Abstract
The preparation of eco-friendly low-cost silkworm feces activated carbon (SFAC) for the removal of oxamyl pesticide from aqueous solution has been investigated in batch experiments. Structure and morphology of SFAC were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), field emission scanning electron microscopy (SEM). The specific surface area and mean pore diameter were obtained as 75.219 and 0.2035 cm3 g−1, respectively. The effect of different physicochemical parameters such as initial oxamyl concentrations, activated carbon dose and contact time has been studied. The results showed that the oxamyl removal on SFAC was unaffected in the pH range of 2–10. The percent removal of oxamyl onto SFAC was 99.48% from aqueous solutions. The adsorption process attained equilibrium within 120 min of contact time. Equilibrium data were analyzed by the Freundlich, Langmuir and Dubinin–Radushkevich (D-R) isotherm models. Freundlich isotherm provided the best fit to the equilibrium data. Adsorption kinetic was fitted well by the pseudo-second-order kinetic model. The results revealed that SFAC could be used a low-cost and eco-friendly alternative to other adsorbents for the oxamyl removal from aqueous solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The common usage of pesticides (hazardous compounds) has some undesirable effects such as toxicity, carcinogenicity and mutagenicity [7, 10, 36]. The presence of pesticides in water can cause serious environmental and human health problems.
Oxamyl (methyl N′-dimethyl-N-[(methyl carbamoyl) oxy]-l-thiooxamimidate) is an oximino carbamate pesticide, systemic and active as an insecticide or a nematicide. It is used for the control of nematodes in vegetables, bananas, pineapple, peanut, cotton, soya beans, potatoes, sugar beet and other crops. Oxamyl is characterized by its high water solubility (280 g/L), has a very low soil sorption coefficient, therefore, and has high potential for movement in the soil profile. In addition, it is characterized by high acute toxicity (LD50 = 2.5 mg/Kg). It can easily cause contamination of both ground and surface water resources. In addition, various amounts of oxamyl have been detected in surface and ground waters not only during actual insecticide application, but also after a long period of use.
The removal of pesticides from water is one of the major environmental concerns nowadays. Photocatalytic degradation [24, 53], ultrasound combined with photo-Fenton treatment [34], advanced oxidation processes [57], ozonation [41] and adsorption [20] are different methods used to remove the pesticides from water. One of the most widely used techniques is adsorption by activated carbon (AC) coming from agricultural wastes which show greater potential for the treatment of wastewaters due to very large quantities, easy to get and very low costs [43, 39]. For the above reasons, a wide range of agricultural wastes including banana and pomegranate peels [44] and rice husk [29], sugar beet pulp [37], corn wastes [1], tea waste and rice husks [54], walnut shells [42], chestnut shells [15], orange peels [23], walnut [33] and citrus limetta peel [49]. Lit et al. [38] studied the abilities of biochars produced from six agriculture wastes (soybeans, corn stalks, rice stalks, poultry manure, cattle manure and pig manure) to remove atrazine pesticide from contaminated water.
Several authors have successfully studied a variety of low-cost, effective and locally available adsorbents for the removal of different types of pesticides, Naushad et al. [45], Tran et al. [52] and Bansal [9].
To the best of our knowledge, there is no any study devoted to the potential applicability of silkworm feces activated carbon for the removal of pesticide from an aqueous solution. Some researchers [19] prepared activated carbons from silkworm feces via chemical activation method and used them as cheap adsorbents for removal cadmium and methylene blue from aqueous solutions.
Silkworm feces are non-toxic, low-cost, effective and environmentally friendly adsorbent. They are effective in the treatment of skin diseases and possess anti-inflammatory characteristics.
The current study aims to use silkworm feces as an inexpensive and environmentally friendly precursor for the production of activated carbon (SFAC) and to test its ability to remove oxamyl pesticide from aqueous solution under different conditions. Adsorption isotherm and kinetic parameters were also calculated and discussed.
Materials and methods
Chemicals
All reagents used in this study were of analytical grade. Before each experiment, all glassware were cleaned with dilute nitric acid and repeatedly washed with deionized water.
