Abstract
Purpose
Preliminary evidence suggests a potential effect of antiviral medication used during the acute COVID-19 phase for preventing long-COVID. This review investigates if having received pharmacological treatment during acute SARS-CoV-2 infection may reduce the risk of long-COVID.
Methods
MEDLINE, CINAHL, PubMed, EMBASE, Web of Science databases, as well as medRxiv/bioRxiv preprint servers were searched up to July 15th, 2023. Articles comparing the presence of long-COVID symptoms between individuals who received or not a specific medication, particularly antivirals, during the acute phase of SARS-CoV-2 infection were included. Methodological quality was assessed using the Newcastle–Ottawa Scale or Cochrane’s Risk of Bias (RoB) tool.
Results
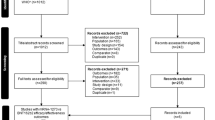
From 517 studies identified, 6 peer-reviewed studies and one preprint met all inclusion criteria. The sample included 2683 (n = 4) hospitalized COVID-19 survivors and 307,409 (n = 3) non-hospitalized patients. The methodological quality was high in 71% of studies (n = 5/7). Two studies investigating the effects of Nirmatrelvir/Ritonavir and three studies investigating the effect of Remdesivir reported conflicting results on effectiveness for preventing long-COVID. Three studies investigating the effects of other medication such as Dexamethasone (n = 2) or Metformin (n = 1) found positive results of these medications for preventing long-COVID.
Conclusion
Available evidence about the effect of medication treatment with antivirals during acute COVID-19 and reduced risk of developing long-COVID is conflicting. Heterogeneous evidence suggests that Remdesivir or Nirmatrelvir/Ritonavir could have a potential protective effect for long-COVID. A limited number of studies demonstrated a potential benefit of other medications such as Dexamethasone or Metformin, but more studies are needed.



Similar content being viewed by others
Data availability
All data derived from the study is presented in the current text.
Change history
07 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s15010-024-02208-x
References
Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med. 2020;17: e1003501.
Rahmah L, Abarikwu SO, Arero AG, Essouma M, Jibril AT, Fal A, Flisiak R, Makuku R, Marquez L, Mohamed K, Ndow L, Zarębska-Michaluk D, Rezaei N, Rzymski P. Oral antiviral treatments for COVID-19: opportunities and challenges. Pharmacol Rep. 2022;74:1255–78.
https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 15 July 2023)
Tian F, Chen Z, Feng Q. Nirmatrelvir-ritonavir compared with other antiviral drugs for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2023;95: e28732.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM, EPIC-HR Investigators. Oral nirmatrelvir for high-risk, non-hospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–408.
Amstutz A, Speich B, Mentré F, et al. Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir Med. 2023;11:453–64.
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA, GS-US-540-9012 (PINETREE) Investigators. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305–15.
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, Zhou X, Wu Q, Zhang X, Feng Z, Wang M, Mao Q. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516–23.
Zadeh F, Wilson D, Agrawal D. Long COVID: complications, underlying mechanisms, and treatment strategies. Arch Microbiol Immunol. 2023;7:36–61.
Fernández-de-las-Peñas C. Long COVID: current definition. Infection. 2022;50:285–6.
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–7.
Global Burden of Disease Long COVID Collaborators, Wulf Hanson S, Abbafati C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328:1604–15.
Rahmati M, Udeh R, Yon DK, Lee SW, Dolja-Gore X, McEVoy M, Kenna T, Jacob L, López Sánchez GF, Koyanagi A, Shin JI, Smith L. A systematic review and meta-analysis of long-term sequelae of COVID-19 2-year after SARS-CoV-2 infection: a call to action for neurological, physical, and psychological sciences. J Med Virol. 2023;95: e28852.
Suran M. VA Finds nirmatrelvir associated with lower risk of long COVID. JAMA. 2022;328:2386.
Peluso MJ, Anglin K, Durstenfeld MS, et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun. 2022;7:95–103.
Sebők S, Gyires K. Long COVID and possible preventive options. Inflammopharmacology. 2023. https://doi.org/10.1007/s10787-023-01204-1.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n7.
Wells GA, Tugwell P, O’Connell D, Welch V, Peterson J, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses 2015.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19. medRxiv 2022.11.03.22281783; https://doi.org/10.1101/2022.11.03.22281783
Boglione L, Meli G, Poletti F, Rostagno R, Moglia R, Cantone M, Esposito M, Scianguetta C, Domenicale B, Di Pasquale F, Borrè S. Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM. 2022;114:865–71.
Badenes Bonet D, Caguana Vélez OA, Duran Jordà X, et al. Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19. J Clin Med. 2023;12:4158.
Nevalainen OPO, Horstia S, Laakkonen S, et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat Commun. 2022;13:6152.
Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med. 2023;183:554–64.
Chuang MH, Wu JY, Liu TH, Hsu WH, Tsai YW, Huang PY, Lai CC. Efficacy of nirmatrelvir and ritonavir for post-acute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection. J Med Virol. 2023;95: e28750.
Bramante CT, Buse JB, Liebovitz DM, et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023;S1473–3099:00299–302.
Milne A, Maskell S, Sharp C, Hamilton FW, Arnold DT. Impact of dexamethasone on persistent symptoms of COVID-19: an observational study. medRxiv; doi: https://doi.org/10.1101/2021.11.17.21266392
National Institute for Health and Care Excellence (NICE), Royal College of General Practitioners, Healthcare Improvement Scotland SIGN. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National institute for health and care excellence, 2020. www.nice.org.uk/ guidance/ng188 (accessed Oct 30, 2022).
Singh RSP, Toussi SS, Hackman F, Chan PL, Rao R, Allen R, Van Eyck L, Pawlak S, Kadar EP, Clark F, Shi H, Anderson AS, Binks M, Menon S, Nucci G, Bergman A. Innovative Randomized phase i study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of Nirmatrelvir. Clin Pharmacol Ther. 2022;112:101–11.
Paxlovid EU. Accessed 15 July 2023. Available from: https://aspr.hhs.gov/COVID-19/Therapeutics/Products/Paxlovid Pages/emergency-use-authorization.aspx
Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J. Remdesivir against COVID-19 and other viral diseases. Clin Microbiol Rev. 2021;34:e00162-e220.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704.
Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol. 2020;11:191.
Fernández-de-las-Peñas C, Rodríguez-Jiménez J, Cancela-Cilleruelo I, Guerrero-Peral A, Martín-Guerrero JD, García-Azorín D, Cornejo-Mazzuchelli A, Hernández-Barrera V, Pellicer-Valero OJ. Post-COVID-19 symptoms 2 years after SARS-CoV-2 infection among hospitalized vs non-hospitalized patients. JAMA Netw Open. 2022;5: e2242106.
Kuri-Ayache M, Rivera-Cavazos A, Pérez-Castillo MF, Santos-Macías JE, González-Cantú A, Luviano-García JA, Jaime-Villalón D, Gutierrez-González D, Romero-Ibarguengoitia ME. Viral load and its relationship with the inflammatory response and clinical outcomes in hospitalization of patients with COVID-19. Front Immunol. 2023;13:1060840.
Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–95.
Bramante CT, Huling JD, Tignanelli CJ, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19. N Engl J Med. 2022;387:599–610.
Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. 2021;51: e13429.
Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9.
Castle BT, Dock C, Hemmat M, Kline S, Tignanelli C, Rajasingham R, Masopust D, Provenzano P, Langlois R, Schacker T, Haase A, Odde DJ. Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets. bioRxiv 2020.05.22.111237
Tran VT, Riveros C, Clepier B, et al. Development and validation of the long COVID symptom and impact tools, a set of patient-reported instruments constructed from patients’ lived experience. Clin Infect Dis. 2022;74:278–87.
Funding
The project was supported by the LONG-COVID-EXP-CM, a grant associated to the Fondo Europeo De Desarrollo Regional—Recursos REACT-UE del Programa Operativo de Madrid 2014–2020, en la línea de actuación de proyectos de I + D + i en materia de respuesta a COVID 19. The sponsor had no role in the design, collection, management, analysis, or interpretation of the data, draft, review, or approval of the manuscript or its content. The authors were responsible for the decision to submit the manuscript for publication, and the sponsor did not participate in this decision.
Author information
Authors and Affiliations
Contributions
All the authors cited in the manuscript had substantial contributions to the concept and design, the execution of the work, or the analysis and interpretation of data; drafting or revising the manuscript and have read and approved the final version of the paper. CF: conceptualization, visualization, methodology, validation, data curation, writing—original draft, writing—review and editing. JT-M: conceptualization, validation, data curation, writing—review and editing. JAC: methodology, validation, data curation, writing—original draft, writing—review and editing. RM: methodology, validation, data curation, writing—original draft, writing—review and editing. JVV: methodology, validation, data curation, writing—original draft, writing—review and editing. SM: methodology, validation, data curation, writing—original draft, writing—review and editing. MC: validation, writing—review and editing. BMH: validation, writing—review and editing. GL: review and editing. AF-M: methodology, validation, writing—review and editing. KIN: conceptualization, visualization, methodology, validation, data curation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest is declared by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández-de-las-Peñas, C., Torres-Macho, J., Catahay, J.A. et al. Is antiviral treatment at the acute phase of COVID-19 effective for decreasing the risk of long-COVID? A systematic review. Infection 52, 43–58 (2024). https://doi.org/10.1007/s15010-023-02154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02154-0