Abstract
This article presents a method for the electrochemical preparation of a coating of nickel–silica nanocomposites on a carbon steel substrate. The incorporation of hydrophilic silica particles into the Ni composite coating during co-electrodeposition is so difficult due to the small size and the hydrophilicity of SiO2 particle, generally less than 2 v% of silica is incorporated into the composite at different current densities, agitation speeds and silica concentrations. The effect of the presence of four surfactants, namely cocamidopropyl betaine (CAPB), decylglycoside (DG), cetyltrimethyl ammonium chloride (CTAC) and ammonium lauryl ether sulfate (ALES), on overcoming this problem was investigated in this research, and the surfactants were found to greatly influence the surface charge of silica, silica incorporation percentage and the microstructure of the composite. In fact, upon increasing the internal stresses, the products prepared in the presence of CAPB and DG were found to crack to some degree. CTAC was found to lead to entrapment mode silica co-deposition in the Ni coating. Furthermore, the addition of ALES into an electrolyte bath negatively supercharged silica surfaces and increased silica dispersion, which led to a dramatic increase in the silica incorporation percentages to around 14 v%. The results showed that Ni–SiO2 composites prepared in the presence of ALES had better corrosion resistance, hardness and wear properties.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, the need for the properties of construction materials is increasing. The materials should be robust and light while offering a combination of several desired mechanical properties such as wear resistance, hardness, self-lubrication (in some cases), and heat resistance while providing good corrosion resistance at the same time [1]. Metal matrix composites (MMCs) could be described as a class of composite materials with two constituents: a metallic matrix that consists of dispersed inert particles. MMCs have been under consideration over the past decades, due to their superior properties such as wear and corrosion resistance, hardness, thermal, electrical and magnetic properties, in comparison with pure metal or alloy coatings, have attracted significant attention in the protective coating industry [1, 2]. Generally, MMCs could be classified into three different microstructures, namely particle-reinforced MMCs, short-fiber or whisker-reinforced MMCs and continuous-fiber or sheet-reinforced MMCs [1, 3]. Copper, aluminum, chromium, zinc, cobalt, iron and nickel act as common matrix materials, with a wide variety of inert particles such as carbon fiber, TiO2 [4, 5], ZNO [6], SiC [7, 8], Al2O3 [9] and SiO2 [10,11,12,13,14] as reinforcement. MMCs have been used in various fields ranging from high-tech industries (e.g., electronic components and computers) to more traditional industries (e.g., general mechanics and automobile, paper mill, textiles and food industries) [14]. There are several routes to prepare and form MMCs, i.e., squeeze casting [15], hydrothermal methods [16], powder metallurgical methods [17], high-velocity oxygen fuel thermal (HVOF) spraying [18], physical and chemical vapor deposition (PVD and CVD) [19] and electrodeposition [5].
Under ambient pressure and temperature, electrodeposition is one of the most important techniques used for producing composites. In addition, electrodeposition as a low-cost technique has a high deposition rate that leads to homogenous distribution of the particle [10,11,12,13,14]. Several models for studying co-deposition of particles in metal matrices could be found in the literature; for example, Guglielmi’s and Celis’s models [5, 20]. As the earliest model of its type, Guglielmi’s model is based on a two-step process involving loose and strong adsorption of the particles.
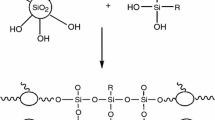
Nickel is a metal with high tensile strength, toughness and corrosion resistance, and is a popular choice as an MMC that hard and soft reinforcements could be dispersed on it, while improving its wear, anti-friction and corrosion resistances. Furthermore, nickel composites are noteworthy alternatives to hard Cr because of their improved resistance towards wearing and high-temperature oxidation. Electrodeposition of nickel is among the widely used surface finishing processes of an industrial scope and also many efforts have been carried out to improve the properties of Ni coatings through co-electrodeposition with different inert particles or fibers over the past decades. The importance of nickel-based particle-reinforced composite coatings in engineering applications has been significantly enhanced gradually, and these are currently applied in various technological fields requesting for corrosion, hardness, wear, friction and thermal resistances [5, 7, 11]. One beneficial particle used in such coating is SiO2 that according to some studies has an enhancing effect on the corrosion and mechanical resistance of composites [21, 22]. SiO2 particles can act as a barrier film, inhibiting the corrosion of the composite and reducing the consumption rate of the metallic portion of the composites. It should be mentioned that SiO2 particles have hydrophilic surfaces and naturally carrying negative charges due to the deprotonation of silanol groups in aqueous solutions (Scheme 1) [12, 13, 21].
