Abstract
Purpose
Reuse of treated wastewater (TWW) for irrigation can be an effective strategy in Morocco to overcome the pressure on freshwater resources. The M’zar wastewater plant is based on percolation infiltration treatment, allowing the purification of the wastewater of Agadir, and with its UV disinfection system, it is now possible to reuse this water for irrigation. In this sense, the aim of our study is to evaluate the microbiological and physicochemical quality of the treated wastewater of this station, used for irrigation of a Golf course as well as to determine its impact on grass and soil.
Methods
A monitoring of TWW quality was carried out monthly on the level of the Ocean’s Golf on water samples, grass and soil. This monitoring is related to the physicochemical (pH, temperature, conductivity, STD, COD, and BOD5) and bacteriological characteristics by counting the indicators of faecal contamination, faecal coliforms (FC), faecal streptococci (FS), Salmonella and Vibrios as well as sulphito-reducers spores (SRS).
Results
The results of microbiological analysis in the three compartments confirm the presence of various organisms such as FC, FS, and SRS in a very significant number with no load in Salmonella and Vibrios during our study period. For physicochemical analyses, we observed that only the conductivity showed fairly a high value of 6.38 dS/m.
Conclusion
The obtained physicochemical and bacteriological results revealed that the treated wastewater with the M’zar plant complies with national and international standards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the increasing competition for high-quality freshwater supplies between agricultural and urban uses, particularly in arid and semi-arid regions with high population densities, the reuse of treated wastewater has been an interesting alternative source of irrigation widespread in several mediterranean countries (Angelakis et al. 1999; Hochstrat et al. 2006). Reusing wastewater is indeed a promising option, many researchers around the globe have investigated the effects of wastewater on soil, seeds germination, and plant growth (Mekki et al. 2013) as well as the risk analysis of using them in agriculture (Ganoulis 2012). Globally, the use of this resource is growing rapidly, at a rate of 10–29% per year, and the major part of this water (70%) is used for agriculture (Aziz and Farissi 2014). Currently, the yearly volume of discharged raw wastewater in Morocco is about 700 million m3, 60% of which is discharged to the sea, the remaining quantity is either drained off and a small part is actually reused on an area of 7000 ha (Choukr-Allah 2012). This could be a way to reduce surface water pollution and provide groundwater for other agricultural fields.
In fact, treated wastewater is often an interesting source of water containing the nutrients necessary for the proper fertilization of agricultural land and plant growth and which is always available (Jiménez-Cisneros 1995). The use of this resource in agriculture is a form of water and nutrient recycling, but can also cause serious environmental problems due to their high levels of toxic chemicals and pathogens (El Addouli et al. 2009) that have become resistant to antibiotics (Meloul and Hassani 1999; El Boulani et al. 2016); as a result, the use of these water in crop irrigation threatens the health of farmers and consumers products (Howard et al. 2003; Oren et al. 2004; Taylor et al. 2004).
This danger is manifested chemically by the uncontrollable rise in levels of salts, pesticides and heavy metals as well as bacteriologically by the indicators of faecal contamination, namely total coliforms, faecal coliforms, and faecal streptococci which can predict the presence of pathogenic germs presenting an increased health risk (Al-Nakshabandi et al. 1997; Toze 2006; El Addouli et al. 2009).
In the south of Morocco, the Agadir region is an agricultural zone characterized by limited water resources, especially since liquid wastewater discharges throughout the Souss basin amount to 25% million m3/year. Consequently, the use of treated wastewater in agriculture would not only preserve water resources, but also significantly reduce the misuse of fertilizers (Mouhanni et al. 2013).
The objective of our work is to evaluate the quality of the treated wastewater of the M’zar station and the impact of their use in the irrigation of the ocean golf course. Such an objective remains dependent on the transition from a treatment and rejection model to a purification and reuse model.
