Abstract
The main objective of this work was to study the degradation of Acid Orange 7 (AO7) dye in aqueous solution by hydrogen peroxide using HFe2.5P2W18O62 23.H2O as a catalyst. HFe2.5P2W18O62 23.H2O is a recyclable DAWSON-type heteropolyanion. Effects of various experimental parameters of the oxidation reaction of the dye were investigated. The studied parameters were the initial pH, the initial H2O2 concentration, the catalyst mass, and the dye concentration. The optimum conditions had been determined, and it was found that efficiency of degradation obtained was about 100%. The optimal parameters were: initial pH 4; [H2O2]0 = 2 mM; catalyst mass 0.01 g; concentration of dye 30 mg/L. Infrared spectroscopy analysis of the HFe2.5P2W18O62 23.H2O catalyst indicates that the catalyst showed good stability for degradation of AO7 even after second cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of textile waste waters has always been a serious problem for these industries. These components sometimes are not biodegradable and are toxic enough for aquatic ecosystems [1]. Some classical methods such as adsorption on activated carbon, ozonation, reverse osmosis, ion exchange on synthetic adsorbent resins, flocculation, and decantation are available for removing dye from water. However, these methods have high operating cost [2] or are inefficient due to complex aromatic structure. Other alternatives to degrade recalcitrant organic pollutants are now being studied including advanced oxidation (AOPs) whose characteristic is to generate hydroxyl radicals (·OH), which are powerful oxidants [3].
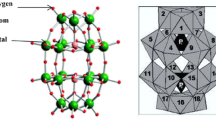
These radicals are generated by Fenton’s reagent (Fe2+/H2O2), which has been the subject of many studies in the degradation of organic matter [4]. However, the disadvantage of the method lies in Fenton rejection of a significant amount of sludge Fe2+ and Fe3+ [5], which requires separation or removal, thus increasing the operational cost. To solve this problem, many studies have focused on the use of a modified Fenton process (Fenton-Like) to treat waste waters. A method which relies on the use iron incorporated in the recyclable compounds [6]. In this work, a new homogeneous Fenton-like system based on hydrogen peroxide and a recyclable Dawson-type heteropolyanion (Fig. 1) [7].
(HFe2.5P2W18O62 23.H2O/H2O2) system was used for decolorization of Acid Orange 7 (AO7) (also known as Orange II).
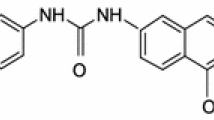
Acid Orange 7 is one of the dyes that are produced in large amounts in the world. It is commonly used in pharmaceutical, food, and cosmetics industries. Acid Orange 7 is an azoique dye used in dyeing of silk and wool. For these qualities, Acid Orange 7 is much used in the textile industry. The molecular structure of azo-dye Orange II is shown in Fig. 2. Acid Orange 7 is not amenable to conventional biological treatment [8].
The aim of the study is to examine the influence of different parameters such as the solution pH, the catalyst mass, the concentration of H2O2, and the initial dye concentration on the degradation of Acid Orange 7 by H2O2 using HFe2.5P2W18O6223.H2O as a catalyst. The mechanism of the reaction and catalytic stability were studied.
Experimental section
Synthesis of catalyst HFe2.5P2W18O62 23.H2O [9]
HFe2.5P2W18O62 23.H2O
5 g (1.088 mol) of H6P2W18O62 was dissolved in 20 mL of water at room temperature and 0.767 g of solid FeCl2,6H2O (3.26 mol) was then added. The mixture was then stirred for 10 min. Yellow powder was obtained after five days by slow evaporation.
H6P2W18O62 was prepared, starting from the substance mother (K6P2W18 O62, nH2O)6-, by extraction with ether in hydrochloric acid medium according to the methods described in the literature [10].
Behavior of AO7 oxidation using HFe2.5P2W18O62 23.H2O/H2O2 system
Reagents
The 4-(2-hydroxy-1-naphthylazo) benzene sulfonic acid sodium salt, commonly named orange II or Acid Orange 7 (AO7) (90%) was obtained from Aldrich (H2O2 35%, W/W) was purchased from Aldrich Chemical Company. All other reagents (NaOH or H2SO4) that used in this study were analytical grade.