Preparation of activated carbon (SFAC)
Silkworms’ feces were kindly obtained from Sericulture Research Department, Agricultural Research Center (ARC), Egypt, were washed repeatedly with distilled water for several times to remove dirt particles and soluble impurities and were allowed to air-dry in an oven at 80 °C for 2 days. The sample was then soaked in orthophosphoric acid (H3PO4) with an impregnation ratio of 1:1 (w/w) for 24 h and dehydrated in an oven overnight at 105 °C. The resultant sample was activated in a closed muffle furnace to increase the surface area at 500 °C for 2 h in the absence of air. The SFAC produced was cooled to room temperature and washed with 0.1 M HCl and successively with distilled water. Washing with distilled water was done repeatedly until the pH of the filtrate reached 6–7. The final product was dried in an oven at 105 °C for 24 h and stored in vacuum desiccators until needed [46].
Instruments
The surface morphology of prepared activated carbon was examined using a scanning electron microscopy, Quanta 250 FEG (Field Emission Gun) with accelerating voltage 30 kV. Surface area and pore size distribution were measured using BELSORP-mini II instrument. The surface functional groups and structure were studied by FTIR spectrophotometer (PerkinElmer 1720) in the range of 400–4000 cm−1. The samples were examined as KBr disks. The crystallographic structure of the activated carbon sample is studied using powder X-ray diffraction analyzer (XPERT PRO, Netherland) with Cu Ka radiation (1.54 A˚) at 40 kV and 40 mA, in 2θ range 4–80°.
Adsorption experiments
All chemicals used in this work were of analytical reagent grade and were used without further purification. Batch adsorption experiments were conducted for the removal of oxamyl using silkworm feces activated carbon as a function of initial pH (2–10), initial oxamyl concentration (100–2500 mg/L), adsorbent dose (0.1–1.5 g/100 mL) and contact time (5–180 min). All the experiments were carried out at room temperature.
The adsorption of oxamyl by SFAC was studied over a pH range of 2–10. The influence of the initial solution pH was studied by shaking 1 g of sorbent and 100 mL of the oxamyl solution (500 mg/L) at different pHs. The flasks were agitated for 2 h. The solution pH was adjusted at the desired value by adding a small amount of HCl (0.1 M) or NaOH (0.1 M).
After adsorption process, the adsorbent was separated from the sample by filtering and the filtrate was transferred to a separatory funnel and extracted successively three times with 20, 15 and 10 mL portions of dichloromethane. The combined extracts were dried on anhydrous sodium sulfate to remove moisture content and evaporated using a rotary evaporator on a water bath at 40 °C. All samples should be cleaned by filtration with target nylon (0.45 µm) prior to analysis in order to minimize the interference of the carbon fines with the analysis. The samples were analyzed using HPLC with DAD (diode array detector). The equilibrium and kinetics data were obtained from batch experiments.
The amount of oxamyl adsorbed (q e) was calculated by using the following mass balance equation:
The removal efficiency of oxamyl was calculated as follows:
where C 0 and C e are the initial and the equilibrium concentrations of oxamyl mg/L, respectively, V is the volume of solution (mL), and W is the amount of adsorbent used (g).
Fitness of the adsorption isotherms models
The isotherm models were evaluated for fitness of the adsorption data by calculating the sum of squared error (SEE). The SEE values were calculated by the equation:
where Y is an actual score, Y’ is a predicted score, and N is the number of data points. In this study, the standard error of estimate was used to confirm the best fitting. If data from the model are similar to the experimental data, error is a small number.
Analysis of oxamyl
The concentrations of oxamyl in the solutions before and after adsorption were determined using an Agilent HPLC 1260 infinity series (Agilent technologies) equipped with a quaternary pump, a variable wavelength diode array detector (DAD) and an autosampler with an electric sample valve. The column was Nucleosil C18 [30 × 4.6 mm (i.d) × 5 μm] film thickness. The mobile phase was 60/40 (V/V) mixture of HPLC grade acetonitrile/water. The mobile phase flow rate was 1 mL/min. The wavelength was 220 nm. The retention time of oxamyl was 2.4 min, and the injection volume was 5 μL under the conditions.