Ni–SiO2 composite could be used from traditional industries from general mechanics, automobiles, paper mills, electrical devices to high-tech industries such as microelectronics, magneto-electronics and nanotechnology, and also for the preparation of high corrosion resistance, good hardness and wear coatings [1, 2, 14]. It is well known that the major challenges in the co-deposition of SiO2 particles are the low amount of nanoparticles in the composite, and also the agglomeration of particles suspended in the electroplating solutions due to high surface free energy of the nanoparticles. Furthermore, the high ionic strength of the electrolyte and also the high concentration of the inert particles in the electroplating bath increase the tendency of the particles to be agglomerated. Various additives have been studied to reduce the particle agglomeration to increase the volume fraction of the particles in the deposit with good dispersion and improved properties [22, 23]. In general, surface-active additives are used to change the surface characteristics of the particles. According to the literature [14], the addition of accelerants and organic surfactants to an electrolytic bath improves the amount and distribution of the co-deposited particles.
Generally, a surfactant molecule has two different parts, namely a hydrophilic and a hydrophobic end. Surfactants present the benefits of helping the inert particles to effectively co-deposit in the metal matrix. When surfactants are added to a suspension, the surfactant molecules may be adsorbed on the surface of the particles. Although surfactants might increase the internal stress of the coating and lead to cracks in the composite structure, they are notable positively as they tend to change the surface charge (zeta potential) of the particles and decrease the tendency of particle agglomeration [23, 24], promoting the co-deposition and uniformity of the particles throughout composite layers [14, 23, 24]. In spite of some researchers having used anionic [25, 26] and cationic surfactants [23, 24] to incorporate hydrophobic particle like SiC into metal matrices through electrodeposition method, no precise and thorough study has been reported on the application of different kinds of surfactants to solve the common problem of low incorporation of hydrophilic silica into Ni coating from aqueous electrolytes during electrodeposition. In this study, four common inexpensive surfactants, including a cationic surfactant: CTAC (C19H42ClN), an anionic surfactant: ALES (C14H33NO5S), a zwitterion surfactant: CAPB (C19H38N2O3) and a non-ionic surfactant: DG (C16H32O6), are used to overcome the low silica incorporation problem. According to the results, ALES and CTAC were chosen to increase the silica content of the coatings. The mechanism of ALES and CTAC effects on the zeta potential and volume percentage of co-deposited SiO2 in Ni coating were studied. Further, hardness, wear and corrosion resistance of the coatings prepared in the presence and absence of the surfactants were investigated.
Experimental
The Ni–SiO2 composites were deposited on 30 × 30 × 2 mm carbon steel substrates, 1 cm2 of which was subjected to electrodeposition, with the remaining area isolated with a resin. The plating bath electrolyte for electrodeposition was similar to Watt’s bath including NiSO4·6H2O, NiCl2·6H2O, H3BO3 and SiO2 particle and the silica particles were added to the solution as is summarized in Table 1. Analytical reagent-grade chemicals and double-distilled water were used in the experiments. Based on the existing literatures [13, 23, 25], the surfactant concentration was 5 × 10−4 M, since at a lower surfactant concentration of 10−5 M, the particles could not be dispersed in the electrolyte at all. On the other hand, at the higher concentrations, micelle formation decreased the monomer concentration of the surfactant in the electrolyte. The silica particles were purchased with industrial grade sizes between 50 and 200 nm. The initial pH values of the electrolyte and bath temperature were 4.0 and 40 ± 0.5 °C, respectively. Particle agglomeration was prevented by suspending SiO2 particles into the bath under magnetic stirring at 400 rpm for 24 h and ultra-sonicating the mixture for 30 min. Then the surfactants (if any) increased and were stirred for 30 min. The stirring was maintained during the electrodeposition. Prior to the electrodeposition, the zeta potential and particle size distribution of the silica particles were measured using a standard laboratory instrument equipped with an optical technique (Zetasizer 3000HS Advance, Malvern Instrument GmbH, UK). The electrodeposition was performed by chronopotentiometry on an AUTOLAB (EcoChemie, The Netherlands) model PGSTAT 30 potentiostat/galvanostat coupled with a PC operated by GPES 4.9 software (EcoChemie) on a three-electrode cell where a platinum plate (15 mm × 15 mm) was used as counter electrode (CE), a saturated Ag/AgCl served as the reference electrode (RE), and the carbon steel element acted as the working electrode. After the electrodeposition, the surfaces were washed with distilled water three times. The particle contents of the Ni–SiO2 composite coatings were measured with an energy-dispersive X-ray analysis (EDX) system connected to a scanning electron microscope (SEM, Rontec model). Four different points across each coating were analyzed and the average volume percentage was calculated.