Procedures and analysis
The experiments were performed in a reactor batch of 500 mL capacity. Various solutions of AO7 were prepared at different concentrations. They were then homogenized by stirring them until the dye is completely dissoluted of the dye. pH was adjusted using 0.1 N H2SO4 or NaOH aqueous solutions. In all experiments 100 mL of dyes (30 mg/L) solution containing the appropriate quantity of catalyst and H2O2 was magnetically stirred at room temperature. The AO7 decreasing concentration of dye was monitored by a 6705 UV visible spectrophotometer JENWAY. The Wave length corresponding to the maximum absorbance is given below: λ max = 486 nm [11].
The resolution of the wave length and bandwidth was 1 and 0.5 nm. The cell used during the experiments was made of 1 cm thick quartz.
Effects of operational parameters on AO7 oxidation
The oxidation of AO7 by H2O2 using (HFe2.5P2W18O61 23H2O) as a catalyst has been studied according to the following factors: initial pH of the solution, H2O2 concentration, mass of the catalyst (HFe2.5P2W18O61 23H2O), and dye concentration.
The oxidation efficiency (discoloration) was determined as it is shown below [12]:
DE: discoloration efficiency; C i : Initial dye concentration; C f : final dye concentration.
Effect of solution pH
The pH value of dye solution has significant influence on the catalytic system efficiency because: (1) it can affect the catalyst stability and (2) it influences the catalytic reaction which controls the production rate of hydroxyl radicals [13]. To find the optimum pH for the decolorization of AO7, a series of experiments at initial pH value in the range 3–8 was conducted.
Figure 3 illustrates the effect of pH on the discoloration efficiency of AO7 in water. It was found that the discoloration efficiency of AO7 is strongly pH dependent. The optimal pH is about 4 giving discoloration efficiency equal to 94.25%. This result can be explained by the stability of the catalyst at this pH. Also, H2O2 molecules are unstable in alkaline solution and therefore, the degradation of dye decreases in alkaline solution [14].
The optimal value is chosen pH 4.
Effect of the nature of the acid used to adjust the pH
The oxidation of the dye by the Fenton-like process can be influenced by the presence of various ions such as SO4 2−, NO3−, Cl−, and PO4 3− [15]. To evaluate the influence of these anions, we adjusted the pH of an aqueous solution of AO7 by different acids H2SO4, HNO3, HCl, and H3PO4 in similar operating conditions to those previously established.
Figure 4 shows the effect of these acid ions (chloride, sulfate, nitrate, and phosphate) on the dye oxidation. It appears that the presence of phosphate ions inhibits the oxidation of the dye using HFe2.5P2W18O62 23.H2O/H2O2 system.
Sulfate and nitrate ions have virtually no effect on the discoloration of AO7. However, depending on the nature of the acids, the discoloration efficiency is about 94.25, 94.35, 96.09, and 03.06% in the presence of H2SO4, HNO3, HCl, and H3PO4 acids, respectively. These results agree with those found at the degradation of other organic pollutants [16]. We can deduce that H2SO4, HNO3, and HCl acids lead almost to the same efficiency, but in the presence of H3PO4 it is too low. The inhibitory effect of phosphate ions may be due to the trapping of ·OH radicals.
Effect of catalyst mass
The effect of the catalyst mass was investigated, keeping operational parameters identical to those of the above-mentioned experiment. The following catalyst quantities have been used: 0.005; 0.008; 0.01; 0.02 g. The results are illustrated in Fig. 5.
These results show that the discoloration efficiency of AO7 oxidation increases when increasing the catalytic mass up to 0.01 g where the decolorization efficiency is optimal. Beyond this value, there is a decrease of the discoloration efficiency. The excess of the catalyst does not appear to ply a positive role in the AO7 oxidation using HFe2.5P2W18O62 23.H2O/H2O2 system. This result is in good agreement with literature [12, 13, 17]: the decrease of the discoloration efficiency of AO7 when increasing the mass of the catalyst can be explained by the presence of side reaction consuming the radicals hydroxyls. The mass of catalyst that gives the best result is 0.01 g.
Effect of H2O2 concentration
The effect of H2O2 concentration on AO7 decolorization was studied in the range 0.04–0.44 mmol. The results are illustrated in Fig. 6.
