Abstract
Background
Currently, there are no standardized guidelines for the diagnosis or management of the complications of urogenital schistosomiasis (UGS). This systematic review of the literature aims to investigate the state of the art in reference to diagnostic approaches and the clinical management of this condition.
Methods
A systematic review of literature published between January 1990 and January 2021 was conducted in the MEDLINE database, scoping for articles regarding diagnostic means or therapeutic options for the complications of UGS, namely obstructive uropathy, bladder cancer, abortion, ectopic pregnancy, infertility, kidney failure, urolithiasis and the need for invasive procedures. Relevant data were then extracted from the articles deemed eligible according to the inclusion criteria.
Main results
In total, 3052 articles were identified by the research query, of which 167 articles fulfilling inclusion criteria after title/abstract screening and full-text evaluation were included, 35% on both diagnostic and therapeutic aspects, and 51% on diagnosis and 14% on therapy. Ultrasound was the most frequently tool employed for the diagnosis of UGS complications showing a good performance. Concerning the management of hydronephrosis, the majority of available evidences came from community-based studies where universal treatment with praziquantel was used leading to decrease of prevalence of obstructive uropathy. Concerning studies on surgical procedures, laser endoureterotomy followed by stenting was mostly employed in adult patients leading to a crude cure rate of 60% (43 of 71 patients). In the case of severe hydronephrosis, surgery consisting of ureteral re-implantation showed excellent results with a crude cure rate of 98% (157 cured patients of 160 treated). Concerning bladder cancer, data on 93 patients with a clear diagnosis of UGS-related bladder were available reporting a variable and sometime combined approach based on disease stage. Available data on diagnosis and management of abortion, ectopic pregnancy, infertility, kidney failure, urolithiasis and the need for invasive procedures due to UGS are also presented.
Conclusions
The review produced a complete picture of the diagnostic and therapeutic options currently available for complicated UGS. These results can be useful both for guiding clinicians towards correct management and for tracing the direction of future research.
Similar content being viewed by others
Background
Schistosomiasis is a parasitic neglected tropical disease (NTD) caused by trematodes belonging to the genus Schistosoma. There are two main clinical forms of the disease, the gastrointestinal and the urogenital. Urogenital schistosomiasis (UGS) is caused by Schistosoma haematobium, mostly endemic in Africa and the Middle East [1]. S. haematobium is prone to hybridization with several zoonotic Schistosoma species and this feature may have important ecological, diagnostic and therapeutic implications, currently not completely understood [2]. S. haematobium globally affects 112 million subjects of which 90% in Sub-Saharan Africa [3]; however, the disease represents a relevant issue also in non-endemic countries since it may affect migrants and travellers returning from endemic countries [4, 5]. Recently, some foci of autochthonous transmission have been identified in the Mediterranean area in Almería, Spain and in Corsica, France, the latter due to a S. haematobium/S. bovis hybrid species [6,7,8,9].
S. haematobium adult worms reside in the peri-vesical venous plexus. Eggs laid by females must reach the urinary tract to be released in the environment with urine and perpetuate the parasite transmission cycle. However, a proportion of eggs is trapped in the tissues of urogenital organs (e.g. bladder wall), and elicits a granulomatous inflammatory reaction followed by fibrosis [10]. Due to the consequences of this chronic inflammation, between 3.5% and 20% of affected subjects develops urogenital complications which include hydronephrosis leading to kidney failure, urolithiasis, ectopic pregnancy, infertility and bladder cancer [11, 12]. The latter is estimated to be responsible of 13,300 death per year [3].
Complicated urogenital schistosomiasis (cUGS) is potentially extremely harmful and carries a significant health burden for patients and health systems in both endemic and non-endemic areas [12, 13]. Its management is complex and often not addressed by international guidelines [14].
In high-resource countries, the management of cUGS may be suboptimal due to low awareness of health care workers in non-endemic setting leading to diagnostic delay and unnecessary use of invasive procedures [15, 16].
A systematic review of the literature was conducted to assess the current level of evidence on cUGS diagnosis and management within the activities of the TropNet Schisto Task Force (http://tropnet.eu/). Two main questions pertaining to the clinical management of cUGS were investigated: (i) What are the diagnostic methods and their performance for cUGS in endemic and non-endemic settings? (ii) What are the treatment strategies and their performance for cUGS in endemic and non-endemic settings?
Materials and methods
Definitions of cUGS used for this review are provided in Fig. 1. The search strategy is available in the Supplementary materials and methods.
Inclusion criteria
Inclusion criteria were:
-
Papers written in Italian, English, French or Portuguese.
-
Case reports, case series, clinical trials, retrospective, prospective and cross-sectional studies reporting original data on the diagnosis and/or clinical management of patients satisfying the definition cUGS.
Exclusion criteria
Narrative and systematic reviews, animal studies, laboratory studies, studies dealing with a species different from S. haematobium, editorials, news reports or articles only reporting radiological data (e.g. pictorial essays) or only data on pathological anatomical aspects were excluded. Potentially eligible papers for which full text was not available and the abstract, when available, did not convey the needed information, were also excluded.
Selection process
Two authors (T.M. and D.M.) conducted an initial independent assessment of the articles by title and abstract screening, followed by evaluation of the full texts. If doubts over eligibility were present, a third author (L.Z.) reviewed the publications to reach a collegial decision. Data extracted can be found in Supplementary Materials and Methods. For studies other than case reports or case series, data are presented as summaries of findings using Effect Direction Plots [17], grouped by paper focus (diagnostic or treatment) and by treatment category. Study type, effect direction of interventions on outcomes, differences between interventions or from baseline (together with statistical significance when reported), and sample size were visually plotted to provide an overall appraisal of the extracted data quality, characteristics and heterogeneity. No meta-analysis and formal quality assessment of extracted data were planned, expecting most articles to be case report or case series.
Results
Article type, setting, populations
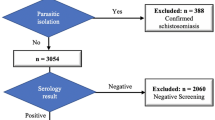
Figure 2 shows search and selection results. Of the initial retrieved references (n = 3052), 167 were included in the review. Eighty-six (51.5%) articles dealt with diagnostic aspects only, 58 (34.7%) dealt with both diagnostic and therapeutic aspects and 23 (13.8%) dealt with therapeutic aspects only. Amongst 144 studies dealing with diagnostic aspects, 60 (41.7%) were carried out in centres in non-endemic areas, 77 (53.5%) were conducted in Africa and 7 (4.9%) in the Middle East. Data on the diagnosis of cUGS were available from 8,093 patients; of these patients, 7997 (98.8%) were residents in endemic areas, 49 (0.6%) were travellers, 45 (0.6%) were migrants and 2 (0.1%) were expatriates.