Results and discussion
Characterization of the prepared adsorbent (SFAC)
Surface morphology of SFAC
The SEM micrographs of SFAC are shown in Fig. 1a, b. The SEM image of SFAC before adsorption (Fig. 1a) illustrates the irregular size and shape of individual grains and heterogeneous surface morphology. The porous structure is appearing with different widths. After adsorption, it is obvious that the pores have been covered by the oxamyl (Fig. 1b).
Silkworm feces activated carbon (SFAC) samples were first degassed under high vacuum at 350 °C for 8 h. Nitrogen adsorption–desorption isotherms of ACs were measured at nitrogen temperature (77 K).
Figure 2 shows the N2 adsorption–desorption isotherms of the SFAC. It is noted that the sample (SFAC) displayed isotherms of type IV, signifying that the sample contained mesopores with sizes ranging from 2 to 50 nm. According to the BET method, the specific surface area and mean pore diameter were obtained as 75.219 m2 g−1 and 0.2035 cm3 g−1, respectively.
Function groups of SFAC
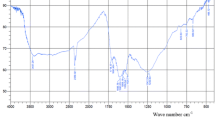
The FTIR spectrum of SFAC is illustrated in Fig. 3. A wide absorption band at 3431 cm−1 is assigned to O–H stretching vibrations of hydrogen bonded hydroxyl group of polymeric compounds, such as alcohols, phenols and carboxylic acids, as in pectin, cellulose groups on the adsorbent surface. The oxygen functional groups on the surface of SFAC greatly enhance its hydrophilic properties and also act as binding sites for the organic pollutant molecules [14].
The peaks at 2816 and 2921 cm−1 are attributed to the symmetric and asymmetric C–H stretching vibration of aliphatic acids. The peak observed at 1622 cm−1 is due to C=C stretching that can be attributed to the aromatic C–C bond, C–N and C–O– (1157 cm−1). The bands located at 1077 cm−1 are attributed to C–H in plane. The bands around 1440 cm−1 are due to the symmetric bending of –CH3. After loading, the most of characteristic peaks corresponding to these groups unchanged. However, there are also different changes in some peaks. The wave number of –OH group blueshifted from 3431 to 3405 cm−1 compared with that of SFAC. Hao et al. [30] had reported that the changes in some peaks of silkworm were seemed to be fact that –OH and–NH2 participate in the adsorption process.
Adsorption of the pesticides was considered to take place mainly by dispersion forces between electrons in the oxamyl pesticide structure and electrons in the SFAC surface. The adsorption of oxamyl on SFAC may be mainly due to dispersion forces and polarization of π electrons (electron-rich portion of the adsorbate).
A covalent bond between the pesticide and the surface of the carbon is formed Al-Qodah and Shawabkah [5] and Guixia et al. [25]. Moreover, the ionization of nitrogen atoms and/or NH groups in pesticide molecules is likely to occur, leading to the adsorption of more pesticide molecules on the surface of the carbon. Figure 4 shows the interaction mechanism of oxamyl pesticide onto activated carbon prepared from silkworm feces.
X-ray diffraction
The XRD pattern of the SFAC sample (Fig. 5) showed that defined and considerably sharp peaks at 23.9° and 42° were attributed to the presence of carbon and graphite [12], while different peaks which can related to specific compounds were found in ash. Meanwhile, peaks at 25°, 27°, 31°, 35° and 42° were attributed to titanium oxide, calcium corresponding silicate magnetite and iron oxide, respectively.
The effect of the initial pH
To study the influence of pH on the adsorption capacity of SFAC for oxamyl, experiments were performed at room temperature and oxamyl initial concentration of 500 mg/L using different initial solution pH values, varying from 2 to 10. The obtained results of removal of oxamyl at different pH solution values are shown in Fig. 6. It is clear that oxamyl was unaffected by varying pH of solution. A similar trend of pH effect was observed for the adsorption of anthracene on activated carbon and P. oceanica [21] and Al-Zaben and Mekhamer [6]. Thus, medium pH was used to study the adsorption isotherms and kinetics.