The results indicate that the degradation of AO7 was increased by increasing the concentration of H2O2 up to a value of concentration of H2O2 equal to 2 mM.
Decolorization of certain dyes, mainly azo, with activated hydrogen peroxide in homogeneous systems, using different soluble catalysts (heteropolyanions) was already studied [18].
The activation of hydrogen peroxide by homogeneous catalysts was attributed to the formation of highly active hydroxyl radicals [19]. High concentrated H2O2 solution undergoes self quenching of OH radicals, with formation of hydroperoxyl radicals HO2. Although HO2 is an effective oxidant itself, its oxidation potential is much lower than that of ·OH radicals [20].
It can be postulated that H2O2 should be added at an optimum concentration to achieve the best degradation. Hence, 0.2 mM/L of H2O2 appears as an optimal.
Radical scavenging experiments and possible reaction mechanism
In general, ·OH radicals are known as a key active species in the catalytic oxidation process. ·OH radicals are powerful oxidants for many organic molecules.
Ethanol, methanol, and 2-propanol are known as OH scavengers [21–24]. These reagents were introduced into the reaction system to capture ·OH in the oxidation of AO7 process. The results are illustrated in Fig. 7.
These results show that the addition of ·OH scavenger reagents causes a sharp decrease in the degradation of AO7, and the discoloration efficiency of AO7 from 100% to 1.6, 2.08, and 2.53% in the presence of methanol, ethanol, and 2-propanol, respectively. According to the above discussion, it is furthermore confirmed that the degradation of AO7 molecules is dependent on the availability of ·OH radical species. ·OH radicals make a major contribution to degradation of AO7. OH scavenger reagents added consume ·OH radical species and less will be available for the degradation of the dye molecules, hence the lower discoloration efficiency.
Also, it was shown [25] that the action of H2O2 on a complex containing Fe3+ resulted in the reduction of Fe3+ to Fe2+ with apparition of HO·2.
The action of H2O2 on the complex of Fe2+ leads to the generation of hydroxyl radicals OH·. These hydroxyl radicals cause the degradation of the dye.
In agreement with the results found and the mechanism proposed below, we can propose the following mechanism:
The degradation of AO7 by H2O2 catalyzed with HFe2.5P2W18O62 23.H2O heteropolyanion can be well supported by the change in the UV–Vis absorption spectrum over the course of degradation (Fig. 8). The AO7 spectrum was characterized by the chromophore that contained azo linkage [26] in the visible range at 484 and 430 nm corresponding to the hydrazone and azo form. In the ultraviolet region, the absorbance peaks at 230 and 310 nm are due to the benzene and naphthalene rings, respectively. These four characteristic bands were markedly weakened during the degradation reaction, tending to disappear completely after 60 min, without the appearance of new absorption bands in the visible or ultraviolet regions due to destruction of the chromophoric and auxochromic structures by the homogeneous Fenton-like reaction.
Effect of the AO7 concentration
It is important from an application point of view to study the dependence of removal efficiency on the initial concentration of the colorant (AO7).
To determine the effect of AO7 concentration on discoloration efficiency, a series of experiments was realized between 5 and 50 mg/L. The variation of the discoloration efficiency of the AO7 dye with H2O2 and HFe2.5P2W18O61 23H2O catalyst as a function of initial dye concentration is represented in Fig. 9. The results show that the discoloration efficiency increases with increasing of dye concentration up to a value equal to 30 mg/L, but above it the discoloration efficiency decreases.
This result can be explained as follows:
At low concentration of dye, the discoloration efficiency is lower (ED 39% for the concentration of AO7 5 mg/L). These results concur with those found in literature [27–29]. This phenomenon can be explained by the effect of H2O2 concentration is in excess compared to latter and can cause the scavenging effect (the reaction of H2O2 and OH· in aqueous solution).
-
At higher concentration of dye, the discoloration efficiency decreases (ED 48% for the concentration of AO7 50 mg/L). This phenomenon can be explained by the fact that increasing the initial concentration of dye leads to an increase in the number of molecules of (AO7), while the number of the radical’s hydroxyls remains constant (H2O2 concentration and catalyst kept constant), thereby causing a decrease in the discoloration efficiency [19, 27].