In studies on treatment, 3648 patients were found to have information relevant to the review for any of the cUGS clinical manifestations. Amongst 81 studies dealing with diagnostic aspects, 34 (42.0%) were carried out in centres in non-endemic areas, 41 (50.6%) were conducted in Africa and 6 (7.4%) in the Middle East. Data on the treatment of cUGS were available for 3648 patients, 3564 (97.7%) of whom were residents in endemic areas, 48 (1.3%) were travellers, 36 (1.0%) were migrants and 1 (0.1%) was an expatriate.
Most articles were case reports or case series (n = 81, 56.2% and n = 57, 70.4% respectively), followed by retrospective or transversal studies (n = 63, 43.8% and n = 8, 9.9%). Prospective studies were present only in the treatment group (n = 14, 17.3%) and only two randomized clinical trials were retrieved in the treatment group (2.5%). Figure 3 summarizes data on study and patient type included in this review, whilst Table 1 shows the different complications described in the included papers. As shown by the data presented in Tables 2 and 3 and in the Supplementary tables, reporting of data on age and sex of patients with UGS is erratic at best, with several papers failing to report information on sex and age. If data were presented, summary metrics used were different between similar sized populations.
Diagnosis
Obstructive uropathy, kidney failure and urolithiasis
Ultrasound (US) was the most commonly used to diagnose obstructive uropathy (OU). US was employed following the Niamey protocol [18] in 19 studies over the 31 found to deal with the diagnosis of OU. Other radiological methods found to be used were CT scans (8 studies), intravenous urography (7 studies), renal scintigraphy (2 studies) and cystography (2 studies). Plain X-rays were used in 8 reports. Whilst 12 studies have used US alone for the diagnosis of OU [12, 19,20,21,22,23,24,25,26,27,28,29], others have used two methods to confirm the complication (intravenous urography in five case reports [30,31,32,33,34], anterior urography in one patient from a case series [33], CT scan in one case report [35]) or an invasive procedure (cystoscopy in one case report [35]). Very few studies compared the performances of radiological methods: only one study compared the performances of intravenous urography (considered the gold standard) and cystography, finding suboptimal performances of the latter (Sensitivity (Se) 26.8%; specificity (Sp) 66.7%; positive predictive value (PPV) 84.6%; negative predictive value (NPV) 11.8%) [36]. US proved superior to intravenous urography as one study found better performances (Se 89.5%; Sp 63.6%; PPV 81.0%; NPV 77.8%) and the ability to show hydronephrosis in patients whose kidneys were not visible via intravenous urography [37]. As said, intravenous urography has mainly been used as a gold standard in evaluating other diagnostic techniques [36, 37] or to confirm ultrasonographic results [30,31,32,33,34]. One study assessed the sensitivity and specificity of cystography for the diagnosis of kidney failure in the setting of OU, but found no statistical difference in renal deterioration between patients with and without vesicoureteral reflux [36]. A case report [38] illustrated a case of S. haematobium-associated glomerulopathy, a phenomenon more frequently associated with S. mansoni [39,40,41,42,43,44,45,46,47]. One study on 10 patients looked at the ability of CT scans to show the typical alterations of UGS, including OU [48]. CT scans were also described with comparable results in another 8 case reports [35, 49,50,51,52,53,54,55].
Some studies tried to use non-radiological means to predict the presence of OU: one study assessed the role of egg burden (proposing a cut-off of 10 eggs/ml of urine). However, the use of this marker showed a very low sensitivity (25%) when compared to US with an expected good specificity (93%) [56]. Another study tried to find a correlation between the genetic composition of the parasitic population and the presence of hydronephrosis, with the presence of some genetic clusters showing a sensitivity of 81.8% and the absence of cluster 7 showing a sensitivity of 90.9% [57].
Stones in the urinary tract were reported as cUGS in three articles [26, 37, 56]. Two reports compared US to plain X-rays to diagnose the presence of stones, with a sensitivity of 66.7 and 70%, respectively, and better specificity (90 and 97%) [26, 37]. As expected, stones in the ureter were less easy to spot by US whilst a sensitivity of 100% was found in searching for stones in the bladder in one study [37]. Supplementary Table 1 shows all the papers dealing with the diagnosis of OU. Visual effect plots for studies with at least 10 participants dealing with the diagnosis of UO are presented in Table 2.
Bladder cancer
Forty-five studies dealt with the diagnosis of bladder cancer. The most commonly employed radiological method was US. All articles reported on the presence of cancers associated to schistosomiasis confirmed by histological examination. Thirty-six studies also reported data on the use of other techniques. Radiological methods included US in five studies, CT scan in six studies, positron emission tomography (PET) in one case report and urography in one case report. Twenty-two studies evaluated the role of urinary markers, whilst one study only relied on urinary cytology. Cystoscopy was used in three studies. Again, studies comparing different methods were scarce, with one study comparing US to cystoscopy on eighty patients with confirmed squamous cell carcinomas and S. haematobium infection showed a good sensitivity (88.9%) and excellent specificity (100%) in detecting masses seen at cystoscopy [58]. The only study evaluating the use of urinary cytology detected alterations in 3 of 32 patients with bladder cancer and schistosomiasis [59]. Supplementary Table 2 shows all the papers dealing with the diagnosis of urinary cancers associated with schistosomiasis.
Amongst studies evaluating non-instrumental tools for the diagnosis of Schistosoma-related bladder cancer, one report evaluated the dosage of urinary CEA. Eighty-six per cent of patients with Schistosoma-related bladder cancer presented elevated value of urinary CEA, compared to 62% of patient with bladder cancer not related to Schistosoma and 0% of controls without cancer nor schistosomiasis [60]. Although the authors did not test for differences between schistosomal and non-Schistosoma-associated bladder cancers, there was no statistical difference applying a t test to the data presented in the article (p = 0.1076, mean CEA 74.27 ± 64.96 SD vs 43.86 ± 55.66 SD). LDH was employed in one study, but no differences were found comparing patients with schistosomiasis-associated cancers with patients with active schistosomiasis and no evidence of cancer, patients with non-Schistosoma associated bladder cancer and uninfected patients with hepatocellular carcinoma [61]. Several studies (Table 3) explored the use of a plethora of urinary markers to distinguish cancers due to chronic schistosomiasis and those due to other causes. However, all these studies were limited by the lack of data on the presence of other risk factors for cancer. A small pilot study (6 patients per group) on differences in the circulating amino acids pattern in patients with schistosomal squamocellular bladder cancer, chronic UGS patients and controls found that there were differences between UGS patients and controls, but no differences between chronic UGS and cancer patients [61]. Another study found an increase in the levels of HPV-17 DNA in the serum of UGS cancer patients compared to both healthy controls and non-UGS cancer patients. However, numbers in this study were also small with 24 patients per group [62]. Excluding studies on schistosomiasis-related bladder cancer which are reported in details in Table 3, visual effect plots for additional eligible studies with at least 10 participants dealing with the diagnosis of bladder cancers are presented in Table 2.