Polarization tests on the composite coatings were conducted at room temperature using an AUTOLAB system coupled with a PC operated using GPES software in a three-electrode cell in 3.5 wt% NaCl solution in a standard three-electrode cell. The corrosion potential (Ecorr) and corrosion current density (icorr) of each sample were deduced from the Tafel plots. The corrosion resistance of the coatings was also investigated with an electrochemical impedance spectroscope using an AUTOLAB instrument coupled with a PC operated by FRA software, using a three-electrode cell in 3.5 wt% NaCl solution within the frequency range of 10−2–105 Hz at an AC signal amplitude of 0.5 mV at open circuit potential (OCP). Microhardness of the composite coatings was found by Vickers indentation with a load of 25 g for 15 s. An average value over four points across the coating surface has been taken as the hardness value. Wear tests were carried out by a pin (microhardness 65 HRC)-on-disk tribometer under a normal load of 10 N, a sliding distance of 200 m, a sliding speed of 0.28 m/s at room temperature. The weight losses during the wear tests were determined at a 0.01 mg weight-scale accuracy. The averages of the three indentation readings of the weight loss tests are reported.
Results and discussion
Co-electrodeposition of silica
As a beginning point, 60 different coatings were prepared at different current densities, agitation speeds, and silica concentrations (2 times repeated), as indicated in column 2 of Table 1. Results of EDX analyses (see, for example, Fig. 1) on these coatings showed that their silica content ranged within 0.2–1.8 volume percent (v%), which is very limited (Fig. 2). Yet it can be seen that the data fail to follow any conceivable trends, and the reproducibility is poor. According to the literature [27, 28], to achieve a composite with good corrosion resistance and mechanical properties, the hard particle content of the coating should be increased. It should be mentioned that electroplating was carried out at various current densities, agitation speeds, and silica concentrations—the parameters which have been reported as important determinants of the particle content of the composite coatings in the literature [14, 29, 30]; however, no acceptable and reproducible changes in the silica volume percentage of the composites were found. The results show the silica content of the composites would be low under this classic situation, and electroplating parameters cause no significant effects on the particle incorporation in the coatings.
An example of EDX analysis of coatings produced in the situation of Table 1 column 2, current density 3 A/dm2, silica 30 g/l and agitation speed 400 rpm
There are some reasons for the low silica content of the composites prepared through the different electroplating parameters (column 2, Table 1). It should be mentioned that silica nanoparticles were used in this work, due to the fact that their smaller particle sizes tend to further improve the corrosion and mechanical resistance of the composite layers, while increasing the hardness and the lifetime of the coatings, as compared to the larger particles [14, 27]. However, silica nanoparticles significantly tend to be agglomerated in the plating bath due to the high surface free energy, van der Waals forces, and effective compression of the diffused double layer surrounding the particles by the high ionic strength, reducing particle suspension in the bath [31,32,33]. Therefore, SiO2 content in the composite is low. Moreover, silica particles are hydrophilic materials with little tendency toward co-deposition, in comparison to hydrophobic materials, due to the fact that a separation of the aqueous film from the particles in Helmholtz layer must occur to allow for the embedding of particles in the metallic matrix. As a result, the separation of the aqueous molecules from hydrophilic SiO2 is too difficult [27, 34]. These may be overcome by adding a surfactant, which increases the zeta potentials of the particles and reduces the surface free energy of the inert particles through adsorbing them on the silica surface. Thus, particle agglomeration would be reduced, promoting the silica incorporation and uniform distribution of the particles through the electrodeposited layer [23,24,25,26]. On the other hand, a surfactant may enhance the incorporation fraction of silica by increasing the hydrophobicity of the particles [27]. This would provide a driving force for the attachment of the particles at the cathode–electrolyte interfaces. To examine this, four surfactants (i.e., CTAC, ALES, CAPB and DG) were selected to be introduced into the electrolyte bath for increasing the silica content of the composite. The electroplating conditions are presented in Table 1 (each experiment was repeated twice and the average results are reported).