Stability and recycling of the catalyst
After reaction, we have tried to recover the catalyst P2W18Fe3+. The method used is its precipitation, as potassium salt, by adding a mass of KCl in the solution after complete discoloration in a basic medium. The resulting precipitate was characterized by infrared spectroscopy. The spectra are illustrated in Fig. 10.
The spectrum of the recovered heteropolyanion is identical to that of the initially added heteropolyanion. We can therefore conclude that the catalyst remains stable and robust after the reaction.
Figure 11 shows the catalytic performance of recovered catalyst after four cycles through the oxidation of AO7 dye, by hydrogen peroxide.
After second cycle, the catalyst did not exhibit significant loss of catalytic activity and the discoloration efficiency was 100. AO7 degradation decreases only from 100 to 95% in three cycles. In four cycles, AO7 degradation decreases from 100 to 89%.
This result is in good agreement with the literature [28]. The loss of activity can be explained by to poisoning of the active catalytic sites due to adsorbed organic species. However, this could be avoided by placing the catalyst in an oven at 180 °C, to enhance the organic solvent.
Conclusion
Fe(III)-phosphotunstate/H2O2 system is a new homogeneous Fenton-like system that is capable of oxidizing organic dye compounds. The experimental results show that the initial pH, the initial concentration of H2O2, Fe(III)P2W18, AO7 concentration, had a great influence on the degradation of AO7 dye. It is approximately 100% dye which has been eliminated the Fe(III)P2W18/H2O2 on the following operating conditions: pH 4, catalyst mass 0.01 g, [H2O2]0 = 2 m M. The molar ratio H2O2/AO7 = 0.02. The possibility of recovery of the catalyst after reaction is verified by adding a mass of KCl in the solution after complete discoloration. The stability of reused catalyst is confirmed after second cycle.
This system constitutes simple and effective method compared to those previously reported for the oxidation of Acid Orange 7 [29, 30].
References
Fernández J, Kiwi J, Baeza J, Freer J, Lizama C, Mansilla HD (2004) Orange II photocatalysis on immobilised TiO2, Effect of the pH and H2O2. Appl Catal B Environ 48:205–211
Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni MVB, Kajitvichyanukul P, Krishnan-Ayer R (2008) Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. Photochemrev 9:171–192
Zhang Y, He C, Deng J, Tu Y, Liu J, Xiong Y (2009) Photo-Fenton-like catalytic activity of nano-lamellar Fe2V4O13 in the degradation of organic pollutants. Res Chem Intermed 35:727–737
Medien HAA, Khalil SME (2010) Kinetics of the oxidative decolorization of some organic dyes utilizing Fenton-like reaction in water. J King Saud Univ 22:147–153
Duesterberg CK, Mylon SE, Waite TD (2008) pH effects on iron-catalyzed oxidation using Fenton’s reagent. Environ Sci Technol 42:8522–8527
Hsueh CL, Huang YH, Wang CC, Chen CY (2005) Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 58:1409–1414
Pope MT (1983) Heteropoly and isopoly oxometalates. Springer, New York
Kuznetsova NI, Kirillova NV, Kuznetsova LI, Smirnova MY, Likholobov VA (2007) Hydrogen peroxide and oxygen oxidation of aromatic compounds in catalytic systems containing heteropoly compounds. J Hazard Mater 46:569–576
Bechiri O, Abbessi M, Belghiche R, Ouahab L (2014) Wells–Dawson polyoxometelates [HP2W18-nMonO62]Fe2.5, xH2O; n = 0, 6: synthesis, spectroscopic characterization and catalytic application for dyes oxidation. C R Chim 17:135–140
Ciabrini JP, Contant R, Fruchart M (1983) Heteropolyblues: relationship between metal-oxygen-metal bridges and reduction behaviour of octadeca (molybdotungsto) diphosphate anions. Polyhedron 2:1229–1233