The effect of adsorbent dose
Adsorbent dose is an important parameter in the determination of adsorption capacity for a given initial concentration of the adsorbate under the operating conditions. The effect of adsorbent dose on removal of oxamyl by SFAC was studied by varying the dose of adsorbent in the range of 0.1–1.5 g/100 mL solution, while all the other variables were kept constant. Batch experiments were conducted at initial concentration 500 mg/L. The results are shown in Fig. 7 for oxamyl. It is evident from the plots that the percentage removal of oxamyl from aqueous solution increases with increase in the adsorbent dose. It was observed that the removal efficiency increased from 99.18 to 99.49% for oxamyl with the adsorbent dose varying from 0.1 to 1 g, and thereafter, it reached a constant value, which is probably due to an increase in the number of binding sites available for biosorption [3, 48]. Further increase in adsorbent dose did not significantly change the biosorption yield. The optimum dose is 1 g/100 mL.
Effect of different concentrations
The adsorption experiments were conducted with different initial concentrations of oxamyl pesticide (100–2500 mg/L), 1 g/100 mL of SFAC for 2 h. The wide range of initial concentration of oxamyl was used to observe the adsorption performance of SFAC for oxamyl at both low and high concentrations of oxamyl. It has been found that the biosorption capacity of the adsorbent SFAC increases from 9.969 to 248.76 mg/g with increasing pesticide concentrations from 100 to 2500 mg/L, respectively. The increase in equilibrium adsorption capacity may be due to the utilization of all available active sites for adsorption at higher oxamyl concentration, a larger mass transfer driving force and increased number of collision between pesticide molecules and SFAC, as shown in Fig. 8.
Effect of contact time
The rate at which adsorption takes place is most important during designing batch adsorption experiments. In order to determine the equilibrium time for maximum uptake of oxamyl, at a contact time study was performed with initial oxamyl concentration of 1000 mg/L, adsorbent dose (1 g for SFAC), at room temperature and contact time 5–180 min. The graphical representation of the contact time is given in Fig. 9. The adsorption of oxamyl is rapid for the first 30 min, and finally, equilibrium is established after about 120 min. The rapid pesticide adsorption at the initial stages of contact time could be attributed to the abundant availability of active sites on the surface of adsorbents. Afterward with the gradual occupancy of these sites, the adsorption became less efficient due to the decreased or lesser number of active sites [35]. Further increase in contact time did not enhance the adsorption, so, the optimum contact time for the adsorbent was selected as 120 min for further experiments. This is in agreement with the results obtained for hazelnut shell [16].
Biosorption kinetics
For analyzing the adsorption kinetics of oxamyl, the pseudo-first-order, pseudo-second-order and the intraparticle diffusion models were applied to the experimental data.
The pseudo-first-order rate equation is one of the most widely used equations for the adsorption of a solute from an aqueous solution and is represented as:
where q e and q t are the amount of pesticide adsorbed (mg/g) at equilibrium and time t, respectively. K 1 is the first-order reaction rate constant (l/min). Examination of the data shows that the pseudo-first-order kinetic model is not applicable to oxamyl adsorption onto SFAC (data not shown) judged by low correlation coefficient.
The pseudo-second-order equation based on adsorption equilibrium capacity may be expressed as follows:
where q e is the equilibrium biosorption capacity and K 2 is the pseudo-second-order rate constant (g/mg min). A plot of (t/q t ) versus t gives a linear relationship for the applicability of the second-order kinetic model as shown in Fig. 10.
As seen in Table 1 due to high R 2, the pseudo-second-order model is predominant kinetic model for the oxamyl removal by silkworm feces activated carbon biosorbent. The similar kinetic result was reported for hazelnut shell, Pyracantha coccinea and drin pesticide on the surface of acid-treated olive stones [4, 18, 50]. According to the high regression coefficient, the adsorptions of oxamyl onto SFAC are best fitted by the pseudo-second-order kinetic model compared to pseudo-first-order kinetic model.