Ectopic pregnancy and infertility
Data on diagnosis of ectopic pregnancy were reported in 13 articles, all case reports or case series. In all instances, the presence of Schistosoma eggs was detected only after surgery, when histology was performed. Diagnoses were made dosing β-HCG levels, clinically or at US [63,64,65,66,67,68,69,70,71,72,73,74,75]. Of note, no women were screened for the presence of Schistosoma eggs in urine before the procedure, and only in two instances, eggs were searched for after the intervention: in one case (a migrant woman recently immigrated to the United States from Africa), eggs were present in urine [63], and in another, patient (a traveller) urine samples were negative [68].
Male infertility was the subject of two case reports. In one instance, the correlation with Schistosoma infection was made due to the presence of granulomas seen at histological examination of a testicular mass [76]. In the other, azoospermia was deemed obstructive after testosterone and gonadotropin dosing and the causal relationship was inferred due to the history of schistosomiasis. This single report does not mention results from serology or parasitological results [77].
Female infertility was the subject of 17 articles, mostly case reports (n = 7) or case series (n = 3). Various techniques were used to characterize the diagnosis of infertility, including US (both transabdominal [78] and transvaginal [79, 80], CT scan, [80] hysterosalpingography [79, 81,82,83,84] and hysteroscopy [65]. In the ten case reports/series, the diagnosis was made after histological examination of either biopsies or surgical specimens of the fallopian tubes [65, 79, 80, 83,84,85,86,87], ovaries [79, 82, 84,85,86], peritoneum [65, 85], and cervix [88]. Reasons for these invasive procedures included ultrasonographic or radiographic evidence of hydrosalpinges and subsequent surgery [65, 79, 80, 83,84,85] and an atypical laparoscopic aspect of a lesion thought to be endometriosis [86]. In five patients, egg search in the urine was performed, but was negative in all instances [65, 85, 86].
In one cross-sectional study of 109 women with infertility, a clinical diagnosis was employed considering a combination of history of haematuria, genital or urological lesions detected by US and a positive eggs count in urine to identify cases, with 13 women having a positive eggs count over the whole cohort of 109 patients (11.9%). All the 42 cervical smears in this study were negative for S. haematobium eggs, whilst two biopsies were positive for the presence of eggs [81]. Another study found that amongst 23 women with infertility, 4 had a smear positive for eggs presence [89]. The latter study concluded that there was a significant association between the presence of eggs and infertility. In another study using histological positivity for S. haematobium eggs in cervical biopsies as the definition for female genital schistosomiasis, 19 of 31 women were found to be infertile. Five women with negative cervical biopsies and eggs in urine were also infertile [90]. Supplementary Table 3 shows a summary of all articles dealing with ectopic pregnancy and male or female infertility. No papers dealing with abortions were retrieved. Visual effect plots for eligible articles dealing with the diagnosis of female infertility are presented in Table 2.
Invasive procedures
Twenty-four patients underwent invasive procedures in the suspect of a disease different than schistosomiasis (e.g. bladder cancer unrelated to schistosomiasis). Most patients (20 of 24, 86%) were migrants from endemic countries. Most cases (19 of 24, 70%) were examined after urogenital manifestations seen clinically or by US (n = 9 granulomatous lesions observed at US examination, n = 5 polyps observed at US, n = 4 masses observed at US, n = 1 persistent haematuria). Serology was performed only in two patients and was positive in both [91, 92]. Urine filtration and eggs search was performed in 15 patients and was positive in nine (60%) [92,93,94,95,96,97,98,99,100,101,102,103,104,105,106]. A summary of this studies is presented in Supplementary Table 4.
Treatment
Obstructive uropathy, urolithiasis and kidney failure
Twenty-three articles concerned the treatment of OU in cUGS patients. Two articles (8.7%) reported the use of medical anthelmintic treatment, with no distinction made over the use of PZQ or metrifonate, eight (34.3%) only reported using PZQ and one (4.3%) only metrifonate. Twelve articles (47.8%) dealt with the use of surgery, one using robotic surgery. Laser endoureterotomy followed by stenting was used in five studies (23%).
Studies on medical therapy showed varying methodologies. Some studies did not allow the use of individual-patient data as authors evaluated the effects of mass drug administration (MDA) on the prevalence of OU by US. One study conducted in Madagascar using PZQ (n = 472) showed the prevalence of OU reducing from 13.6 to 2.6% twelve months after the MDA campaign [107]. Another study in Niger staged an MDA intervention in adolescents and adults of two villages with a total population of 2570 people, 72.3% were treated using a single dose of 40 mg/kg PZQ. This led to a reduction of OU prevalence from 17 to 4% 36 months after treatment [108]. Studies with longer follow-up periods showed contrasting results. In a study involving a cohort of patients aged 14–19 and remotely treated with a single dose of PZQ or metrifonate in the context of an RCT followed-up for ten years (n = 132), the prevalence (0.7%) of hydronephrosis was the same as the untreated cohort [109]. Another study followed a cohort of 194 patients for thirteen years after receiving either PZQ or metrifonate. In this study, prevalence dropped to zero, starting from 14% [110].
One study analyzed the efficacy of the Boari flap technique in ureteral re-implantation surgeries for patients with chronic stenosis. In this study, 2 out of 150 patients (1.3%) had complications (one urinoma, one sepsis post-surgery) [111]. Nephrectomy was reported for two patients, but no surgical outcomes are presented in the two articles [13, 50]. In two patients, a bladder reconstruction with the creation of an ostomy, with the patient improving in the long term [31, 53].
Concerning the use of endoscopic techniques, one randomized study compared the use of two ipsilateral double-J stents vs a single double-J stent after laser endoureterotomy for the treatment of ureteral stenosis due to schistosomiasis. The use of two stents improved outcomes and was found to be most useful when the stenotic tract was longer than 1.5 cm [112].
A total of eleven patients treated with combination therapy were present in 9 case reports or series. In seven cases, a combined treatment of surgical and medical therapy was employed [30, 32,33,34, 49,50,51], the other patients were only treated surgically [35, 113]. Surgical techniques included all those found in prospective and retrospective studies (i.e. ureteral re-implantation or reconstruction or nephrectomy), and only two patients had a worsening of OU after treatment or a complication [33, 49].