Djaneye-boundjou G, Amouzou E, Kodom T, Tchakala I, Anodi K, Bawa LM (2012) Photocatalytic degradation of orange II using mesoporous TiO2 (P25) and fenton reactive (Fe/H2O2). IJESMER 1:91–96
Bechiri O, Abbessi M, Ouahab L (2012) The oxidation study of methyl orange dye by hydrogen peroxide using Dawson-type heteropolyanions as catalysts. Res Chem Intermed 39:2945–2954
Bechiri O, Abbessi M, Samar ME (2013) Decolorization of organic dye (NBB) using Fe(III)P2W12Mo5/H2O2 system. Desalin Water Treat 51:31–33
Gould DM, Spiro M, Griffith WP (2005) Mechanism of bleaching by peroxides: part 7. The pH dependence of the oxometalate catalysed bleaching of methyl orange. J Mol Cat A Chem 242:176–181
Fan HJ, Huang ST, Chung WH, Jan JL, Lin WY, Chen CC (2009) Degradation pathways of crystal violet by Fenton and Fenton-like systems: condition optimization and intermediate separation and identification. J Hazard Mater 71:1032–1044
Modirshahla N, Behnajady MA, Ghanbary F (2007) Decolorization and mineralization of C.I. Acid Yellow 23 by Fenton and photo-Fenton processes. Dyes Pigments 73:305–310
Mylon SE, Quan S, Waite TD (2010) Process optimization in use of zero valent iron nanoparticles for oxidative transformations. Chemosphere 81:127–131
Strukul G (1992) Catalytic oxidations with hydrogen peroxide as oxidant. Kluwer Academic, Dordrecht
Lucas MS, Peres JA (2006) Decolorization of the azo dye reactive black 5 by Fenton and photo-Fenton oxidation. Dyes Pigments 71:236–244
Sun JH, Shi SH, Lee YF, Sun SP (2009) Fenton oxidative decolorization of the azo dye Direct Blue 15 in aqueous solution. Chem Eng J 155:680–683
Tadolini B, Cabrini L (1988) On the mechanism of OH. scavenger action. Biochem J 253:931–933
Zheng J, Gao Z, He H, Yang S, Sun C (2016) Efficient degradation of Acid Orange 7 in aqueous solution by iron ore tailing Fenton-like process. Chemosphere 150:40–48
Lindsey M, Tarr M (2000) Quantitation of hydroxyl radical during Fenton oxidation following a single addition of iron and peroxide. Chemosphere 41:409–417
Overend R, Paraskevopoulos G (1978) Rates of OH radical reactions. 4. Reactions with methanol, ethanol, I-propanol, and 2-propanol at 296 K. J Phys Chem 82:12
Ramirez HJ, Costa CA, Madeira LM, Mata G, Vicente MA, Rojas-Cervantes ML, Lopez-Peinado AJ, Martin-Aranda RM (2007) Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl Catal B 71:44–56
Bauer C, Jacques P, Kalt A, Photochem J (2001) Photodegradation of an azo dye induced by visible light incident on the surface of TiO2. Photobiol A Chem 140:87–92
Ji F, Li C, Zhang J, Deng L (2011) Efficient decolorization of dye pollutants with LiFe(WO4)2 as a reusable heterogeneous Fenton-like catalyst. Desalination 269:284–290
Catrinescu C, Teodosiu C, Macoveanu M, Miehe-Brendle J, Dred RL (2003) Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res 37:1154–1160
Zhang F, Feng C, Li W, Cui J (2014) Indirect electrochemical oxidation of dye wastewater containing acid orange 7 using Ti/RuO2-Pt electrode. Int J Electrochem Sci 9:943–954
Wang J, Liu G, Lu H, Jin R, Zhou J (2012) Biodegradation of acid orange 7 and its auto-oxidative decolorization product in membrane-aerated biofilm reactor. Int Biodeterior Biodegrad 67:73–77
Acknowledgements
This work was supported by the Engineering Environmental Laboratory of Badji Mokhtar University (Annaba-Algeria). This work is a tribute to the late Professor Mostefa Abbessi, God rest his soul.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tabaï, A., Bechiri, O. & Abbessi, M. Degradation of organic dye using a new homogeneous Fenton-like system based on hydrogen peroxide and a recyclable Dawson-type heteropolyanion. Int J Ind Chem 8, 83–89 (2017). https://doi.org/10.1007/s40090-016-0104-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-016-0104-x