The adsorption mechanism
In order to identify the diffusion mechanism, the intraparticle diffusion model described by Weber and Morriss is [55].
where K i is the intraparticle diffusion rate constant (mg/g min1/2) and C (mg/g) is a constant that gives an idea about the thickness of the boundary layer, i.e., the larger the value of C, the greater the boundary layer effect. The value of Ki was calculated from the slope of the linear plot of q t versus t 1/2. According to this model, if the regressions of q t versus t 1/2 is linear and pass through the origin, then intraparticle diffusion is the rate controlling step. Examination of the data showed that the regression was linear, but the plot did not pass through the origin (data not shown), suggesting that removal of oxamyl on silkworm feces activated carbon involved intraparticle diffusion but not the only rate controlling step. Other kinetic models may control the adsorption rate.
It could be stated that this process is complex and may involve more than one mechanism. This is in accordance with the results obtained for Araucaria angustifolia [13] and garlic peel, Hameed and Ahmad [28].
Adsorption isotherm
Adsorption isotherm expresses the relationship between pesticide adsorbed onto the adsorbents and pesticide in the solution and provides important design parameters for adsorption system. Several equilibrium models have been used to describe the adsorption data. Langmuir, Freundlich and Dubinin–Radushkevich (D-R) models are the most widely used.
The Freundlich adsorption model deals with non-ideal sorption onto heterogeneous surfaces involving multilayer sorption. The linear form of the Freundlich adsorption isotherm is
where K f is adsorption capacity and 1/n is related to the degree of surface heterogeneity (smaller value indicates more heterogeneous surface, whereas value closer to or even 1.0 indicates a material with relatively homogenous binding sites). The value of n obtained as a result of plotting of log C e versus log q e is shown in Fig. 11. Data given in Table 2 suggest that adsorption of oxamyl onto SFAC is well represented by Freundlich isotherm model and support the assumption that adsorption takes place on heterogeneous surfaces (H.M.F 1906).
The values of ‘n’ indicate the degree of nonlinearity between solution concentration and adsorption. If the value of n is equal to unity, the adsorption is linear; if the value is below unity, this implies that adsorption process is chemical; if the value is above unity, adsorption is a favorable physical process [27, 40].
The Langmuir model suggests that uptake occurs on a homogeneous surface by monolayer sorption without interaction between the adsorbed molecules [56]. The linear form of Langmuir adsorption isotherm is
where q e is the amount adsorbed onto adsorbent at equilibrium, b is the Langmuir constant, and q m is the monolayer adsorption capacity. The plot of C e /q e versus C e is employed to generate the intercept value of 1/bq m and slope of 1/q m as shown in Fig. 11.
One of the essential characteristics of this model can be expressed in terms of the dimensionless separation factor for equilibrium parameter, R L, defined by [22]
The value of R L indicates the type of isotherm to be irreversible (R L = 0), favorable (0 < R L < 1), linear (R L = 1) or unfavorable (R L > 1). The value of R L in the present investigation was found to be 0.007, indicating that the adsorption of oxamyl on SFAC is favorable. Adsorption capacity from the present study was compared with other adsorbents from previous studies [8] which is shown in Table 2. q max of oxamyl in silkworm feces was almost 4 times greater than that in apricot stone which is shown in Table 2 according to the source of the activated carbon. Also the starting material significantly influences physical and chemical properties of activated carbon as a result.
Based on the correlation coefficient (R 2) shown in Table 2, the adsorption isotherms of oxamyl on SFAC can be slightly better described by the Freundlich equation. Thus, the results of the present study indicate that biosorption of oxamyl onto SFAC is heterogeneous in nature. However, the sum of squared error SEE test (Table 2) for Langmuir isotherm model exhibited lower values than for Freundlich isotherm model. Similar results have been reported for the adsorption of phosphate ions by pine cone [11].
