Abstract
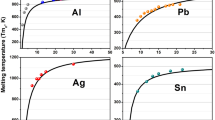
The size-dependent cohesive energy, melting temperature, Debye temperature and lattice heat capacity are investigated using density functional theory within generalized gradient approximation of Sn nanoparticles. The analyses of the obtained total energies are presented by considering effect of mean bond length and the ratio number of surface atoms to that of its internal with size. The cohesive energy is calculated for Sn nanoparticles and the obtained data are used to determine melting temperature, Debye temperature and lattice heat capacity. The cohesive energy, melting point and Debye temperature drop while the lattice specific heat rise when the size is decreased due to the effects of the elevated bond length stretch. The results obtained are in excellent agreement with the available experimental results for melting point and Debye temperature of Sn nanoparticles. Also, the same trend variations of the lattice heat capacity obtained for Sn nanoparticles to that calculated theoretically in Se and Cu.




Similar content being viewed by others
References
Gleiter H (2000) Nanostructured materials: basic concepts and microstructure. Acta Mater 48(1):1–27
Chamaani A, Marzbanrad E, Rahimipour MR, Yaghmaee MS, Aghaei A, Kamachali RD, Behnamian Y (2011) Thermodynamics and molecular dynamics investigation of a possible new critical size for surface and inner cohesive energy of Al nanoparticles. J Nanopart Res 13(11):6059–6067
Yang CC, Li S (2007) Investigation of cohesive energy effects on size-dependent physical and chemical properties of nanocrystals. Phys Rev B 75(16):165413
Langreth DC, Perdew JP (1980) Theory of nonuniform electronic systems. I. Analysis of the gradient approximation and a generalization that works. Phys Rev B 21(12):5469–5493
Payne MC, Teter MP, Allan DC, Arias TA, Joannopoulos JD (1992) Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients. Rev Mod Phys 64(4):1045–1097
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(8):3865–3968
Gonze X, Beuken J-M, Caracas R, Detraux F, Fuchs M, Rignanese G-M, SindicL VM, Zerah G, Jollet F, Torrent M, Roy A, Mikami M, Ghosez P, Raty J-Y, Allan DC (2002) First-principles computation of material properties: the ABINIT software project. Comput Mater Sci 25(3):478–492
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane–wave calculations. Phys Rev B 43(3):1993–2006
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192
Lesar R (2013) Introduction to computational materials science: fundamentals to applications. Cambridge University Press, New York, pp 62–92
Fiolhais C, Nogueira F, Marques MA (2003) A primer in density functional theory. Springer, Berlin, pp 246–254
Omar MS (2012) Models for mean bonding length, melting point and lattice thermal expansion of nanoparticle materials. Mater Res Bull 47(11):3518–3522
Jiang Q, Shi HX, Zhao M (1999) Melting thermodynamics of organic nanocrystals. J Chem Phys 111(5):2176
Zhang Z, Li JC, Jiang Q (2000) Modelling for size-dependent and dimension-dependent melting of nanocrystals. J Phys D Appl Phys 33(20):2653–2656
Omar MS, Taha HT (2009) Lattice dislocation in Si nanowires. Physica B 404(23–24):5203–5206
Liang LH, Shen CM, Du SX, Liu WM, Xie XC, Gao HJ (2004) Increase in thermal stability induced by organic coatings on nanoparticles. Phys Rev B 70(20):205419
Avramov I, Michailov M (2008) Specific heat of nanocrystals. J Phys Condens Matter 20(29):295224
Sun CQ (2007) Size dependence of nanostructures: impact of bond order deficiency. Prog Solid State Chem 35(1):1–159
Shandiz MA (2008) Effective coordination number model for the size dependency of physical properties of nanocrystals. J Phys Condens Matter 20(32):325237
Vanithakumari SC, Nanda KK (2008) A universal relation for the cohesive energy of nanoparticles. Phys Lett A 372(46):6930–6934
Abdullah BJ, Omar MS, Jiang Q (2016) Effects of size on mass density and its influence on mechanical and thermal properties of ZrO2 nanoparticles in different structures. Bull Mater Sci 39(5):1295–1302
Yang CC, Xiao MX, Li W, Jiang Q (2006) Size effects on Debye temperature, Einstein temperature, and volume thermal expansion coefficient of nanocrystals. Solid State Commun 139(4):148–152
Lai SL, Guo JY, Petror V, Ramanath G, Allen LH (1996) Size-dependent melting properties of small tin particles: nanocalorimetric measurements. Phys Rev Lett 77(1):99–102
Hong LB, Ahn CC, Fultz B (1995) The Debye temperature of nanocrystalline β–Sn measured by X-ray diffraction. J Mater Res 10(10):2408–2410
Zhu YF, Lian JS, Jiang Q (2009) Modeling of the melting point, debye temperature, thermal expansion coefficient, and the specific heat of nanostructured materials. J Phys Chem C 113(39):16896–16900
Haas P, Tran F, Blaha P (2009) Erratum: Calculation of the lattice constant of solids with semilocal functionals. Phys Rev B 79(20):085104
Acknowledgements
The work is supported by Salahaddin-Erbil University in Kurdistan region, Iraq under Grant No. 7/54/3146-1092017 as program cooperation with Jilin University in China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdullah, B.J., Omar, M.S. & Jiang, Q. Size Effects on Cohesive Energy, Debye Temperature and Lattice Heat Capacity from First-Principles Calculations of Sn Nanoparticles. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 88, 629–632 (2018). https://doi.org/10.1007/s40010-017-0417-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-017-0417-y