNMR (75 MHz, DMSO-d6): δ 35.5, 58.5, 88.3, 119.8, 122.8, 122.9, 130.7, 135.3, 147.3, 148.6, 150.4, 153.5, 158.7, 163.4 ppm.
7-Amino-6-cyano-5-(3-hydroxyphenyl)-5H-pyrano[2,3-d]pyrimidinone (4n)
IR (KBr, cm−1): 3439, 3337, 3193, 2206, 1677; 1HNMR(300 MHz, DMSO-d6): δ 4.10 (s, 1H), 6.56 (br s, 2H, NH2), 6.59 (m, 1H, Ar), 7.04–7.10 (m, 3H, Ar), 9.33 (br s, 1H, OH) 11.09 (br s, 1H, NH), 12.07 (br s, 1H, NH) ppm; 13CNMR (75 MHz, DMSO-d6): δ 35.6, 59.9, 89.5, 114.7, 114.9, 118.8, 120.1, 130.1, 146.5, 150.4, 158.1, 158.5, 163.3 ppm.
Results and discussion
Characterization of prepared of BF3 supported on coconut shell (nano-BF2O-coc)
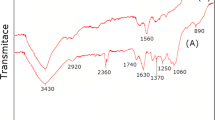
Fourier transform infrared (FT-IR) analysis
The chemical composition of coconut shell consists of lignin, cellulose, and hemicelluloses [33, 34]. Cellulose is composed of a long chain of glucose molecules, lignin is a complex polymer composed of phenylpropane units, and hemicelluloses are branched polymers composed of xylose, arabinose, galactose, mannose, and glucose [34].
Figure 1 shows the FT-IR spectra related to BF3 supported on coconut shell (nano-BF2O-coc). As it is seen, absorption band around the broad peak at 3452 cm−1 implies the presence of hydroxyl group. The peak appeared at 2922 cm−1 is attributed to the C–H stretching vibrations of CH, CH2, and CH3 groups. In addition, the absorption bands at nearly 1513 and 1643 cm−1 indicate the C=C bonds in aromatic rings. After supporting the coconut shell with BF3, the absorbtion of C–O and B–O is observed in 1152 and 664,598 cm−1 respectively.
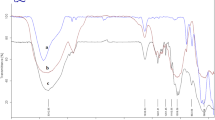
X-ray diffraction (XRD)
Figure 2a, b shows the XRD patterns related to coconut shell and BF3 supported on coconut shell (nano-BF2O-coc) respectively. The major index diffraction peaks for coconut shell (Fig. 2a) are 10°, 20°, and 35° with their inter-planar distance and their relative intensity of X-ray scattering are 63, 320, and 220, respectively. After supporting BF3 on coconut shell (nano-BF2O-coc) (Fig. 2b), the representative peaks are broadened and also shifted to some extent, owing to the alternation in the crystalline structure of the supported coconut shell with BF3.
Field-emission scanning electron microscope (FESEM)
Figure 3A, B indicates FESEM images of coconut shell and BF3 supported on coconut shell. According to Fig. 3A, the surface topography of coconut shell is shown. In fact, the pore structures and complete opening of network pores on its surface are observed and the average of diameter coconut shells is 282 nm. In addition, after supporting BF3 on coconut shell (nano-BF2O-coc) (Fig. 3B), average of its diameter is reduced to about 32 nm.
Energy-dispersive X-ray (EDX)
The EDX analysis of coconut shell and BF3 supported on coconut shell is presented in Fig. 4a, b. Coconut shell is composed of C, O, Na, Cl, and K (Fig. 4a), while BF3 supported on coconut shell (Fig. 4b) is composed of C, O, B, and F, mentioning that BF3 has been supported on coconut shell due to the presence of B and F.
Transmission electron microscopy (TEM)
Figure 5 shows the TEM of images related to BF3 supported on coconut shell (nano-BF2O-coc). As it can be seen from TEM images, the average particle size distribution is 30 nm which is consistent with the obtained results by FESEM images.
Catalytic application of BF2O-coc nano-catalyst
At first, to figure out the optimized conditions, the three-component reaction of barbituric acid, benzaldehyde, and ethyl cyanoacetate in the presence of the BF2O-coc as nano-catalyst in ethanol as the solvent was chosen as model. The reaction was performed with different amount of BF2O-coc as nano-catalyst (10, 15, and 20 mg) in various temperatures (25 and 78 °C). It is concluded that optimal condition was 15 mg of BF2O-coc at 78 °C in 5 ml ethanol (Table 1, entry 4).