Morphological and structural analysis
Figure 3 shows the SEM image (surface morphology) of a Ni–SiO2 composite coating deposited on carbon steel surface in the absence and presence of surfactants using a 30 g/l suspension of SiO2 particles. The results of the EDX analysis (Fig. 1) and the SEM image (Fig. 3a) show that in the absence of a surfactant, the particle content is 0.82 v%, but the deposit has a relatively homogenous structure (Fig. 4a). It can be seen from Fig. 3b, c that using CAPB and DG the composites are cracked, indicating increased internal stress of composites, which makes them fragile [35, 36]. Yet, there are no cracks in the structure of the composites prepared using CTAC and ALES (Fig. 3d, e). Also, it seems that the surfactants have a great influence on the silica content of the composites. The SEM images (Fig. 4b) of the composites prepared in the presence of CTAC show SiO2 particles (30 g/l in electrolyte) have formed aggregated grains on the surface of the coating, which create an inhomogeneous composite. Moreover, the EDX analysis of this composite (Fig. 5a, b) shows that the silica percent on the deposit is different in various locations through the composite. According to the obtained results, the amount of SiO2 on the aggregated grains (p part) was 24–27 v%, while this was 7–9 v% in other locations (s part).
It seems that the co-electrodeposition of the modified silica in the presence of CTAC led to the entrapment of the particles, as aggregates, inside the coating, which has been referred to as the entrapment mode of co-deposition [28]. The zeta potential of the silica surface in the presence of CTAC (Table 2) indicates a decrease in the negative surface charge of the particles due to the absorption of the cationic surfactant that gave rise to the agglomeration of the particles. As seen in Fig. 5a, b, the presence of carbon indicates that CTAC is co-deposited in the composite structure. This composite mode may cause better mechanical and corrosion properties than those of the composite prepared in the absence of any surfactant [28]. According to the negative charge of the silica surface, the increasing of the silica content in the composite can be attributed to improved hydrophobicity of the particles as CTAC molecules adhere to silica surface. Additionally, the presence of a small positive charge on the particles enhances the migration toward the negatively charged cathode [23, 24].
Figures 3e and 4c, d show that the silica particles’ size of the Ni–SiO2 composite is smaller and the particles are more uniform in this composite, when using ALES instead of CTAC. Also, it can be seen from the EDX results (Fig. 5c) that the silica content on the deposit is relatively constant all across the composite (12–14 v%), i.e., the silica particles were homogeneously dispersed throughout the composite. In fact, using ALES as a dispersion agent in electrolyte bath leads to more uniform composite coating containing well-dispersed particle, it can be referred to as an incorporated mode co-deposition [28, 37]. Corrosion resistance and mechanical properties of this type of composite are expected to be the best among the three types studied [28]. As seen, the absence of carbon in Fig. 5c demonstrates that ALES is not co-deposited.
SEM images of a cross-sectional area of the Ni–SiO2 coatings prepared in the absence of surfactant (Fig. 6a), in the presence of CTAC (Fig. 6b), and in the presence of ALES (Fig. 6c) show that increasing the anionic and cationic surfactants in the electrolyte bath significantly affects silica co-deposition in the Ni layers during the electrodeposition. As illustrated, the silica content of the composite coating is increased in the presence of anionic and cationic surfactant. Also, the particle dispersion was more uniform in the presence of ALES rather than CTAC, confirming the entrapment and incorporation mode co-depositions in the presence of CTAC and ALES, respectively.
One reason for the increase of the negatively charged silica content in the composite prepared in the presence of the anionic surfactant could be the improvement in the particle suspension stability. In fact, upon adding the surfactant, some ALES molecules are adsorbed on the surface of the particles creating a negatively supercharged system (Table 2), through increasing the effective charge on silica surface, which creates high repulsion forces among the silica particles, hence decreasing the agglomeration of the particles. As a consequence, the probability of silica suspension enhanced and particle incorporation rate in the metal deposit is increased [38, 39]. Figure 7a–c shows the size distribution of the silica particles after dispersion in the absence of a surfactant and in the presence of CTAC and ALES, respectively. It can be seen that after using ALES as the dispersing agent, the size range of the SiO2 particles was narrow and most of the particles were below 400 nm in diameter due to the supercharged nature of the system and strong repulsion forces among the particles decreased the probability of the agglomeration of the particles. This was while the silica grain size distribution was relatively bigger in the presence of CTAC, due to the decrease in the zeta potentials among particles, which led to particle agglomeration.
There are two reasonable interpretations for the adsorption of the anionic surfactant on the negatively charged silica surfaces. First, due to the acidity of the electrolyte (pH 4), the probability of the deprotonation of the silanol group is low and hence the negative charge of silica surface is small. In addition, upon adding a certain amount of the surfactant to the electrolyte, several kinds of cationic ions including Ni+, NH4+, and H+ surrounded the negatively charged silica particles, and as a result, the double layer of particle decreased the repulsive electrostatic forces among the negative charges on the surface of the particles and the anionic surfactants [38, 39]. According to the work of Luo et al. [39], it can be said that the hydrophobic tails and hydrophilic heads of ALES molecules tend to orient, so they are closer to the silica surfaces and the surfactant molecules lay adjacent to the particles, leading a supercharged system. Second, it is possible that the adsorption process was not completely decided by the electrostatic forces and only a minor part of the interactions among the SiO2 particles is determined by the electrostatic force, and then it is dominated by other forces such as London, van der Waals, hydrophilic and hydrophobic interaction, hydration and dehydration, and surface interaction among the surfactant and particles, known as DLVO and non-DLVO forces [40].