Materials and methods
Study site
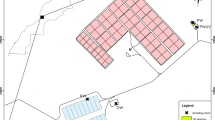
Ocean’s Golf (30°21′51.1″ N, 9°34′35.0″ W) is located in the south of Agadir, Morocco (Fig. 1). This space has an exceptional view on the valley of the Souss and the mountains of the high atlas, and it is a sporting and tourist place, spread over 90 ha. The golf area is covered with grass, besides a various trees of eucalyptus, tamaris, cypresses, mimosas, and palm trees, and this vegetation is irrigated by treated wastewater provided by the station M’zar, within the framework of a convention between the water district of Agadir (RAMSA) and the Golf course since 2010.
Sampling method
Water, grass, and soil samples were collected monthly in sterile containers and transported to the laboratory under refrigeration, two sampling points were retained by our study with a distance of 683 m, the first site denoted S1 is considered as a reference site related to the sports area and is irrigated by water coming directly from the wastewater treatment plant, and the second site denoted S2 composed of plant form developed by the Agricultural and Veterinary Institute of Agadir and irrigated by the waters of the same station after storage. At each point, three compartments were sampled: for grass (G1, G2) and soil (So1, So2), samples were taken in sterile plastic bags, using, respectively, scissors and a sterile coring tool. For the water samples (W1, 2), sterile borosilicate glass bottles (1L) were filled in such a way as to keep the sample away from any source of contamination.
Physico–chemical analyses
The pH, temperature, conductivity, and total dissolved salts were determined in situ by a pH meter equipped with a multi-parameter probe-type SELECTA CD 2004. The chemical oxygen demand (COD) and 5-day biological oxygen demand (BOD5) was determined according to AFNOR standard (NF T90-101).
Bacteriological analysis
Bacteriological analysis was performed on composite samples. The purpose of these analysis can be highlighted in two main objectives:
-
The enumeration of faecal coliforms (FC), faecal streptococci (SF), and anaerobic sulphite-reducing spores of the genus Clostridium (SRS).
-
The detection of pathogenic germs of the genus Vibrio and Salmonella spp. according to the Moroccan standard NM ISO 9308-1 2007.
Water
The enumeration of the indicator germs of faecal contamination was performed using the membrane filtration method on Slanetz and Bartley medium, Tergitol TTC, and TSC medium, respectively, for FS, FC, and SRS (Rodier et al. 2009). For FS and FC, the incubation was carried out at a temperature of 44 ± 1 °C 24 h and 48 h, respectively. For SRS, the samples were pre-heated to 80 °C for 10–15 min to destroy the vegetative forms and keep only sporulated forms, and incubated at 37 °C for 48 h. The results were expressed as number of colonies forming units (CFU) per ml.
For the detection of Salmonella spp., 5 l of water were filtered through 0.45 μm cellulose acetate filter, and the filter was then placed in 225 ml of pre-enrichment medium (buffered peptone water), and incubated at 37 °C for 18–24 h. A 0.1 ml enrichment of the pre-enrichment was transferred to 10 ml of RV10 Rappaport–Vassiliadis broth and incubated at 44 °C for 18–24 h. The isolation was done on a selective medium, and it consists of seeding the Hektoen and XLD medium from the enrichment broth and then incubating at 37 °C for 24–48 h. Typical colonies were selected and streaked onto nutrient agar at 37 °C for 24 h and identified biochemically by the API 20E gallery. The results are expressed in presence/absence by filtered volume.
For the detection of Vibrio cholerae, 450 ml of water were filtered (cellulose acetate 0.45 μm). The filter was then transferred to 225 ml of saline peptone water and incubated at 37 °C for 24 h. The surface layer is seeded on thiosulfate citrate bile sucrose (TCBS) medium and incubated at 37 °C for 24–48 h. Typical colonies were purified on 2% nutrient agar at 37 °C for 24 h. The biochemical identification of the suspicious strains was carried out by the API 20 E gallery. The results are expressed in presence/absence by filtered volume.