Dubinin–Radushkevich (D-R) proposed another equation used in the analysis of isotherms. D-R model was applied to estimate the porosity apparent free energy and the characteristic of adsorption [17]. The D-R isotherm does not assume a homogeneous surface or constant sorption potential, and it has commonly been applied in the following form Eq. (8) and its linear form can be shown in Eq. (9):
where K is a constant related to the adsorption energy, q e (mg/g) is the amount of pesticide adsorbed per g of adsorbent, q m represents the maximum adsorption capacity of adsorbent, β (mol2/J2) is a constant related to adsorption energy, while ε is the Polanyi potential that can be calculated from Eq. (10):
The values of β and q m can be obtained by plotting ln q e vs. ε 2. The mean free energy of adsorption (E, J/mol), defined as the free energy change when one mole is transferred from infinity in solution to the surface of the sorbent, is calculated using the following relation Eq. (11):
The calculated values of D-R parameters are given in Fig. 11 and Table 2. The saturation adsorption capacities qm obtained using D-R isotherm model for adsorption of oxamyl SFAC are 169.44 mg/g. The mean energy of adsorption is the free energy change when one mole of pesticide is transferred to the surface of the solid from infinity in the solution. The value of this parameter can give information about adsorption mechanism. When one mole of pesticide is transferred, its value in the range of 1–8 kJ/mol suggests physical adsorption [47], the value of E is between 8 and 16 kJ/mol, which indicates the adsorption process, follows by ion-exchange [31], while its value in the range of 20–40 kJ/mol is indicative of chemisorption [51]. So, the values of E calculated is 1.11 kJ/mol, indicating that weak physical forces such as van der Waals and hydrogen bonding affect the adsorption process of oxamyl onto SFAC. These E values are in agreement with [2] for the adsorption of dyes by loofa activated carbon and drin pesticides onto acid-treated olive stones [32].
Adsorption capacity from the present study was compared with other adsorbents from previous studies [8] are shown in Table 3. The maximum adsorption capacity qmax of oxamyl by silkworm feces was almost 4 times greater than that in Apricot stone in Table 3 according to the source of the activated carbon. Also the starting material significantly influences physical and chemical properties of activated carbon as a result.
Conclusion
The present study focused on removal of oxamyl pesticide from aqueous solution using eco-friendly silkworm feces-based activated carbon. The adsorption has been examined with the variations in the parameters of activated carbon dose, initial oxamyl concentration and contact time. The experimental data were analyzed using Langmuir, Freundlich and Dubinin–Radushkevich (D-R) isotherm models. The Freundlich model provides the best correlation of the experimental equilibrium data. The adsorption system obeys the pseudo-second-order kinetic model. The results indicated that the SFAC could be a promising biosorbent for the removal of oxamyl from aqueous solution.
References
Abdel-Ghani N, El-Chaghaby G, Zahran E (2015) Pentachlorophenol (PCP) adsorption from aqueous solution by activated carbons prepared from corn wastes. Int J Environ Sci Tech 12(1):211–222
Abdelwahab O (2008) Evaluation of the use of loofa activated carbons as potential adsorbents for aqueous solutions containing dye. Desalination 222:357–367
Ahmad R (2009) Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J Hazard Mater 171:767–773
Akar T, Celik S, Akar ST (2010) Biosorption performance of surface modified biomass obtained from Pyracantha coccinea for the decolonization of dye contaminated solutions. Chem Eng J 160(2):466–472
Al-Qodah Z, Shawabkah R (2009) Production and characterization of granular activated carbon from activated sludge. Braz J Chem Eng 26:127–136
Al-Zaben MI, Mekhamer WK (2013) Removal of 4-chloro-2-methyl phenoxy acetic acid pesticide using coffee wastes from aqueous solution. Chem, Arabian J. doi:10.1016/j.arabjc.2013.05.003 (In press, corrected proof)