However, there still is a question to answer on how a negatively supercharged particle goes from bulk to the surface of electrode (cathode) with negative charge? The significant force which moves the silica particles from the bulk toward the electrode is governed almost entirely by the convection instigated due to the dispersion and stirring forces, while the electrophoresis and double layer force can be ignored [13]. Near the electrode surface, the van der Waals forces and electrostatic repulsion among the electrode and silica particles could be important as they cause a barrier and develop loose adsorption of the particles on the electrode surface. Inspired by Bund’s idea [41], the first possible explanation for increasing the particle content of the composite due to adding anionic surfactants (e.g., ALES) with the same charge as the cathode could be the fact that the positively charged electric double layer of the cathode attracts negatively supercharged particles. The silica particles move towards the cathode and due to the repulsive force of the cathode, a double layer of the particles is deformed. The center of its adsorbed anionic surfactant moves to the side of silica particle which is far from the electrode. Within the intense electric field at the interface, the ALES molecules adsorbed on the particle surfaces are stripped, allowing the incorporation of the particles into the growing metallic matrix (Scheme 2). The absence of a carbon signal in Fig. 5c demonstrates that ALES was not co-deposited, confirming the argument in Scheme 2. The second reason for increasing silica incorporation could be the decrease in the interfacial energy between the silica particles and metal (σP/M) to lower than the interfacial energy between the silica particle and electrolyte (σP/E) [42] by rendering the hydrophobicity property to silica surface due to the adsorption of ALES on the particles that increased the stripping tendency of the particles from aqueous which ends up with a driving force moving the particles toward the electrode–electrolyte interfaces. In other words, the incorporation of the silica particles into the Ni coating is possible if σP/E is large enough, and such a condition can be accessible by making the particle hydrophobic. In fact, the solvation force could be seen as an effective factor for creating the driving force for treated silica particles with surfactant [13, 34]. The solvation force arises from the orientation of the solvent molecules near the interfaces and is only observed in concentrated electrolytes. An increase in the ordering of the solvent molecules leads to a larger solvation force which is repulsive for hydrophilic surfaces and attractive for hydrophobic surfaces causing modified silica particles with hydrophobic surfaces to have better suspension ability during electrolysis, which is a prerequisite for homogeneous co-electrodeposition [13, 34]. Hence, due to its hydrolysable surface nature, hydrophilic silica has a small tendency for co-deposition. On the other hand, treated hydrophobic silica (with surfactant agent) demonstrates a high degree of co-deposition. Also, there are chances that other possible forces such as the London, van der Waals forces among the treated silica particle and cathode surface to overcome the barrier resulted from the repulsive electrostatic force between them, and keep the particles from being adsorbed on the cathode. Such a chain of interactions suggests that the co-electrodeposition of SiO2 particles in Ni coating is not completely governed by the electrostatic forces and it is complicated. All of these processes can intensify loose adsorption of silica particles on the electrode, increasing the incorporation probability (strong adsorption) of silica particles into the coating [5].
In addition, an inhomogeneous composite with agglomerated SiO2 is prepared (Fig. 8) when the concentration of the silica particles increases to 40 g/l in the electrolyte in the presence of CTAC. This can be attributed to the higher possibility of agglomeration at higher particle concentrations. EDX analysis (Fig. 9) of the coating prepared in the presence of ALES shows that, upon increasing silica particles concentration in electrolyte to 40 g/l, the SiO2 content of the composite is decreased to 10 v% due to the agglomeration of the particles in the electrolyte.
Polarization measurements
The anodic polarization curves in a 3.5% NaCl solution for the Ni–SiO2 composites are produced in the absence and presence of the surfactants are shown in Fig. 10. The results indicate that, using CTAC and ALES while preparing the composite, the corrosion resistance will be higher due to the higher percentages of silica particles in the coatings. It can be seen that, in the presence of ALES in the electrolyte, the corrosion potential of the prepared Ni–SiO2 composite coating shifts to more positive values and the corrosion current decreases more than in the case of the presence of CTAC. This can be attributed to the smaller size and more uniform distribution of silica particles in the composite.