Grass and soil
The hygienic quality of the soil and grass compartments has also been the subject of this study. Samples addressed to the counting of FC and FS were prepared by mixing 10 g of each compartment with 90 ml of physiological water. Subsequently, spreading was carried out on a Bile medium with azide and esculin and Tergitol medium with TTC, respectively, for FS and F Cat 44 ± 1 °C for 24 h; the results were expressed in number colony forming units (CFU) per g.
For Vibrio and Salmonella spp., the same steps were followed by placing 25 g of each sample in 225 ml of buffered peptone water for Salmonella and saline alkaline peptone water for Vibrio spp.
Results and discussion
Physico–chemical analyses of the water samples
Table 1 highlights the obtained physico-chemical values of water samples W1 and W2. The average values of the physicochemical variables of wastewater treated and reused by the Golf Ocean show that these parameters are provided in an acceptable quantity in accordance with the standards of the irrigation water (WHO 1989; CNS 1994) and in agreement with similar studies (El Addouli et al. 2009; Mouhanni et al. 2013). Both samples W1 and W2 have the same trend in the course of the sampling year, the conductivity, COD, DBO5 increases as a function of the water temperature (Fig. 2). This can be explained by the increase of the salts in the samples in the high-temperature months (summer): the increase of salt ions is responsible of the increase of the conductivity in both samples; inversely, the opposite happens in the low-temperature months. High levels of salinity can also be explained by mixing domestic wastewater with industrial effluents during fish transformation process (Mouhanni et al. 2013). The specific electrical conductivity which is brought in excessive quantity and corresponds to the limit value of the direct discharge in the receiving environment. Again, relatively high values for this parameter showed that the waters studied are moderately saline (Mouhanni et al. 2013; Bourouache et al. 2019). This imposes some restrictions on their use for irrigation of sensitive crops.
There is also an increase in the values of these parameters in the water sample of the second site (W2), which could be explained by exposure of the storage tank to the sun causing evaporation and concentration of salts. Thus, the addition of fertilizers to the irrigation water of the second site can also be at the origin of this increase.
The bacteriological analysis of these waters (Table 2) indicates an average faecal coliform (FC) load of 1.14 log 10 CFU/100 ml and 1.23 log 10 CFU/100 ml, respectively for W1 and W2. Those of fecal streptococci (FS) are 0.82 log 10 CFU/100 ml for E1 and 0.97 log 10 CFU/100 ml for E2. For sulphito-reducing spores (SRS), Clostridium, the average loads are 1.37 log 10 CFU/100 ml and 1.75 log 10 CFU/100 ml for W1 and W2, respectively.
This difference in charge between W1 and W2 might be due to the fact that the storage pond which feeds the second site with irrigation water is exposed to external biotic factors. For example, faeces from animals and birds and probably from the aforementioned abiotic factors of the natural environment. In addition, water stagnation causes poor aeration, eutrophication, and, therefore, a large bacterial proliferation. The duration of water storage in the pond can also explain the difference in bacterial load.
These levels are in accordance with the Moroccan standard, WHO, and are consistent with the results of Bourouache et al. (2019) (< 1000 CFU/100 mL).
Origin of faecal pollution
To explain the presence of fecal contamination indicators, the ratio R = FC/FS was used as a basic element to determine the origin of faecal pollution (Fig. 3). In our case, 0.7 < R < 2.1 showing a mixed predominantly animal pollution to a pollution of uncertain origin for the four seasons which could be explained by the presence of natural fertilizers (animal origin), besides the phenomenon of soil leaching, the urbanization of the municipality, and the exposure of the basin to different sources of contamination, including the excrement of animals and birds. In addition to animal and human origin, the physico-chemical parameters, as temperature and pH, can also contribute to variations in bacterial activity (Chigbu et al. 2004).