It should be pointed out that the reaction was conducted with BF3 as the liquid catalyst. Regardless of difficulties in its handling, the chemical yield is very low (Table 1, entry 6). The catalyst activity is extremely improved with supporting the BF3 on the nano-crystalline structure due to the remarkable increase in the catalyst surface area.
Then, different aryl aldehydes were applied in the reactions that led to obtaining the corresponding products in high-to-outstanding yields (Table 2). As it can be displayed in Table 2, all the aryl aldehydes including electron-donating or electron-withdrawing substituents result, the desired products, in high yields (Table 2).
A proposed mechanism for synthesis of pyrano[2,3-d]pyrimidine derivatives using barbituric acid, arylaldehydes, and ethyl cyanoacetate or malononitrile in the presence of BF2O-coc as nano-catalyst was shown in Scheme 3.
First, the ethyl 2-cyano-3-arylacrylate or 2-benzylidenemalononitrile (5), containing the electron-poor C=C double bond, is formed quantitatively by Knoevenagel addition of ethyl cyanoacetate or malononitrile (2) to the aromatic aldehyde (1) in the presence of BF2O-coc as nano-catalyst. Then, the barbituric acid C-alkylation reacts with the electrophilic C=C double bond and gives the intermediate (6). Tautomerization convert intermediate (6) to intermediate (7). After, the intermediate (7) was cyclized by the nucleophilic attack of OH group on the cyano (CN) moiety and gave the intermediate (8). The intermediate (8) by the 1,3-proton transfer produced the desired product (4).
To establish a better catalytic activity of nano-BF2O-coc, the synthesis of pyrano[2,3-d]pyrimidine derivatives was compared with other catalysts reported in the literature [8,9,10,11,12,13,14,15,16]. As shown in Table 3, synthesis of these compounds catalyzed by many of various methods, but some of them suffer from disadvantages such as harsh reaction conditions, long reaction times, complex working and purification procedures, long volume of catalyst loading, and moderate yields. Therefore, the development of a simple, mild, and efficient method is still needed.
We also studied the recyclability of the BF2O-coc as the nano-catalyst utilizing the model reaction of barbituric acid, benzaldehyde, and ethyl cyanoacetate in ethanol (Table 2, entry 1). When the reaction is completed, the mixture was filtered to separate the catalyst. Then, catalyst washed several times with the hot ethanol and reused four times in the model reaction to evaluate the catalyst reusability. The results indicated that BF2O-coc is stable with no decrease in its catalytic activity (Fig. 6).
Conclusions
In summary, we have elaborated a new attractive and effective methodology for preparation of pyrano[2,3-d]pyrimidine derivatives via one-pot three-component condensation reaction strategy of barbituric acid with aromatic aldehydes and ethyl cyanoacetate or malononitrile at refluxing ethanol utilizing of BF3 supported on coconut shell (BF2O-coc) as a nano-catalyst. BF2O-coc was successfully prepared and characterized by FT-IR, XRD, EDX, FESEM, and TEM techniques. In comparison with the previous investigations (Table 3), this method is among the advantages such as lower reaction times, high yield, clean and fume free reaction, and reusability for a several times without any impressive loss of activity, and the formed compound is filtered and purified just by simple crystallization. The present study is a good addition to the application hypothesis of plants or fruits consisting of cellulose, which enables the binding the Boron Lewis acidic compounds with them, to prepare new solidified and easy-to-handle catalysts for chemical reactions facilitations.