Electrochemical impedance spectroscopy (EIS)
EIS measurements were carried out to obtain further information on the corrosion behavior of Ni–SiO2 composite coatings, upon immersion in a 3.5% NaCl solution for 30 min. The corresponding Nyquist diagrams are shown in Fig. 11. The polarization and EIS measurements show that the composite prepared in the presence of ALES had better corrosion resistance than other composites. Although the exact role of the inert particles in the corrosion reaction has not been well understood, the enhanced corrosion resistance of the composites containing uniform particles has been attributed to various reasons in the literature [6, 21, 27, 37, 43]. In this case, this can be attributed to smaller size and more uniform distribution of the silica particles in the composite. In fact, the uniform dispersion of the silica particles in the composite, in the presence of ALES, improves the filling of the surface defects, e.g., pores, gaps, and micro-holes, developing more finely grained, dense and compact structures on the part of the composite and strong interfacial bonding between the silica particles and the Ni metal in the composite [12, 43]. However, in the presence of CTAC, due to the agglomeration of silica in the composite, a porous site with irregular surface is formed. It seems that there is no significant difference between the composites prepared in the absence/presence of CTAC in terms of corrosion resistance [12].
Microhardness and wear study
Table 3 presents the microhardness values of the Ni–SiO2 composites prepared in the presence and absence of surfactants. Accordingly, an increase in the hard phase, i.e., SiO2, in the coatings produced in the presence of anionic and cationic surfactants led to greater hardness [44]. Clearly, the Ni–SiO2 nanocomposite coating fabricated in the presence of ALES gave rise to higher microhardness, which can be attributed to the incorporation mode of the dispersed silica particles in comparison to the entrapment mode in the presence of CTAC in the electrolyte.
The anti-wearing performance of the Ni–SiO2 composite coatings was estimated from the weight loss of the coatings (Fig. 3) [45]. The wearing weight loss of Ni–SiO2 composite coatings prepared in the absence of surfactant as well as in the presence of CTAC and ALES is shown in Table 3. Ni–SiO2 composite coatings prepared in the presence of ALES exhibit lowest wear weight loss. This directly corresponds to the rise in the hardness of the composite due to the incorporation mode of the high volume percent of the embedded silica particles in the Ni layer.
Conclusion
The study indicated that adding ionic surfactants to the electrolyte bath, prior to electroplating, results in an appropriate dispersion and effective embedment of silica particles in the Ni coating. In fact, the surfactants tend to change the surface charge (zeta potential) and hydrophilicity–hydrophobicity balance of the particles and change their agglomeration and incorporation tendencies. It was also observed that adding ALES and CTAC to the electrolyte bath improved the silica content of the composite prepared through co-electrodeposition. The results further showed that the addition of CTAC and ALES caused entrapment- and incorporated mode of silica particle co-deposition in Ni coating, respectively. It should be mentioned that the corrosion resistance, hardness and wearing properties of the Ni–SiO2 composite prepared in the presence of ALES are several times better than those prepared with CTAC.
References
Natarajan, N., Krishnaraj, V., Davim, P.: Metal Matrix Composites, Synthesis, Wear Characteristics, Machinability Study of MMC Brake Drum. Springer, Berlin (2014)
Lynch, C.T., Kershaw, J.P.: Metal Matrix Composites. CRC Press, Boca Raton (2018)
Walsh, F.C., Ponce de León, C.: A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying coatings technology. Trans. IMF 92, 83–98 (2014)
Camargo, M.K., Schmidt, U., Grieseler, R., Wilke, M., Bund, A.: Electrodeposition of Zn–TiO2 dispersion coatings: study of particle incorporation in chloride and sulfate baths. J. Electrochem. Soc. 161, 168–175 (2014)