Two articles concerned the treatment of urolithiasis in cUGS. One case series described the stone removal by surgical lithotomy in 17 patients, whilst two required ureteral re-implantation and two had to undergo a nephrectomy. All 21 patients improved after treatment, despite 3 developing surgical-site infections [114]. One article illustrated the use of percutaneous suprapubic cystolithotripsy as the treatment of choice for bladder stones secondary to schistosomiasis in a prospective cohort of 5 paediatric patients; all of them fully recovered without complications [115].
Four articles concerned the treatment of kidney failure. In one report on a patient with OU and AKI due to hydronephrosis, dialysis was employed followed by a percutaneous ostomy to treat the patient [54]. In another report, CKD led to the patient starting dialysis and subsequently died of sepsis [12].
A single case–control study, intended to examine the safety of three monthly doses of 60 mg/kg PZQ and their efficacy in reducing the eggs count, also examined the effect of this therapy on kidney function of 28 S. haematobium-infected patients with no renal damage. The study showed improvements of post-treatment organ function (increase of the eGFR) without significant adverse effects [116].
In a case series of four patients with CKD due to OU, two patients were managed by ureter replacement by ureteroileoplasty, whilst in two cases nephrectomy was employed. All four patients then went on to require transplantation [117].
One case of nephrotic syndrome due to S. haematobium was found. The patient was managed using cyclophosphamide, methylprednisone, and PZQ but still reached end-stage disease and required transplantation [38]. Of note 9 articles reporting data on glomerular disease were excluded because the etiological agent was S. mansoni [39,40,41,42,43,44,45,46,47]. Supplementary Table 5 shows a summary of all articles regarding the treatment of UO. Visual effect plots for eligible articles dealing with the treatment of UO are presented in Table 4.
Cancer
Reports on the treatment of schistosomiasis-related cancers were scarce. Only fourteen articles dealt with the treatment of cancer in cUGS patients, of which ten were case reports. Surgery was the most frequently employed treatment, followed by immunotherapy with Bacillus Calmette–Guérin (BCG, 1 article) or haemocyanin (1 article), an immunomodulator used to treat other urothelial tumours. Three case reports illustrated the use of combination therapy, particularly the use of chemotherapy followed by surgery and radiotherapy with lack of disease evidence at 19 months post-operation [118], trans-urethral resection of bladder (TURB) plus intra-vesical chemotherapy for a histologically mixed carcinoma [119] and finally surgery and adjuvant chemotherapy for a urothelial carcinoma, although outcome and follow-up information were not provided [120].
Two studies on immunomodulators showed promising results for both BCG (reduction of tumour recurrence, prolongation of the disease-free period by a median time of nine months) and haemocyanin, with a reduction of the relapse rate by 60% compared to endoscopic procedures not followed by immunotherapy [121, 122].
Supplementary Table 6 shows a summary of articles dealing with the treatment of bladder cancer associated with schistosomiasis. Visual effect plots for eligible articles dealing with the treatment of cancers associated with schistosomiasis are presented in Table 4.
Abortion, ectopic pregnancy and infertility
No papers dealing with abortions were retrieved. Twenty-one patients had ectopic pregnancies linked to S. haematobium infections in 15 articles, all consisting of case reports or case series [63,64,65,66,67,68,69, 71,72,73,74,75, 84, 123, 124]. All cases were managed by salpingectomy with the employment of anthelmintics in 10 patients (47.6%, 9 patients treated with PZQ, 1 with niridazole) [63, 65, 67,68,69, 71,72,73,74, 123].
Both laparotomy [64, 67, 71,72,73,74,75, 84, 123] and laparoscopy [63, 65, 66, 68, 69, 75] were employed depending on settings and resources, as well as on the degree of urgency. In one case report, methotrexate was used given the increase of b-HCG post-surgery [68].
Concerning female infertility, 11 articles were found, all case reports describing a single patient. Surgical procedures of varying nature were employed (salpingectomies, neosalpingectomies, adhesiolysis and cyst excisions) [79, 80, 82,83,84,85]. For none of these therapies, fertility outcomes were reported. In two cases, besides surgical therapy, intra-cytoplasmic in vitro injection (ICSI) was also employed to attempt a fecundation. The procedure did not work in one case and its outcome was not reported in the other [80, 84].
Intracytoplasmic Sperm Injection (ICSI) was also employed in one of the two case reports on male infertility, where it allowed management of obstructive azoospermia linked to S. haematobium infection in a young male [77]. In another male patient, orchiectomy and PZQ were used to manage a granulomatous orchitis found after a testicular mass had been misdiagnosed as neoplastic. Sperm count and motility index, initially altered, returned to normality after treatment [76]. Supplementary Table 7 shows a summary of articles on the treatment of male and female genital complications associated with schistosomiasis.
Patients diagnosed after invasive procedures
All the articles fitting this inclusion criteria were case reports or case series [85, 125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140] (Supplementary Table 8). One study [135] (Table 4) dealt with patient whose cUGS diagnosis was made after failure of one 40 mg/kg PZQ dose and a bladder biopsy showed the presence of granulomas with viable eggs. The authors argue that this points towards the adoption of a repeated dosing scheme in areas where PZQ resistance is known, but the study has limitations connected to the small number of patients included.
Discussion
This review shows that the approaches for the diagnosis and management of cUGS are extremely variable, reflecting the protean clinical entity of the disease, able to affect different organs with different degrees of severity and requiring a case-by-case multidisciplinary approach in most patients.
Systematic data collection efforts are lacking: whilst we focussed on cUGS, the lack of standardization in the treatment of uncomplicated schistosomiasis and the lack of data gathering on treatment efficacy is also present [141]. Shared recommendations on the use of PZQ are for instance needed and should be widely adopted to increase the amount of usable data, with particular regard to their use in non-endemic settings where several different regimens are used (single or repeated doses with different timing) without solid efficacy data in support. [141]. Furthermore, in our review, most papers were either case reports or case series. Only two small RCTs were found [112, 142]. Absent outcome reporting is also problematic, as information is often lacking or incomplete (39 of 87 articles did not report follow-up data). When reported, its length rarely exceeded twelve months post intervention, which complicates efficacy estimates. Even basic demographic information was often lacking. Whilst a formal analysis of data on the participant’s age is impossible due to data heterogeneity, studies on OU diagnosis and treatment focus on younger subjects. Our findings on OU diagnosis and treatment might be less applicable to older patients. The same distinction emerges when we look at studies conducted in endemic countries versus studies in travellers, the former group being younger. This distinction is important as patients with a chronic infection are known to have poorer response to treatments in parasitic diseases [143, 144].