Origin of pollution according to the report “faecal coliforms/faecal streptococci” (R = CF/SF). R < 0.7 mainly or entirely of animal origin, R between 0.7 and 1 mixed predominantly animal, R between 1 and 2 uncertain origin, R between 2 and 4 mixed predominantly human, R > 4 source exclusively human
Characterization of the soil and the grass studied
The bacteriological analyses of soil and grass are presented in Tables 3 and 4. We have presented the data of the minimum and maximum faecal pollution to better understand the trend of its presence in both media. Thus, we have observed that the bacteriological quality of soil and grass is moderately normal: the average number of faecal coliforms obtained is 1.94 log 10 CFU/g and 2.45 log 10 CFU/g for G1 and G2, with 2.27 log 10 CFU/g and 2.3 log 10 CFU/g for So1 and So2, respectively. It is the same for fecal streptococci, and the average values obtained are 2.28 log 10 CFU/g and 2.54 log 10 CFU/g for G1 and G2, and 1.85 log 10 CFU/g and 2.46 log 10 CFU/g,, respectively for So1 and So2. According to these results, the faecal contamination varies largely depending on the nature of the irrigated substrate (soil or grass) and the type of water used (water coming directly from the station or water from the reservoir).
The bacterial load is higher in the compartments of site 2 than those of the first site, and this could be explained by the fact that the water (E2) used in the irrigation of these compartments is more loaded with bacteria, since it is water stored in basins exposed to several sources of contamination. Some samples have zero charge, the influence of climatic conditions could be the origin, since the plots are open air and exposure to the sun can destroy the fungi contamination germs (FGIC) by UV to which they are exposed. In addition, stopping irrigation during periods of rain can cause a decrease in bacterial load.
The absence of germs indicating a faecal contamination (GIFC) in both compartments and during different periods can be explained by the lack of favorable conditions for their survival in these substrates, since they are very demanding in terms of alkalinity. There is also the possibility of competition between soil or turf bacteria and exogenous bacteria (GIFC), which can induce the decrease of the number of these microorganisms in the soil, on the other hand, the existence of predators of these microorganisms, especially in the first centimeters of soil (Sou 2009). The presence of grass covering the ground could also act as a filter that prevents the passage of germs. The GIFC load is higher in the turf than in the soil because of its exposure. (Al-Lahlam et al. 2003)
Results of Salmonella and Vibrios
Pathogen analysis showed negative results for all samples analyzed for water, grass, and soil at both sampling sites despite the presence of fecal contamination indicator bacteria. The probable existence of these viable non-cultivable organisms would call into question the conventional culture techniques used. However, the absence of these germs would probably be related to the presence of antimicrobial substances (polyphenols, tannins, and fatty acids) (El Addouli et al. 2009).
These results are in agreement with the Moroccan water standards for irrigation and are consistent with the previous reports (El Addouli et al. 2009). However, a report published earlier showed the presence different strains of salmonella even after the installation of the tertiary UV treatment (El Boulani et al. 2016). This can be explained by the difference in the sampling period.
It is also noted that biochemical tests have revealed the presence of other pathogenic species for humans such as E. Coli, Providencia rettgeri, Klebsiella pneumonia, Proteus mirabilis, and Yersinia pseudotuberculosis (Fig. 4). These germs belong to the family Enterobactereacea, colonizing the digestive tract of humans; they can cause gastrointestinal, pulmonary or urinary infections, and could also be involved in nosocomial infections (Podschun and Ullmann 1998). They can be isolated from wastewater or soil and considered as an indicator of faecal contamination. Other studies have confirmed the presence of this family in the soil after sewage irrigation (Ahmed and Müller 1984).
Valorisation of spent wastewater
Based on our results, treated wastewater parameters (pH, BOD5 and faecal coliforms) of M’zar station analyzed comply with WHO and USEPA standards (also based on a goal of zero pathogen in reused waters) (Table 5), and therefore, the sanitary quality of these waters would be acceptable in the case of reuse for irrigation. It should, therefore, be noted that other factors may directly or indirectly affect the efficiency and effectiveness of the treated wastewater reuse such as irrigation type (surface irrigation or sprinkler irrigation), the nature of the soil to irrigate and the type of crop to be grown (FAO 2003).
