Abstract
Purpose
Fexofenadine is an antihistamine drug used to treat various allergic diseases. Despite long-term clinical application, research and knowledge on setting dosage regimen based on quantitative pharmacometrics prediction are still very lacking. This study aimed to explore the dosing regimen of fexofenadine through establishment of a fexofenadine population pharmacokinetic (PK)–pharmacodynamic (PD) model and model simulation. We also assessed whether ATP-binding cassette-subfamily-B-member-1 (ABCB1) gene polymorphism is associated with interindividual variability in fexofenadine PKs.
Methods
For this study, the results of fexofenadine bioequivalence study on healthy Korean males and previously reported PK and PD results were used. Based on these data, a fexofenadine population PK–PD model was established.
Results
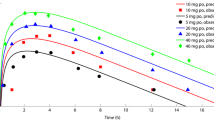
ABCB1 gene polymorphisms were not significantly correlated with interindividual PK diversity of fexofenadine. This result suggests that considering ABCB1 gene polymorphisms would not be significantly beneficial in individualized therapy using fexofenadine. The established model well explained the PK–PD outcomes of fexofenadine at various doses in healthy adults. Model simulation revealed that the maximum PD (Rmax) values at approximately 200–4000 mg doses of fexofenadine did not differ largely (approximately 10%); however, fivefold to eightfold differences were noted between Rmax and the subsequent minimum PD (Rmin) values. Moreover, the degree of decrease in the average fexofenadine PDs in the steady–state according to increase in the dosing interval (6–24 h) for same dose was within 5%, whereas the difference between Rmax and Rmin for prolonging PD response was as large as 7%. This result suggests that effective therapy for allergy could involve increasing oral dosage of fexofenadine and reducing dosing interval to prolong the PD effect.
Conclusion
Our findings can provide a clearer direction for personalized therapy using fexofenadine in clinical setting through qualitative–quantitative predictions.




Similar content being viewed by others
Data availability
All data and related materials are accessible in this manuscript and supplementary materials.
References
Akamine Y, Miura M, Sunagawa S, Kagaya H, Yasui-Furukori N, Uno T (2010) Influence of drug-transporter polymorphisms on the pharmacokinetics of fexofenadine enantiomers. Xenobiotica 40:782–789
Cho H-Y, Kang H-A, Kim S-M, Lee Y-B (2008) Bioequivalence test of fexofenadine hydrochloride 120 mg tablets. Yakhak Hoeji 52:188–194
Craun KL, Schury MP (2021) In: StatPearls. StatPearls Publishing
Deschamps C, Dubruc C, Mentre F, Rosenzweig P (2000) Pharmacokinetic and pharmacodynamic modeling of mizolastine in healthy volunteers with an indirect response model. Clin Pharm Therap 68:647–657
Devillier P, Roche N, Faisy C (2008) Clinical pharmacokinetics and pharmacodynamics of desloratadine, fexofenadine and levocetirizine. Clin Pharmacokinet 47:217–230
Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF (2002) MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol 53:526–534
Imanaga J, Kotegawa T, Imai H, Tsutsumi K, Yoshizato T, Ohyama T, Shirasaka Y, Tamai I, Tateishi T, Ohashi K (2011) The effects of the SLCO2B1 c. 1457C> T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics 21:84–93
Jang J-H, Jeong S-H, Cho H-Y, Lee Y-B (2019) Population pharmacokinetics of cis-, trans-, and total cefprozil in healthy male Koreans. Pharmaceutics 11:531–550
Jauregizar N, De La Fuente L, Lucero ML, Sologuren A, Leal N, Rodríguez M (2009) Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) effect of bilastine. Clin Pharmacokinet 48:543–554
Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B (2020) Population pharmacokinetic analysis of tiropramide in healthy Korean subjects. Pharmaceutics 12:374–393
Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B (2021a) Population pharmacokinetic analysis of cefaclor in healthy Korean subjects. Pharmaceutics 13:754–773
Jeong S-H, Jang J-H, Lee Y-B (2021b) Pharmacokinetic comparison between methotrexate-loaded nanoparticles and nanoemulsions as hard-and soft-type nanoformulations: a population pharmacokinetic modeling approach. Pharmaceutics 13:1050–1075