Exams found to be used for the diagnosis of cUGS in this review are characterized by low costs in most instances, as in the case of US for the visualization of the urinary tract [12, 19,20,21,22,23,24,25,26,27, 30,31,32,33, 35, 37]. US allows for a first level screening of OU and Schistosoma-related bladder cancers, with good sensitivities according to available data. US is also a diagnostic tool fitting the needs of resource-limited settings due to being cheap, repeatable and since it requires little logistics. Some authors have described the use of endo-venous uretherography for the diagnosis of OU. In this review, the technique was used as a gold standard to evaluate US as a diagnostic tool [36, 37], or alone in studies without comparator [30,31,32,33,34]. CT scans are also employed for the study of OU and other complications of the urinary tract [35, 48, 50,51,52,53,54]: their widespread application in endemic areas is hampered by costs and logistical issues. US has also been employed for the follow-up of patients with good performances in detecting disease regression for OU [108,109,110, 142, 145,146,147]. The same is true for bladder lesions, as reported by some authors [93, 148]. Altogether, available data support the use of US as an initial tool for the screening of cUGS including OU and bladder lesions. Pooled sensitivity data above 85% suggest that US could replace cystoscopy as a first-line exam in patients with a diagnosis of schistosomiasis, differently from previously stated in European guidelines [14].
The review also highlights that, even though late diagnosis and mismanagement were not the focus of this search, in several cases, the diagnosis of schistosomiasis was made only through histology whilst the most sensitive diagnostic test for the infection (which is serology) was often overlooked or performed after the diagnosis was already achieved.
Serology is used erratically in the diagnosis of patients with cUGS-related manifestations. In several reports [n = 7], patients underwent invasive procedures and were also found to have a positive serology for S. haematobium [85, 91, 92, 105, 137, 139, 149]. On the contrary, in several reports [n = 11], eggs were not present in the urine of patients with chronic schistosomiasis [92, 98, 100, 102, 103, 106, 126, 134, 137, 139, 140]. This is a known problem, as many of the pathologic manifestations in cUGS depend from chronic inflammation due to the formation of granulomas around eggs, rather than the continuous secretion of eggs from live parasites. No study found in this review employed the newest tool used in the diagnosis of Schistosoma infections, the circulating anodic antigen (CAA) assay [150]. This test has suggested to provide clinicians information on the presence of live adult parasites rather than just the presence of eggs, and its use could help guide treatment in patients with chronic schistosomiasis. Altogether, the results suggest the use of serology as a systematic tool for cUGS diagnosis, before the use of invasive procedures, whilst egg count in urine or other samples has shown low sensitivity for the diagnosis of cUGS. It is known that serology is the most sensible tool for the diagnosis of schistosomiasis [151] and may be employed as a cost-effective screening test in migrants recently arrived from highly endemic countries in order to identify those infected and treat them preventing the evolution to more advanced complicated disease [152]. The results of this review together with a recently published European multicenter case series showed that serology is positive in the majority of patients with cUGS confirming that this tool is also useful for the diagnosis of advanced disease [16]. Recently, molecular techniques have been increasingly used to diagnosed schistosomiasis; however, we did not find studies on cUGS where PCR was used, suggesting the need to study the role of molecular biology techniques in this cohort of patients.
A vast number of markers were used to diagnose S. haematobium-related cancers. A large part of the studies were carried out by the same group of authors from Egypt. Employed markers range from chemical compounds found to be altered in other pathological conditions (e.g. nitrates) to miRNAs (Table 3). External validation of these markers is required before they gain a place in clinical practice.
Several studies were carried out during elimination programmes, and PZQ was the most frequently employed drug to treat cUGS. Throughout this review, it was noted that the drug was employed at varying dosages, from the 40 mg/kg single dose recommended by the WHO to repeated courses used to treat more severe manifestations. Whilst data on the use of PZQ is lacking for genital cUGS, data on OU mostly coming from studies in the context of elimination programmes supports its use. A more systematic approach on data collection is needed to compare the overall efficacy of different regimes and dosage and effects of treatment on poorly explored outcome such as fertility.
This is even more important considering that available data suggests that the use of PZQ can revert some of the manifestations of cUGS, as shown by response rates for OU found in this review. Moreover, studies, especially case reports, did not report data on the follow-up of patients in 21.7% of articles [12, 13, 50, 53, 113]. 9.8% of patients with OU (333/3415) did not have data on follow-up, either because it is not mentioned in the article or because they did not show up to follow-up visits.
Concerning the effect of PZQ on OU, we found 8 eligible studies, 7 from endemic areas and 1 from Spain [33, 107, 108, 142, 145,146,147]. The largest study was carried out in Niger on 2570 subjects, about half under 15 years and half over 15 years of age. The study reveals that 3 years after a single treatment with PZQ 40 mg/kg, the prevalence of OU significantly decreases from 22 to 4.5% in children and from 12.3 to 3.6% in adults [108]. Another study carried out in Madagascar on 547 subjects reveals similar results decrease on prevalence of OU 12 months after treatment [107]. The remaining 6 studies involved a much smaller number of subjects, but confirmed that OU resolution may be observed 6–12 months after the treatment with PZQ [33, 142, 145,146,147]. However, several studies not matching our inclusion criteria suggest a positive effect of PZQ in patients whose OU grade was not definable [153,154,155,156].
The only trial found in this review compared two ipsilateral double-J stents and a single double-J stent for OU in schistosomiasis (in both cases stenting was performed after laser endoureterotomy). The first technique was found to be superior, concordantly with results on the treatment of OU causes by other etiologies (94). Despite the low number of patients considered, this trial may suggest that the management of patients with cUGS can be informed by results on complications caused by other etiologies.
In the few studies on infertile patients, screening was not done with serology, the most sensitive diagnostic method widely available to date [65, 70, 76,77,78,79,80,81,82,83,84, 86,87,88,89,90, 128, 157]. Most cases were diagnosed after invasive procedures. This also explains why only one case report described the use of PZQ as treatment for Schistosoma-related infertility [76].
This review has several limitations: we could not carry out a meta-analysis of collected data due to the nature of included studies. We included case reports to gather data on some of the most neglected manifestations of cUGS (e.g. infertility and ectopic pregnancy).
Overall, this survey shows an urgent need for prospective studies on several aspects of cUGS and that patients often suffer from a condition preventable by employing appropriate screening protocols for patients arriving in endemic areas (both migrants and returning travellers) as well as public health programmes in endemic areas.