Guglielmi, N.: Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 119, 1009–1012 (1972)
Popoola, A.P.I., Aigbodion, V.S., Fayomi, O.S.I.: Anti-corrosion coating of mild steel using ternary Zn–ZnO–Y2O3 electrodeposition. Surf. Coat. Technol. 306, 448–454 (2016)
Walsh, F.C., Low, C.T.J., Bello, J.O.: Influence of surfactants on electrodeposition of a Ni-nanoparticulate SiC composite coating. Trans. IMF 93, 147–156 (2015)
Aghdam, M.M., Shahbaz, M.: Effects of interphase damage and residual stresses on mechanical behavior of particle reinforced metal-matrix composites. Appl. Compos. Mater. 21, 429–440 (2014)
Roventi, G., Giuliani, G., Pisani, M., Bellezze, T.: Electrodeposition of Zn–Ni–ZrO2, Zn–Ni–Al2O3 and Zn–Ni–SiC nanocomposite coatings from an alkaline bath. Int. J. Electrochem. Sci. 12, 663–678 (2017)
Fazlolahzadeh, O., Rouhollahi, A., Dolati, A., Ghahramanifard, F.: Co-electrodeposition of Ni/SiO2(CH2)3SH composite: modified Guglielmi’s model and mechanism of nucleation. J. Electrochem. Soc. 164, 472–474 (2017)
Li, R., Houa, Y., Liang, J.: Electro-codeposition of Ni–SiO2 nanocomposite coatings from deep eutectic solvent with improved corrosion resistance. Appl. Surf. Sci. 367, 449–458 (2016)
Tuaweri, T.J., Wilcox, G.D.: Behaviour of Zn–SiO2 electrodeposition in the presence of N,N-dimethyldodecylamine. Surf. Coat. Technol. 200, 5921–5930 (2006)
Terzieva, V., Fransaer, J., Celis, J.P.: Codeposition of hydrophilic and hydrophobic silica with copper from acid copper sulfate bath. J. Electrochem. Soc. 147, 198–202 (2000)
Ahmad, Y.H., Mohamed, A.M.A.: Electrodeposition of nanostructured nickel-ceramic composite coatings: a review. Int. J. Electrochem. Sci. 9, 1942–1963 (2014)
Bharathi, V., Ramachandra, M., Srinivas, S.: Influence of fly ash content in aluminum matrix composite produced by stir-squeeze casting on the scratching abrasion resistance, hardness and density levels. Mater. Today Proc. 4, 7397–7405 (2017)
Ghiamaty, Z., Ghaffarinejad, A., Faryadras, M., Abdolmaleki, A., Kazemi, H.: Synthesis of palladium–carbon nanotube–metal organic framework composite and its application as electrocatalyst for hydrogen production. J. Nanostruct. Chem. 6, 299–308 (2016)
Ghasali, E., Alizadeh, M., Niazmand, M., Ebadzadeh, T.: Fabrication of magnesium-boron carbide metal matrix composite by powder metallurgy route: comparison between microwave and spark plasma sintering. J. Alloy. Compd. 697, 200–207 (2017)
Li, B., Gao, Y., Han, M., Guo, H., Jia, J., Wang, W., Deng, H.: Tribological properties of NiAl matrix composite coatings synthesized by plasma spraying method. J. Mater. Res. 32, 1674–1681 (2017)
Abidin, A.Z., Kozera, R., Höhn, M., Endler, I., Knaut, M., Boczkowska, A., Czulak, A., Malczyk, P., Sobczak, N., Michaelis, A.: Preparation and characterization of CVD-TiN-coated carbon fibers for applications in metal matrix composites. Thin Solid Films 589, 479–486 (2015)
Celis, J.P., Roos, J.R., Buelens, C.: A mathematical model for the electrolytic codeposition of particles with a metallic matrix. J. Electrochem. Soc. 134, 1402–1408 (1987)
Ghorbani, M., Salehi, F., Razavizadeh, O.: Enhanced hardness and corrosion resistance of Zn/SiO2 films by electrodeposition. J. Electrochem. Soc. 162, 480–485 (2015)
Sadreddini, S., Ardakani, S.R., Rassaee, H.: Corrosion behavior and microhardness of Ni–P–SiO2–Al2O3 nano-composite coatings on magnesium alloy. J. Mater. Eng. Perform. 26, 2032–2039 (2017)
Rudnik, E., Skal, B., Ski, L.D., Misiak, M.: Electrodeposition of nickel/SiC composites in the presence of cetyltrimethylammonium bromide. Appl. Surf. Sci. 256, 7414–7420 (2010)
Kilic, F., Gul, H., Aslan, S., Alp, A., Akbulut, H.: Effect of CTAB concentration in the electrolyte on the tribological properties of nanoparticle SiC reinforced Ni metal matrix composite (MMC) coatings produced by electrodeposition. Colloids Surf. A Physicochem. Eng. Asp. 419, 53–60 (2013)
Jiang, S.W., Yang, L., Pang, J.N., Lin, H., Wang, Z.Q.: Electrodeposition of Ni–Al2O3 composite coatings with combined addition of SDS and HPB surfactants. Surf. Coat. Technol. 286, 197–205 (2016)