References
Gore, R.P., Rajput, A.P.: A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent. Today 5, 148–152 (2013)
Kundu, S.K., Bhaumik, A.: Triazine-based porous organic polymer: a novel heterogeneous basic organocatalyst for facile one-pot synthesis of 2-amino-4H-chromenes. RSC Adv. 5, 32730–32739 (2015)
Ballini, R., Bigi, F., Conforti, M.L., Santis, D.D., Maggi, R., Oppici, G., Sartori, G.: Multicomponent reactions under clay catalysis. Catal. Today 60, 305–309 (2000)
Akocak, S., Şen, B., Lolak, N., Şavk, A., Koca, M., Kuzu, S., Şen, F.: One-pot three-component synthesis of 2-amino-4H-chromene derivatives by using monodisperse Pd nanomaterials anchored graphene oxide as highly efficient and recyclable catalyst. Nano Struct. Nano Objects 11, 25–31 (2017)
Ravikanth, S., Reddy, G.V., Maitraie, D., Rao, V.V.V.N.S., Rao, P.S., Narsaiah, B.: Synthesis of novel 5-trifluoromethyl-2,4,7-trisubstituted pyrido,3-d]pyrimidines. Synth. Commun. 34, 4463–4469 (2004)
Liu, Y., Zhang, X.H., Jin, G.Y.: Synthesis of novel dihydropyrimidine fused heterotricyclic compounds. Chin. J. Chem. 23, 182–184 (2005)
Bagley, M.C., Hughes, D.D., Lubinu, M.C., Merritt, E.A., Taylor, P.H., Tomkinson, N.C.O.: Microwave-assisted synthesis of pyrimidine libraries. QSAR Comb. Sci. 23, 859–867 (2004)
Bhat, A.R., Shalla, A.H., Dongre, R.S.: Synthesis of new annulated pyrano[2,3-d]pyrimidine derivatives using organo catalyst (DABCO) in aqueous media. J. Saudi. Chem. Soc. 21, S305–S310 (2017)
Safaei, H.R., Shekouhy, M., Shirinfeshan, A., Rahmanpur, S.: CaCl2 as a bifunctional reusable catalyst: diversity-oriented synthesis of 4H-pyran library under ultrasonic irradiation. Mol. Divers. 16, 669–683 (2012)
Safaei, H.R., Shekouhy, M., Rahmanpur, S., Shirinfeshan, A.: Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three-component synthesis of 4H-pyrans. Green Chem. 14, 1696–1704 (2012)
Yu, J., Wang, H.: Green synthesis of pyrano[2,3-d]-pyrimidine derivatives in ionic liquids synthetic. Synth. Commun. 35, 3133–3140 (2005)
Balalaie, S., Abdolmohammadi, S.H., Bijanzadeh, H.R., Amani, M.: Diammonium hydrogen phosphate as a versatile and efficient catalyst for the one-pot synthesis of pyrano[2,3-d]pyrimidinone derivatives in aqueous media. Mol. Diver. 12, 85–91 (2008)
Bararjanian, M., Balalaie, S., Movassagh, B., Amani, A.M.: One-pot synthesis of pyrano[2, 3-d]pyrimidinone derivatives catalyzed by l-proline in aqueous media. J. Iran. Chem. Soc. 6, 436–442 (2009)
Heravi, M.M., Ghods, A., Derikvand, F., Bakhtiari, K., Bamoharram, F.F.: H14[NaP5W30O110] catalyzed one-pot three-component synthesis of dihydropyrano[2,3-c]pyrazole and pyrano[2,3-d]pyrimidine derivatives. J. Iran. Chem. Soc. 7, 615–620 (2010)
Mohammadi Ziarani, G., Faramarzi, S., Asadi, S., Badiei, A., Bazi, R., Amanlou, M.: Three-component synthesis of pyrano[2,3-d]-pyrimidine dione derivatives facilitated by sulfonic acid nanoporous silica (SBA-Pr-SO3H) and their docking and urease inhibitory activity. DARU J. Pharm. Sci. 21, 3–13 (2013)
Gao, Y., Tu, S., Li, T., Zhang, X., Zhu, S., Fang, F., Shi, D.: Effective synthesis of 7-amino-6-cyano-5-aryl-5H-pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones under microwave irradiation. Synth. Commun. 34, 1295–1299 (2004)
Shirini, F., Atghia, S.V., Ghazi Jirdehi, M.: Nanocrystalline TiO2–HClO4: a novel, efficient and recyclable catalyst for the chemoselective N-Boc protection of amines under solvent-free conditions. Chin. Chem. Lett. 24, 34–36 (2013)
Mirjalili, B.F., Bamoniri, A., Zamani, L.: One-pot synthesis of 1, 2, 4, 5-tetrasubstituted imidazoles promoted by nano-TiCl4·SiO2. Sci. Iran. 19, 565–568 (2012)
Mirjalili, B.F., Bamoniri, A., Mirhoseini, M.A.: Nano-SnCl4·SiO2: an efficient catalyst for one-pot synthesis of 2,4,5-tri substituted imidazoles under solvent-free conditions. Sci. Iran. 20, 587–591 (2013)
