Abstract
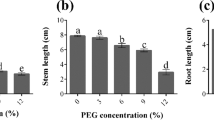
The aim of this research was to investigate the expression of important oxidoreductase genes involved in drought stress tolerance in two Brassica napus cultivars. For this purpose, morphological traits and the expression of four oxidoreductase genes within 0, 3, 6, 12, 24, 48 and 72 h after culture in deep culture medium containing 0, 3, 6, 12 and 15% polyethylene glycol 6000 (PEG 6000) were investigated. Results revealed that in both sensitive and tolerant cultivars, there was a declining trend in not only the leaf weight and area but also the stem weight and length with increasing the PEG level. In both cultivars, changes in the expression of NDH were approximately the same at different PEG levels. The tolerant cultivar showed higher expression of FNR than the sensitive cultivar. The expression of GR1 and GPX1 genes was higher in Hyola 308 than SLM 046.








Similar content being viewed by others
References
Panda SK, Khan MK (2004) Changes in growth and superoxide dismutase activity in Hydrilla verticillata L. under abiotic stress. Braz J Plant Physiol 16(2):115–118
Sairam R, Saxena D (2000) Oxidative stress and antioxidants in wheat genotypes: possible mechanism of water stress tolerance. J Agron Crop Sci 184(1):55–61
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Arakaki A, Ceccarelli EA, Carrillo N (1997) Plant-type ferredoxin-NADP+ reductases: a basal structural framework and a multiplicity of functions. FASEB J 11(2):133–140
Shinohara F, Kurisu G, Hanke G, Bowsher C, Hase T, Kimata-Ariga Y (2017) Structural basis for the isotype-specific interactions of ferredoxin and ferredoxin: NADP+ oxidoreductase: an evolutionary switch between photosynthetic and heterotrophic assimilation. Photosynth Res 134(3):281–289
Hanke G, Mulo P (2013) Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ 36(6):1071–1084
Mulo P (2011) Chloroplast-targeted ferredoxin-NADP+ oxidoreductase (FNR): structure, function and location. Biochimica et Biophysica Acta (BBA)-Bioenergetics 17(8):927–934
Takabayashi A, Endo T, Shikanai T, Sato F (2002) Post-illumination reduction of the plastoquinone pool in chloroplast transformants in which chloroplastic NAD(P)H dehydrogenase was inactivated. Biosci Biotechnol Biochem 66(10):2107–2111
Yamamoto H, Peng L, Fukao Y, Shikanai T (2011) An Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 23(4):1480–1493
Mir-Hosseini-Dehabadi SR (1994) The effect of water stress on water relations, carbon isotype discrimination, and shoot and root growth of sainfoin (Onobrychis viciifolia Scop.) and lucerne (Medicago sativa L.): a thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Department of Plant Science at Massey University
Tausz M, Sircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid. J Exp Bot 55(404):1955–1962
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197(1–4):323
Bela K, Horváth E, Gallé Á, Szabados L, Tari I, Csiszár J (2015) Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol 176:192–201
Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43(1):83–116
Noctor G, Dutilleul C, De Paepe R, Foyer CH (2004) Use of mitochondrial electron transport mutants to evaluate the effects of redox state on photosynthesis, stress tolerance and the integration of carbon/nitrogen metabolism. J Exp Bot 55(394):49–57
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Shan C, Liang Z (2010) Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci 178(2):130–139
Rane J, Maheshwari M, Nagarajan S (2001) Effect of pre-anthesis water stress on growth, photosynthesis and yield of six wheat cultivars differing in drought tolerance. Indian J Plant Physiol 6(1):53–60
Sheykh F, Tourchi M, Valizadeh M, Shakiba MR, Pasban Eslam B (2005) Drought resistance evaluation in spring rapeseed cultivars. J Agric Sci 15(1):163–174
Anjum F, Yaseen M, Rasool E, Wahid A, Anjum S (2003) Water stress in barley (Hordeum vulgare L.). II. Effect on chemical composition and chlorophyll contents. Pak J Agric Sci 40:45–49
Movahhedy DM, Modares Sanavi SAM, Soroushzadeh A, Jalali M (2004) Changes in proline, total soluble sugars, SPAD and chlorophyll fluorescence in winter safflower cultivars under drought stress and foliar application of zinc and manganese. Desert 9(1):93–109
Lal B, Kaushik S, Gautam R (1994) Effect of soil-moisture regime, kaolin spray and phosphorus fertilizer on growth, yield and economics of lentil (Lens culinaris). Indian J Agron 39(2):241–245
Ahmad S, Ahmad R, Ashraf MY, Ashraf M, Waraich EA (2009) Sunflower (Helianthus annuus L) response to drought stress at germination and seedling growth stages. Pak J Bot 41(2):647–654
Nayyar H, Kaushal S (2002) Chilling induced oxidative stress in germinating wheat grains as affected by water stress and calcium. Biol Plant 45(4):601–604
Endo T, Shikanai T, Takabayashi A, Asada K, Sato F (1999) The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett 457(1):5–8
Casano LM, Zapata JM, Martín M, Sabater B (2000) Chlororespiration and poising of cyclic electron transport plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J Biol Chem 275(2):942–948
Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. In: Abiotic stress responses in plants. Springer, New York, NY, pp 149–158
Ding S, Lu Q, Zhang Y, Yang Z, Wen X, Zhang L, Lu C (2009) Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol Biol 69(5):577–592
Dixon DP, Cummins L, Cole DJ, Edwards R (1998) Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol 1(3):258–266
Yang SL, Yu PL, Chung KR (2016) The glutathione peroxidase-mediated reactive oxygen species resistance, fungicide sensitivity and cell wall construction in the citrus fungal pathogen Alternaria alternata. Environ Microbiol 18(3):923–935
Acknowledgements
The authors are grateful to The Guilan University, Biotechnology Laboratory collaborators for the constant support throughout this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to publish this manuscript.
Additional information
Significance statement
The authors tried to simulate bioreactor conditions by using suspension deep culture. For understanding exact gene expression and phenotypic changes, drought levels (0, 3, 6, 12 and 15% w/v) and harvesting times (0, 3, 6, 12, 24, 48 and 72 h) were exclusively extended.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebrahimi, F.S.S., Kumleh, S.H.H. & Rezadoost, M.H. Morphological Changes and the Expression of Oxidoreductase Genes of Brassica napus in Deep Culture Media Containing PEG. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 1311–1318 (2019). https://doi.org/10.1007/s40011-018-1050-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-018-1050-5