Ayranc JE, Hoda N (2005) Adsorption of phthalic acid and its esters onto high-area activated carbon-cloth studied by in situ UV-spectroscopy. Chemosphere 60:1600–1607
Ahmed SM, Mohammad SG (2014) Egyptian apricot stone (Prunus armeniaca) as a low cost and eco-friendly biosorbent for oxamyl removal from aqueous solutions. Am J Exp Agri 4(3):302–321
Bansal OP (2004) Kinetics of interaction of three carbamate pesticides with Indian soils: aligarh district. Pest Manag Sci 60(11):1149–1155
Becker DL, Wilson SC (1980) Carbon adsorption Handbook In: Chereminisoff PN, Ellebush F (eds) Ann Harbor Science Publishers, Michigan. 167–212
Benyoucef S, Amrani M (2011) Adsorption of phosphate ions onto low cost Aleppo pine adsorbent. Desalination 275:231–236
Bouchelta C, Medjram MS, Bertrand O, Bellat JP (2008) Preparation and characterization of activated carbon from date stones by physical activation with steam. J Anal Appl Pyrolysis 82:70–77
Calvete T, Lima EC, Cardoso NF, Dias SLP, Pavan FA (2009) Application of carbon adsorbents prepared from the Brazilian-pine fruit shell for removal of Procion Red MX 3B from aqueous solution-kinetic, equilibrium and thermodynamic studies. Chem Eng J 155:627–636
Chen H, Wang X, Li J, Wang X (2015) Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J Mater Chem A 3:6073–6081
Cobas M, Meijide J, Sanromán MA, Pazos M (2016) Chestnut shells to mitigate pesticide contamination. J Taiwan Inst Chem Eng 61:166–173
Dogan M, Abak H, Alkan M (2009) Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. J Hazard Mater 164(1):172–181
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons. I. Adsorption of organic vapors. ZhFizKhim. 21:1351–1362
El Bakouri H, Usero J, Morillo J, Ouassini A (2009) Adsorptive features of acid-treated olive stones for drin pesticides: equilibrium, kinetic and thermodynamic modeling studies. Bioresour Technol 100:4147–4155
ElShafei GMS, ElSherbiny IM, Darwish AS, Philip C (2014) Silkworms’ feces-based activated carbons as cheap adsorbents for removal of cadmium and methylene blue from aqueous solutions. Chem Eng Res Des 92(3):461–470
ElShafei GMS, Nasr IN, Ayman SM, Mohammad SG (2009) Kinetics and thermodynamics of adsorption of cadusafos on soils. J Hazard Mater 172:1608–1616
El Khames M, Ramzi K, Elimame E, Younes M (2014) Adsorption of anthracene using activated carbon and Posidonia oceanica. Arabian J Chem 7:109–113
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents-a review of the recent literature. Bioresour Technol 101:5043–5053
Fernandez ME, Nunell GV, Bonelli PR, Cukierman AL (2014) Activated carbon developed from orange peels: batch and dynamic competitive adsorption of basic dyes. Ind Crops Products 62:437–445
Gong J, Yang C, Zhang W (2011) Liquid phase deposition of tungsten doped TiO2 films for visible light photoelectrocatalytic degradation of dodecyl benzenesulfonate. Chem Eng J 167:190–197
Guixia Z, Lang J, Yudong H, Li Jiaxing, Huanli D, Xiangke W, Wenping H (2011) Sulfonated graphene for persistent aromatic pollutant management. Adv Mater 23:3959–3963
Freundlich HMF (1906) Uber die adsorption in lasungen. Z Phys Chem 57(1906):385–470
Hadi M, Samarghandi MR, McKay G (2010) Equilibrium two parameter isotherms of acid dyes sorption by activated carbons: study of residual errors. Chem Eng J 160:408–416
Hameed BH, Ahmad AA (2009) Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J Hazard Mater 164:870–875
Han R, Ding D, Xu Y, Zou W, Wang Y, Li Y, Zou L (2008) Use of rice husk for the adsorption of Congo red from aqueous solution in column mode. Bioresour Technol 99:2938–2946
Hao C, Jie Z, Guoliang D (2011) Silkworm exuviae—A new non-conventional and low-cost adsorbent for removal of methylene blue from aqueous solutions. J Hazard Mater 186:1320–1327
Helfferich F (1962) Ion Exchange. McGraw-Hill Book Co, New York
El Hicham, Jose U, Jose M, Abdelhamid O (2009) Adsorptive features of acid-treated olive stones for Drin pesticides: equilibrium, kinetic and thermodynamic modeling studies. Bioresour Tech. 100:4147–4155
Heibati B, Rodriguez-Couto S, Amrane A, Rafatullah M, Hawari A, Al-Ghouti MA (2014) Uptake of Reactive Black 5 by pumice and walnut activated carbon: chemistry and adsorption mechanisms. J Ind Eng Chem 20(5):2939–2947