Conclusion
The concern of this study is to determine the reusability of the treated wastewater for the irrigation of the Golf course by monitoring the physicochemical and bacteriological quality of the M’zar-treated wastewater and hence reused in the irrigation of green areas of the Ocean Golf course. The results of microbiological analysis confirm the presence of various germs such as faecal coliforms, faecal streptococci, and clostridia sulphito-reducers in appreciable numbers with an absence total of Salmonella and vibrio. The comparison of the physicochemical and microbiological quality of these waters with WHO and USEPA standards has shown the conformity of these waters to irrigate without major negative impacts on the environment. The salinity levels in the treated wastewater indicate high values, which should be considered for the selected species to be grown. This also indicates that the M’zar system based on sand infiltration–percolation and UV disinfection has a significantly higher treatment performance: the effluent produced contains very low suspended solids, bacteria and far less organic compounds.
Considering these results, we can conclude that using treated wastewater from the Agadir’s M’zar Plant station is safe but adequate safeguards draw attention to monitor salinity in the soil as the conductivity of the wastewater is over 3.5 dS/m.
References
Ahmed RE, Müller HE (1984) Distribution of enterobacteria in soil following irrigation with waste water. Zentralbl Bakteriol Mikrobiol Hyg B 179:248–258
Al-Lahlam O, El Assi NM, Fayyad M (2003) Impact of treated wastewater irrigation on quality attributes and contamination of tomato fruit. Agric Water Manag 61:51–62. https://doi.org/10.1016/S0378-3774(02)00173-7
Al-Nakshabandi GA, Saqqar MM, Shatanawi MR, Fayyad M, Al-Horani H (1997) Some environmental problems associated with the use of treated wastewater for irrigation in Jordan. Agric Water Manag 34:81–94. https://doi.org/10.1016/S0378-3774(96)01287-5
Angelakis A, Marecos Do Monte MH, Bontoux L, Asano T (1999) The status of wastewater reuse practice in the Mediterranean basin: need for guidelines. Water Res 33:2201–2217. https://doi.org/10.1016/S0043-1354(98)00465-5
Aziz F, Farissi M (2014) reuse of treated wastewater in agriculture: solving water deficit problems in arid areas. Ann West Univ Timişoara ser Biol 17:95–110
Bourouache M, Mimouni R, Ait Alla A, Hamadi F, El Boulani A, Bihadassen B (2019) Bacteriological and physicochemical quality of treated wastewater of the M’zar treatment plant. Appl Water Sci 9:1–8. https://doi.org/10.1007/s13201-019-0958-0
Chigbu P, Gordon S, Strange T (2004) Influence of inter-annual variations in climatic factors on fecal coliform levels in Mississippi Sound. Water Res 38:4341–4352. https://doi.org/10.1016/j.watres.2004.08.019
Choukr-Allah R (2012) Perspectives of wastewater reuse in the Mediterranean region. In: Choukr-Allah R, Ragab R, Rodriguez-Clemente R (eds) Integrated water resources management in the Mediterranean region. Springer, Dordrecht, pp 125–137. https://doi.org/10.1007/978-94-007-4756-2_8
Comite Normes et Standards CNS (1994) Ministere de l’environnement. Rabat, Maroc
El Addouli J, Chahlaoui A, Berrahou A, Chafi A, Ennabili A, Karrouch Lahcen (2009) Influence de rejets d’eaux usées sur les qualités physicochimique et bactériologique d’eaux utilisées en irrigation. Rev Francoph Ecol Ind 4:23–28. https://doi.org/10.4267/dechets-sciences-techniques.1100
El Boulani A, El Mimouni R, Chaouqy NE, Hamadi F, Azelmad K, Le Hello S (2016) Characterization and antibiotic susceptibility of Salmonella strains isolated from wastewater treated by infiltration percolation process. Moroc J Biol 13:44–51. https://doi.org/10.5772/67298