Conclusions
The diagnostic and therapeutic strategies for the management of cUGS are extremely various and usually require a multidisciplinary cooperation. Teams should include tropical diseases physicians, microbiologists, urologists, gynaecologists and radiologists. Currently available data force clinicians to adopt a case-by-case approach. However, we highlight some general points: (1) US is the first-line diagnostic for OU and Schistosoma-related bladder cancer; (2) PZQ can contribute to OU regression. Beneficial approaches in irreversible OU include a surgical approach using a Boari flap and a two ipsilateral double-J stenting after laser endoureterotomy. (3) No specific approaches for the Schistosoma-related bladder cancer were noted and as such it should be managed like bladder cancer due to other causes (4) Infertile patients with risk factors for Schistosoma infection should be screened by serology, and positive subjects should be treated. However, no data on the effect of PZQ on infertility were found. The conclusions are summarized in Table 5.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
WHO. Schistosomiasis. https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis. Accessed 13 July 2022.
Kincaid-Smith J, Tracey A, de CarvalhoAugusto R, Bulla I, Holroyd N, Rognon A, et al. Morphological and genomic characterisation of the Schistosoma hybrid infecting humans in Europe reveals admixture between Schistosoma haematobium and Schistosoma bovis. PLoS Negl Trop Dis. 2021;15: e0010062. https://doi.org/10.1371/journal.pntd.0010062.
WHO. Control of neglected tropical diseases - Schistosomiasis. https://www.who.int/teams/control-of-neglected-tropical-diseases/schistosomiasis/epidemiology. Accessed 13 July 2022.
Asundi A, Beliavsky A, Liu XJ, Akaberi A, Schwarzer G, Bisoffi Z, et al. Prevalence of strongyloidiasis and schistosomiasis among migrants: a systematic review and meta-analysis. Lancet Glob Health. 2019;7:e236–48. https://doi.org/10.1016/s2214-109x(18)30490-x.
Lingscheid T, Kurth F, Clerinx J, Marocco S, Trevino B, Schunk M, et al. Schistosomiasis in European travelers and migrants: analysis of 14 years TropNet surveillance data. Am J Trop Med Hyg. 2017;97:567–74. https://doi.org/10.4269/ajtmh.17-0034.
Holtfreter MC, Mone H, Muller-Stover I, Mouahid G, Richter J. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Euro Surveill. 2014. https://doi.org/10.2807/1560-7917.es2014.19.22.20821.
Wellinghausen N, Mone H, Mouahid G, Nebel A, Tappe D, Gabriel M. A family cluster of schistosomiasis acquired in Solenzara River, Corsica (France) - Solenzara River is clearly a transmission site for schistosomiasis in Corsica. Parasitol Res. 2022. https://doi.org/10.1007/s00436-022-07574-9.
Salas-Coronas J, Bargues MD, Lozano-Serrano AB, Artigas P, Martinez-Orti A, Mas-Coma S, et al. Evidence of autochthonous transmission of urinary schistosomiasis in Almeria (southeast Spain): an outbreak analysis. Travel Med Infect Dis. 2021;44: 102165. https://doi.org/10.1016/j.tmaid.2021.102165.
Beltrame A, Zammarchi L, Zuglian G, Gobbi F, Angheben A, Marchese V, et al. Schistosomiasis screening of travelers from Italy with possible exposure in Corsica, France. Emerg Infect Dis. 2015;21:1887–9. https://doi.org/10.3201/eid2110.150869.
Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. https://doi.org/10.1016/s0140-6736(06)69440-3.
Clerinx J, Van Gompel A. Schistosomiasis in travellers and migrants. Travel Med Infect Dis. 2011;9:6–24. https://doi.org/10.1016/j.tmaid.2010.11.002.
Salas-Coronas J, Vázquez-Villegas J, Lozano-Serrano AB, Soriano-Pérez MJ, Cabeza-Barrera I, Cabezas-Fernández MT, et al. Severe complications of imported schistosomiasis, Spain: a retrospective observational study. Travel Med Infect Dis. 2020;35: 101508. https://doi.org/10.1016/j.tmaid.2019.101508.
Tilli M, Gobbi F, Rinaldi F, Testa J, Caligaris S, Magro P, et al. The diagnosis and treatment of urogenital schistosomiasis in Italy in a retrospective cohort of immigrants from Sub-Saharan Africa. Infection. 2019;47:447–59. https://doi.org/10.1007/s15010-019-01270-0.
Bichler KH, Savatovsky I, Naber KG, Bischop MC, Bjerklund-Johansen TE, Botto H, et al. EAU guidelines for the management of urogenital schistosomiasis. Eur Urol. 2006;49:998–1003. https://doi.org/10.1016/j.eururo.2006.02.022.
Comelli A, Riccardi N, Canetti D, Spinicci M, Cenderello G, Magro P, et al. Delay in schistosomiasis diagnosis and treatment: a multicenter cohort study in Italy. J Travel Med. 2020. https://doi.org/10.1093/jtm/taz075.
Basile G, Tamarozzi F, Salas-Coronas J, Soriano-Perez MJ, Luzon-Garcia P, Moro L, et al. Management of imported complicated urogenital schistosomiasis in Europe: a TropNet retrospective study. J Travel Med. 2022. https://doi.org/10.1093/jtm/taac150.
Thomson HJ, Thomas S. The effect direction plot: visual display of non-standardised effects across multiple outcome domains. Res Synth Methods. 2013;4:95–101. https://doi.org/10.1002/jrsm.1060.
Richter J, Hatz C, Campagne G, Jenkins J. Ultrasound in schistosomiasis: a practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. World Health Organization/TDR/STR/SCH/WHO -Document: 1–41; 2000.
Dabo A, Traoré HA, Diakité M, Kouriba B, Camara F, Coulibaly CO, et al. Echographic morbidity due to Schistosoma haematobium in a peripheral district of Bamako in Mali, Missabougou. Bull Soc Pathol Exot. 1995;88:11–4.
Garba A, Campagne G, Poda JN, Parent G, Kambire R, Chippaux JP. Schistosomiasis in the region of Ziga (Burkina Faso) before the construction of a dam. Bull Soc Pathol Exot. 1999;92:195–7.
Rasendramino MH, Rajaona HR, Ramarokoto CE, Ravaoalimalala VE, Leutscher P, Cordonnier D, et al. Prevalence of uro-nephrologic complications of urinary bilharziasis in hyperendemic focus in Madagascar. Nephrologie. 1998;19:341–5.
Remppis J, Verheyden A, Bustinduy AL, Heller T, García-Tardón N, Manouana GP, et al. Focused Assessment with Sonography for Urinary Schistosomiasis (FASUS)-pilot evaluation of a simple point-of-care ultrasound protocol and short training program for detecting urinary tract morbidity in highly endemic settings. Trans R Soc Trop Med Hyg. 2020;114:38–48. https://doi.org/10.1093/trstmh/trz101.
Salah MA. Ultrasonography of urinary tract lesions caused by bilharziasis in Yemeni patients. BJU Int. 2000;86:790–3. https://doi.org/10.1046/j.1464-410x.2000.00921.x.