Sameti, M.R., Nadali, S., Falahatpisheh, A., Rakhshi, M.: The effects of sodium dodecyl sulfate and sodium saccharin on morphology, hardness and wear behavior of Cr–WC nano composite coatings. Solid State Commun. 159, 18–21 (2013)
Zamblau, I., Varvara, S., Muresan, L.M.: Corrosion behavior of Cu–SiO2 nanocomposite coatings obtained by electrodeposition in the presence of cetyltrimethyl ammonium bromide. J. Mater. Sci. 46, 6484–6490 (2011)
Khan, T.R., Erbe, A., Auinger, M., Marlowand, F., Rohwerder, M.: Electrodeposition of zinc–silica composite coatings: challenges in incorporating functionalized silica particles into a zinc matrix. Sci. Technol. Adv. Mater. 12, 55005–55014 (2011)
Sassia, W., Dhouibi, L., Bercot, P., Rezrazi, M.: The effect of SiO2 nanoparticles dispersion on physico-chemical properties of modified Ni–W nanocomposite coatings. Appl. Surf. Sci. 324, 369–379 (2015)
Bapu, G.N.K.R., Jayakrishnan, S.: Development and characterization of electro deposited nickel–titanium carbonitride (TiCN) metal matrix nanocomposite deposits. Surf. Coat. Technol. 206, 2330–2336 (2012)
Narasimann, M.P., Pushpavanam, M., Periasamy, V.M.: Synthesis, characterization and comparison of sediment electro-codeposited nickel–micro and nano SiC composites. Appl. Surf. Sci. 258, 590–598 (2011)
Fransaer, J., Leunis, E., Hirato, T., Celis, J.P.: Aluminium composite coatings containing micrometre and nanometre-sized particles electroplated from a non-aqueous electrolyte. J. Appl. Electrochem. 32, 123–128 (2002)
Wang, S.C., Wei, W.C.: Kinetics of electroplating process of nano-sized ceramic particle/Ni composite. J. Mater. Chem. Phys. 78, 574–580 (2003)
Fransaer, J., Celis, J.P., Roos, J.R.: Analysis of the electrolytic codeposition of non-Brownian particles with metals. J. Electrochem. Soc. 139, 413–425 (1992)
Gu, C., Lian, J., Jiang, Q.: Layered nanostructured Ni with modulated hardness fabricated by surfactant-assistant electrodeposition. Scr. Mater. 57, 233–236 (2007)
Wang, L.M.: Effect of surfactant-assistant electrodeposition in Ni matrix. J. Electrochem. Soc. 156, 204–206 (2009)
Hashimoto, S., Abe, M.: The characterization of electrodeposited Zn–SiO2 composites before and after corrosion test. Corros. Sci. 36, 2125–2137 (1994)
Ahualli, S., Iglesias, G.R., Wachter, W., Dulle, M., Minami, D., Glatter, O.: Adsorption of anionic and cationic surfactants on anionic colloids; supercharging and destabilization. Langmuir 27, 9182–9192 (2011)
Luo, M., Song, Y., Dai, L.L.: Heterogeneous or competitive self-assembly of surfactants and nanoparticles at liquid–liquid interfaces. Mol. Simul. 35, 773–784 (2009)
Cosgrove, T.: Colloid Science: Principles, Methods and Applications, 2nd edn. Wiley, West Sussex (2012)
Bund, A., Thiemig, D.J.: Influence of bath composition and pH on the electro-codeposition of alumina nanoparticles and copper. Appl. Electrochem. 37, 345–351 (2007)
Khan, T.R., Erbe, A., Auinger, M., Marlowand, F., Rohwerder, M.: Existence of lower critical radius for incorporation of silica particle into zinc during electro-codeposition. Appl. Mater. Interfaces 4, 6221–6227 (2012)
Malatji, N., Popoola, A.P.I., Fayomi, O.S.I., Loto, C.A.: Multifaceted incorporation of Zn–Al2O3/Cr2O3/SiO2 nanocomposite coatings: anti-corrosion, tribological, and thermal stability. Int. J. Adv. Manuf. Technol. 82, 1335–1341 (2016)
Wang, Y., Zhou, Q., Li, K., Zhong, Q., Bui, Q.B.: Preparation of Ni–W–SiO2 nanocomposite coating and evaluation of its hardness and corrosion resistance. Ceram. Int. 41, 79–84 (2015)
Hou, K.H., Sheu, H.H., Ger, M.D.: Preparation and wear resistance of electrodeposited Ni–W/diamond composite coatings. Appl. Surf. Sci. 308, 372–379 (2014)
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rouhollahi, A., Fazlolahzadeh, O., Dolati, A. et al. Effects of different surfactants on the silica content and characterization of Ni–SiO2 nanocomposites. J Nanostruct Chem 8, 139–152 (2018). https://doi.org/10.1007/s40097-018-0259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-018-0259-4