Jeong S-H, Jang J-H, Cho H-Y, Lee Y-B (2022a) Population Pharmacokinetic (Pop-PK) analysis of torsemide in healthy korean males considering CYP2C9 and OATP1B1 genetic polymorphisms. Pharmaceutics 14:771–795
Jeong S-H, Jang J-H, Lee Y-B (2022b) Population pharmacokinetic analysis of lornoxicam in healthy Korean males considering creatinine clearance and CYP2C9 genetic polymorphism. J Pharm Investig 52:109–127
Jeong S-H, Jang J-H, Lee Y-B (2022c) Physiologically Based Pharmacokinetic (PBPK) modeling of lornoxicam: exploration of doses for CYP2C9 genotypes and patients with cirrhosis. J Pharm Sci 111:3174–3184
Kim S-M, Park S, Cho H-Y, Lee Y-B (2008) Haplotype analysis of MDRI gene (Exon 12, 21 and 26) in Korean. J Pharm Investig 38:365–372
Krishna R, Krishnaswami S, Kittner B, Sankoh AJ, Jensen BK (2004) The utility of mixed-effects covariate analysis in rapid selection of doses in pediatric subjects: a case study with fexofenadine hydrochloride. Biopharm Drug Dispos 25:373–387
Martinez J-M, Khier S, Morita S, Rauch C, Fabre D (2014) Population pharmacokinetic analysis of fexofenadine in Japanese pediatric patients. J Pharmacokinet Pharmacodyn 41:187–195
Mason J, Reynolds R, Rao N (1999) The systemic safety of fexofenadine HCl. Clin Exp Allergy 29:163–170
Niemi M, Kivistö KT, Hofmann U, Schwab M, Eichelbaum M, Fromm MF (2005) Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1). Br J Clin Pharmacol 59:602–604
Russell T, Stoltz M, Weir S (1998) Pharmacokinetics, pharmacodynamics, and tolerance of single-and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharm Therap 64:612–621
Sharma A, Jusko WJ (1998) Characteristics of indirect pharmacodynamic models and applications to clinical drug responses. Br J Clin Pharmacol 45:229–239
Shipkova M, Christians U (2019) Improving therapeutic decisions: pharmacodynamic monitoring as an integral part of therapeutic drug monitoring. Ther Drug Monit 41:111–114
Shon JH, Yoon YR, Hong WS, Nguyen PM, Lee SS, Choi YG, Cha IJ, Shin JG (2005) Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharm Therap 78:191–201
Simpson K, Jarvis B (2000) Fexofenadine. Drugs 59:301–321
Yi SY, Hong KS, Lim HS, Chung JY, Oh DS, Kim JR, Jung HR, Cho JY, Yu KS, Jang IJ (2004) A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharm Therap 76:418–427
Zimprich F, Sunder-Plassmann R, Stogmann E, Gleiss A, Dal-Bianco A, Zimprich A, Plumer S, Baumgartner C, Mannhalter C (2004) Association of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsy. Neurology 63:1087–1089
Acknowledgements
This work was supported by a Research promotion program of SCNU.
Author information
Authors and Affiliations
Contributions
S-HJ: Conceptualization, investigation, methodology, writing—original draft, writing—review and editing, software, data analysis, and visualization; J-HJ: investigation, methodology, writing—review and editing, software, and data analysis; and Y-BL: conceptualization, methodology, writing—review and editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
All authors (J.H. Jang, S.H. Jeong, and Y.B. Lee) have no conflicts of interest relevant to this study to disclose.
Human and animal rights
This study protocol was thoroughly reviewed and approved by the Institutional Review Board of the Institute of Bioequivalence and Bridging Study, Chonnam National University, Gwangju, Republic of Korea. The bioequivalence study permit number was as follows: 926; 04.22.2006.
Consent to participate
All subjects provided written informed consent prior to their participation in bioequivalence and pharmacokinetic studies.
Consent for publication
All data were anonymized and participants were informed that the results of this study may be subject to publication and presentation in meetings.
Clinical trial registration
The clinical study protocol used in this study was thoroughly reviewed and approved by the Institutional Review Board of the Institute of Bioequivalence and Bridging Study, Chonnam National University, Gwangju, Republic of Korea. The bioequivalence study permit numbers are as follows: 926; 04.22.2006.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, JH., Jeong, SH. & Lee, YB. Establishment of a fexofenadine population pharmacokinetic (PK)–pharmacodynamic (PD) model and exploration of dosing regimens through simulation. J. Pharm. Investig. 53, 427–441 (2023). https://doi.org/10.1007/s40005-023-00615-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00615-0