Serieye J, Boisier P, Ravaoalimalala VE, Ramarokoto CE, Leutscher P, Esterre P, et al. Schistosoma haematobium infection in western Madagascar: morbidity determined by ultrasonography. Trans R Soc Trop Med Hyg. 1996;90:398–401. https://doi.org/10.1016/s0035-9203(96)90521-0.
Vester U, Kardorff R, Traoré M, Traoré HA, Fongoro S, Juchem C, et al. Urinary tract morbidity due to Schistosoma haematobium infection in Mali. Kidney Int. 1997;52:478–81. https://doi.org/10.1038/ki.1997.356.
Salas-Coronas J, Vázquez-Villegas J, Villarejo-Ordóñez A, Sánchez-Sánchez JC, Espada-Chavarría J, Soriano-Pérez MJ, et al. Radiological findings in patients with imported schistosomiasis. Enferm Infecc Microbiol Clin. 2013;31:205–9. https://doi.org/10.1016/j.eimc.2012.04.003.
Bocanegra García C, Pintar Z, Serres X, Mendioroz J, Moreno M, Gallego S, et al. Ultrasound findings and associated factors to morbidity in Schistosoma haematobium infection in a highly endemic setting. Trop Med Int Health. 2018;23:221–8. https://doi.org/10.1111/tmi.13020.
Brouwer KC, Ndhlovu PD, Wagatsuma Y, Munatsi A, Shiff CJ. Epidemiological assessment of Schistosoma haematobium-induced kidney and bladder pathology in rural Zimbabwe. Acta Trop. 2003;85:339–47. https://doi.org/10.1016/s0001-706x(02)00262-0.
Richter J, Wagatsuma Y, Aryeetey M, Feldmeier H. Sonographic screening for urinary tract abnormalities in patients with Schistosoma haematobium infection: pitfalls in examining pregnant women. Bull World Health Organ. 1996;74:217–21.
Badmos KB, Popoola AA, Buhari MO, Abdulkadir AY. Ureteric schistosomiasis with obstructive uropathy. J Coll Physicians Surg Pak. 2009;19:456–8.
Bakari AA, Gadam IA, Aliyu S, Suleiman I, Ahidjo AA, Pindiga UH. Use of mitrofanoff and yang-monti techniques as ureteric substitution for severe schistosomal bilateral ureteric stricture: a case report and review of the literature. Niger J Surg. 2012;18:30–3. https://doi.org/10.4103/1117-6806.95490.
Olajide AO, Olajide FO, Aremu AA, Komolafe AO. Ureteric obstruction secondary to schistosomiasis 2 years after praziquantel therapy: a case report. Pan Afr Med J. 2012;12:32.
Antwi S, Aboah KE, Sarpong CK. The unacknowledged impact of urinary schistosomiasis in children: 5 cases from Kumasi, Ghana. Ghana Med J. 2014;48:228–33. https://doi.org/10.4314/gmj.v48i4.11.
Oranusi CK, Nwofor A, Onyiaorah IV, Ukah CO. Schistosomal stricture of the ureter-diagnostic dilemma. Niger J Clin Pract. 2011;14:495–8. https://doi.org/10.4103/1119-3077.91765.
Lorca J, Hevia V, Diez Nicolás V, González A, Sánchez Guerrero C, Burgos Revilla FJ. Minimmally invasive resolution of a left ureteral stenosis after Schistosoma haematobium infection. Urol Case Rep. 2019;25: 100889.
Ibrahim AI, Patil KP, el Tahir MI, Shetty SD, Anandan N. Bilharzial vesicoureteric reflux and bladder neck stenosis: fact or fiction? Br J Urol. 1991;68:582–5. https://doi.org/10.1111/j.1464-410x.1991.tb15419.x.
Abdel-Wahab MF, Ramzy I, Esmat G, el Kafass H, Strickland GT. Ultrasound for detecting Schistosoma haematobium urinary tract complications: comparison with radiographic procedures. J Urol. 1992;148:346–50. https://doi.org/10.1016/s0022-5347(17)36590-4.
Seck SM, Sarr ML, Dial MC, Ka EF. Schistosoma hematobium-associated glomerulopathy. Indian J Nephrol. 2011;21:201–3.
Dias CB, Testagrossa L, Jorge L, Malheiros D, Woronik V. Clinical and histological features of patients with membranoproliferative glomerulonephritis classified by immunofluorescence findings. J Bras Nefrol. 2017;39:447–53. https://doi.org/10.5935/0101-2800.20170078.
Halleux D, Moerman F, Gavage P, Carpentier M, Van Esbroeck M, Craenen S, et al. A nephrotic syndrome of tropical origin: case report and short review of the aetiology. Acta Clin Belg. 2014;69:379–81. https://doi.org/10.1179/2295333714y.0000000057.
Mohammad Ibrahim WH, Aly MG, Abdo MK, Ismail W. Primary membranous glomerulonephritis-associated with schistosomal nephropathy. Indian J Nephrol. 2019;29:140–2.
Barsoum R, Nabil M, Saady G, Genin C, Saleh E, Francis M, et al. Immunoglobulin-A and the pathogenesis of schistosomal glomerulopathy. Kidney Int. 1996;50:920–8. https://doi.org/10.1038/ki.1996.392.
Dos-Santos WLC, Sweet GMM, Azevêdo LG, Tavares MB, Soares MFS, Melo CVB, et al. Current distribution pattern of biopsy-proven glomerular disease in Salvador, Brazil, 40 years after an initial assessment. J Bras Nefrol. 2017;39:376–83. https://doi.org/10.5935/0101-2800.20170069.
Gonçalves FO, Fontes TM, Canuto AP. Schistosoma mansoni associated glomerulopathy with IgA mesangial deposits: case report. J Bras Nefrol. 2017;39:86–90. https://doi.org/10.5935/0101-2800.20170015.
Martinelli R, Pereira LJ, Brito E, Rocha H. Clinical course of focal segmental glomerulosclerosis associated with hepatosplenic schistosomiasis mansoni. Nephron. 1995;69:131–4. https://doi.org/10.1159/000188427.
Neves PD, Bridi RA, Ramalho JA, Jorge LB, Watanabe EH, Watanabe A, et al. Schistosoma mansoni infection as a trigger to collapsing glomerulopathy in a patient with high-risk APOL1 genotype. PLoS Negl Trop Dis. 2020;14: e0008582. https://doi.org/10.1371/journal.pntd.0008582.
Rodrigues VL, Otoni A, Voieta I, Antunes CM, Lambertucci JR. Glomerulonephritis in Schistosomiasis mansoni: a time to reappraise. Rev Soc Bras Med Trop. 2010;43:638–42. https://doi.org/10.1590/s0037-86822010000600007.