Sadeghi, B., Zarepour, I.: Nano-sawdust–BF3 as a new, cheap, and effective nano-catalyst for one-pot synthesis of 2-amino benzo[h]chromene derivatives. J. Nanostruct. Chem. 5, 305–311 (2015)
Sadeghi, B., Bouslik, M., Shishehbore, M.R.: Nano-sawdust-OSO3H as a new, cheap and effective nanocatalyst for one-pot synthesis of pyrano[2,3-d]pyrimidines. J. Iran. Chem. Soc. 12, 1801–1808 (2015)
Sadeghi, B.: Silica supported boron triuoride nanoparticles (BF3–SiO2 NPs): an efficient and reusable catalyst for one-pot synthesis of benzo[a]xanthene-11-one derivatives. Sci. Iran. 21, 708–714 (2014)
Sadeghi, B., Shirkhani, M., Hassanabadi, A.: HClO4-SiO2 nanoparticles: an efficient and versatile catalyst for synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles. J. Chem. Res. 39, 112–114 (2015)
Sadeghi, B., SowlatTafti, M.H.: Nano-cellulose-OSO3H as a new, green, and effective nano-catalyst for one-pot synthesis of pyrano [4,3-b] pyrans. J. Iran. Chem. Soc. 13, 1375–1384 (2016)
Sadeghi, B., Ghasemi Pirbaluti, M., Farokhi Nezhad, P., Abbasi Nezhad, R.: A clean and expedient synthesis of spirooxindoles catalyzed by silica–sulfuric acid nanoparticles as an efficient and reusable reagent. Res. Chem. Intermed. 41, 4047–4055 (2015)
Sadeghi, B., Ghorbani Rad, M.: SbCl5/SiO2 nanoparticles: an efficient and reusable reagent for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol) derivatives in water. Synth. React. Inorg. Met. Org. Chem. 45, 1723–1727 (2015)
Daşdelen, Z., Yıldız, Y., Eris, S., Sen, F.: Enhanced electrocatalytic activity and durability of Pt nanoparticles decorated on GO-PVP hybride material for methanol oxidation reaction. Appl. Catal. B 219, 511–516 (2017)
Şen, B., Akdere, E.H., Şavk, A., Gültekin, E., Paralı, Ö., Göksu, H., Şen, F.: A novel thiocarbamide functionalized graphene oxide supported bimetallic monodisperse Rh–Pt nanoparticles (RhPt/TC@GO NPs) for Knoevenagel condensation of aryl aldehydes together with malononitrile. Appl. Catal. B 225, 148–153 (2018)
Aday, B., Pamuk, H., Kaya, M., Sen, F.: Graphene oxide as highly effective and readily recyclable catalyst using for the one-pot synthesis of 1,8-dioxoacridine derivatives. J. Nanosci. Nanotechnol. 16, 6498–6504 (2016)
Erken, E., Esirden, I., Kaya, M., Sen, F.: A rapid and novel method for the synthesis of 5-substituted 1H-tetrazole catalyzed by exceptional reusable monodisperse Pt NPs@AC under the microwave irradiation. RSC Adv. 5, 68558–68564 (2015)
Corma, A., Garcia, H.: Silica-bound homogenous catalysts as recoverable and reusable catalysts in organic synthesis. Adv. Synth. Catal. 348, 1391–1412 (2006)
Buasri, A., Chaiyut, N., Loryuenyong, V., Rodklum, C., Chaikwan, T., Kumphan, N., Jadee, K., Klinklom, P., Wittayarounayut, W.: Transesterification of waste frying oil for synthesizing biodiesel by KOH supported on coconut shell activated carbon in packed bed reactor. Sci. Asia 38, 283–288 (2012)
Liyanage, C.D., Pieris, M.: A physic-chemical analysis of coconut shell powder. Proc. Chem. 16, 222–228 (2015)
Agunsoye, J.O., Talabi, S.I., Bello, S.A., Awe, I.O.: The effects of cocos nucifera (coconut shell) on the mechanical and tribological properties of recycled waste aluminum can composites. Tribol. Ind. 36, 155–162 (2014)
Acknowledgements
The research Council of the Islamic Azad University of Yazd is gratefully acknowledged for the financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Molaei, H., Sadeghi, B. Preparation and characterization of BF3 supported on coconut shell as a novel and effective nano-catalyst for one-pot synthesis of pyrano[2,3-d]pyrimidines. J Nanostruct Chem 8, 197–206 (2018). https://doi.org/10.1007/s40097-018-0268-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-018-0268-3