FAO (2003) L’irrigation avec les eaux usées traitées. Bureau Régional pour le Proche Orient et l’Afrique du Nord. https://www.pseau.org/outils/ouvrages/fao_irrigation_avec_des_eaux_usees_traitees_2003.pdf. Accessed 17 Nov 2019
Ganoulis J (2012) Risk analysis of wastewater reuse in agriculture. Int J Recycl Org Waste Agric 1:3. https://doi.org/10.1186/2251-7715-1-3
Hochstrat R, Wintgens T, Melin T, Jeffrey P (2006) Assessing the European wastewater reclamation and reuse potential—a scenario analysis. Desalin 188:1–8. https://doi.org/10.1016/j.desal.2005.04.096
Howard G, Pedley S, Barrett M, Nalubega M, Johal K (2003) Risk factors contributing to microbiological contamination of shallow groundwater in Kampala, Uganda. Water Res 37:3421–3429. https://doi.org/10.1016/S0043-1354(03)00235-5
Jiménez-Cisneros B (1995) Wastewater reuse to increase soil productivity. Water Sci Technol 32:173–180. https://doi.org/10.1016/0273-1223(96)00152-7
Mekki A, Dhouib A, Sayadi S (2013) Review: effects of olive mill wastewater application on soil properties and plants growth. Int J Recycl Org Waste Agric 2:15. https://doi.org/10.1186/2251-7715-2-15
Meloul AA, Hassani L (1999) Antibiotic resistance of Salmonella strains isolated from children living in the wastewater-spreading field of Marrakesh city (Morocco). World J Microbiol Biotechnol 15:81–85. https://doi.org/10.1023/a:1008874630153
Mouhanni H, Bendou A, Houari M (2013) Study of the wastewater purifying performance in the M’Zar plant of Agadir. Moroc Environ Pollut 2:20. https://doi.org/10.5539/ep.v2n3p20
Oren O, Yechieli Y, Böhlke JK, Dody A (2004) Contamination of groundwater under cultivated fields in an arid environment, central Arava valley, Israel. J Hydrol 290:312–328. https://doi.org/10.1016/j.jhydrol.2003.12.016
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. https://doi.org/10.1128/cmr.11.4.589
Rodier J, Legube B, Merlet N (2009) L’Analyse de l’ Eau (9ème édition). Dunod
Sou YM (2009) Recyclage des eaux usées en irrigation : potentiel fertilisant, risques sanitaires et impacts sur la qualité des sols. Dissertation, école polytechnique fédérale de Lausanne (EPFL)
Taylor R, Cronin A, Pedley S, Barker J, Atkinson T (2004) The implications of groundwater velocity variations on microbial transport and wellhead protection—review of field evidence. FEMS Microbiol Ecol 49:17–26. https://doi.org/10.1016/j.femsec.2004.02.018
Toze S (2006) Reuse of effluent water—benefits and risks. Agric Water Manag 80:147–159. https://doi.org/10.1016/j.agwat.2005.07.010
USEPA (2004) Guidelines for water use. U.S. environmental protection agency. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NRMRL&dirEntryId=129543. Accessed 17 Nov 2019
WHO (1989) L’utilisation des eaux usées en agriculture et en aquaculture: recommandations à visées sanitaires. World Health Organization. https://apps.who.int/iris/handle/10665/37549. Accessed 17 Nov 2019
Acknowledgements
Authors acknowledge the assistance of Faculty of Sciences Ibn Zohr University and the help of Golf course staff for their help to perform this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chahouri, A., Ouahmani, N.E., Choukrallah, R. et al. Physico-chemical and microbiological quality of M’Zar wastewater treatment plant effluents and their impact on the green irrigation of the Golf course. Int J Recycl Org Waste Agricult 8 (Suppl 1), 439–445 (2019). https://doi.org/10.1007/s40093-019-00316-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-00316-5