Katsumata H, Kobayashi T, Kaneco S, Suzuki T, Ohta K (2011) Degradation of linuron by ultrasound combined with photo-Fenton treatment. Chem Eng J 166:468–473
Kannan N, Karrupasamy K (1998) Low cost adsorbents for the removal of phenyl acetic acid from aqueous solution. Indian J Environ Prot 18:683–690
Kouras A, Zouboulis A, Samara C, Kouimtzis T (1998) Environ Pollut 103:193–202
Li D, Yan J, Liu Z, Liu Z (2016) Adsorption kinetic studies for removal of methylene blue using activated carbon prepared from sugar beet pulp. Int J Environ Sci Technol 13:1815–1822
Naa Liu, Alberto BC, Chih HW, Xiaoling Y, Feng D (2015) Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: a comparativestudy. Bioresource Tech. 198:55–62
Mahmoud MS, Ahmed SM, Mohamamd SG, Abou Elmagd AM (2014) Evaluation of Egyptian Banana Peel (Musa sp.) as a green sorbent for groundwater treatment. Int J Eng Tech 4(11):648–659
Mahmoodi NM, Arami M (2008) Modeling and sensitivity analysis of dyes adsorption onto natural adsorbent from colored textile wastewater. J Appl Polym Sci 109:4043–4048
Maldonado MI, Malato S, Perez-Estrada LA, Gernjak W, Oller I, Domenech X (2006) Partial degradation of five pesticides and an industrial pollutant by ozonation in a pilot-plant scale reactor. J Hazard Mater 38:363–369
Memon GZ, Moghal M, Memon JR, Memon NN, Bhanger M (2014) Adsorption of selected pesticides from aqueous solutions using cost effective walnut shells. J Eng 4:43–56
Mohammad SG (2013) Biosorption of pesticide onto a low cost carbon produced from Apricot Stone (Prunus armeniaca): equilibrium, kinetic and thermodynamic studies. J Appl Sci Res 9(10):6459–6469
Mohammad SG, Ahmed SM, Badawi AM (2015) A comparative adsorption study with different agricultural waste adsorbents for removal of oxamyl pesticide. Desalination Wat Treat 55(8):2109–2120
Naushad M, Alothman ZA, Khan MR (2013) Removal of malathion from aqueous solution using De-Acidite FF-IP resin and determination by UPLC-MS/MS: equilibrium, kinetics and thermodynamics studies. Talanta 15(115):15–23
Njoku VO, Hameed BH (2011) Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption. Chem Eng J 173:391–399
Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent cation exchanged zeolite F-9. J Colloid Interface Sci 279:341–350
Saeed A, Sharif M, Iqbal M (2010) Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J Hazard Mater 179:564–572
Sadia S, Nasar Abu (2016) Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J Taiwan Inst Chem Eng 66:154–163
Safa Y, Bhatti HN (2011) Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination 272(1–3):313–322
Tahir SS, Rauf N (2006) Removal of cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Tran VS, Ngo HH, Guo W, Zhang J, Liang S, Ton-That C, Zhang X (2015) Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour Technol 182:353–363
Uğurlu M, Karaoğlu MH (2011) TiO2 supported on sepiolite: preparation, structural and thermal characterization and catalytic behaviour in photocatalytic treatment of phenol and lignin from olive mill wastewater. Chem Eng J 166:859–867
Vithanage M, Mayakaduwa SS, Herath I, Sik YO, Dinesh M (2016) Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 150:781–789
Weber WJ, Morriss JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 9:31–60
Zainal IG (2010) Biosorption of Cr(VI) from aqueous solution using new adsorbent: equilibrium and thermodynamic study. E J Chem 7:S488–S494
Zhou T, Lim TT, Chin SS, Fane AG (2011) Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: feasibility test of advanced oxidation processes with/without pretreatment. Chem Eng J 166:932–939
Acknowledgements
Many thanks to all the members of Central Agricultural Pesticides Laboratory (CAPL), Agricultural Research Center, Dokki, Giza, Egypt, for their valuable assistance and facilities they provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mohammad, S.G., Ahmed, S.M. Preparation of environmentally friendly activated carbon for removal of pesticide from aqueous media. Int J Ind Chem 8, 121–132 (2017). https://doi.org/10.1007/s40090-017-0115-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-017-0115-2