Fataar S, Rudwan M, Bassiony H, Satyanath S. CT of genitourinary calcification due to schistosomiasis. Australas Radiol. 1990;34:234–7. https://doi.org/10.1111/j.1440-1673.1990.tb02638.x.
Pal PO, Smith RD, Allen S, Ratynska M, Edwards S, Gothard P, et al. Schistosomiasis-A disobedient ureter, a disobedient diagnosis. J Endourol Case Rep. 2017;3:114–8.
Vancauwenberghe T, Oyaert M, Termote JL, Mulkens T, Bellinck P. Ureteral obstruction caused by schistosomiasis. Jbr-btr. 2013;96:292–4. https://doi.org/10.5334/jbr-btr.412.
Kazmi Z, Ashfaq MA, Umer D, Idrees R, Ather MH. Snail fever of the bladder in a non-endemic area. J Coll Physicians Surg Pak. 2020;30:874–6.
Pallangyo P, Bhalia S, Simelane NN, Lyimo F, Swai HJ, Mkojera ZS, et al. Massive bilateral hydroureteronephrosis and end-stage renal disease ensuing from chronic schistosomiasis: a case report. J Investig Med High Impact Case Rep. 2020;8:2324709620910912. https://doi.org/10.1177/2324709620910912.
Pollock GR, Meiklejohn KM, Zeng J, Chipollini J. Robotic cystoprostatectomy with intracorporeal ileal conduit diversion in a patient with chronic schistosomiasis. Urology. 2020;141:e8–9. https://doi.org/10.1016/j.urology.2020.04.052.
Srougi V, Gallucci FP, Mattedi RL, Srougi M. Carcinosarcoma of the bladder following local schistosomiasis infection. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2016-218642.
Alvarez Kindelan J, Alameda Aragoneses V, Carmona Campos E, Anglada Curado F, Prieto Castro R, Regueiro López JC, et al. Bilharziasis and bladder cancer. A case report. Actas Urol Esp. 1999;23:60–3.
Abdel-Wahab MF, Esmat G, Ramzy I, Fouad R, Abdel-Rahman M, Yosery A, et al. Schistosoma haematobium infection in Egyptian schoolchildren: demonstration of both hepatic and urinary tract morbidity by ultrasonography. Trans R Soc Trop Med Hyg. 1992;86:406–9. https://doi.org/10.1016/0035-9203(92)90241-4.
Brouwer KC, Ndhlovu PD, Wagatsuma Y, Munatsi A, Shiff CJ. Urinary tract pathology attributed to Schistosoma haematobium: does parasite genetics play a role? Am J Trop Med Hyg. 2003;68:456–62.
Santos J, Chaves J, Araújo H, Vale N, Costa JM, Brindley PJ, et al. Comparison of findings using ultrasonography and cystoscopy in urogenital schistosomiasis in a public health centre in rural Angola. S Afr Med J. 2015;105:312–5. https://doi.org/10.7196/samj.8564.
Akinwale OP, Oliveira GC, Ajayi MB, Akande DO, Oyebadejo S, Okereke KC. Squamous cell abnormalities in exfoliated cells from the urine of Schistosoma haematobium-infected adults in a rural fishing community in Nigeria. World Health Popul. 2008;10:18–22. https://doi.org/10.12927/whp.2008.19581.
Saied GM, El-Metenawy WH, Elwan MS, Dessouki NR. Urine carcinoembryonic antigen levels are more useful than serum levels for early detection of Bilharzial and non-Bilharzial urinary bladder carcinoma: observations of 43 Egyptian cases. World J Surg Oncol. 2007;5:4. https://doi.org/10.1186/1477-7819-5-4.
Ahmed SA, Gad MZ. Diagnostic value of serum lactate dehydrogenase isoenzyme and amino acid patterns in several schistosomal and non-schistosomal disorders as compared to other biochemical parameters. Dis Markers. 1996;13:19–29. https://doi.org/10.1155/1996/214869.
Yang H, Yang K, Khafagi A, Tang Y, Carey TE, Opipari AW, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A. 2005;102:7683–8. https://doi.org/10.1073/pnas.0406904102.
Bahrami S, Alatassi H, Slone SP, O’Connor DM. Tubal gestation and schistosomiasis: a case report. J Reprod Med. 2006;51:595–8.
Bugalho A, Strolego F, Benussi G, Pregazzi R, Osman N. Schistosomiasis: possible cause of ectopic pregnancy. Four clinical cases. Minerva Ginecol. 1991;43:577–9.
Ekoukou D, Luzolo-Lukanu A, Mulard C, Bazin C, Ng Wing Tin L. Peritoneal and tubal Schistosoma haematobium bilharziasis. Two case reports. J Gynecol Obstet Biol Reprod (Paris). 1995;24:819–24.
Eogan M, O’Malley A, Flavin R, Gillan J, McKenna P, Coulter-Smith S. Ectopic pregnancy associated with tubal schistosomiasis. Ir Med J. 2002;95:250.
Garba M, Almoustapha T, Garba A, Nouhou H. Extra uterine pregnancy associated with a tubal schistosomiasis due to Schistosoma haematobium. A case report from Niger. Bull Soc Pathol Exot. 2004;97:41–2.
Laroche J, Mottet N, Malincenco M, Gay C, Royer PY, Riethmuller D. Successive ectopic pregnancies associated with tubal shistosomiasis in a French traveler. Pan Afr Med J. 2016;23:18. https://doi.org/10.11604/pamj.2016.23.18.8845.
Laxman VV, Adamson B, Mahmood T. Recurrent ectopic pregnancy due to Schistosoma hematobium. J Obstet Gynaecol. 2008;28:461–2. https://doi.org/10.1080/01443610802164896.
Nouhou H, Sève B, Idi N, Moussa F. Schistosomiasis of the female genital tract: anatomoclinical and histopathological aspects Apropos of 26 cases. Bull Soc Pathol Exot. 1998;91:221–3.
Odubamowo KH, Akinpelu OM, Lawal OO, Okolo CA, Odukogbe AA, Adekunle AO. Bilateral tubal gestation associated with schistosomiasis in an African woman. Case Rep Obstet Gynecol. 2014;2014: 674514. https://doi.org/10.1155/2014/674514.
Okonofua FE, Ojo OS, Odunsi OA, Odesanmi WO. Ectopic pregnancy associated with tubal schistosomiasis in a Nigerian woman. Int J Gynaecol Obstet. 1990;32:281–4. https://doi.org/10.1016/0020-7292(90)90359-s.
Sahu L, Tempe A, Singh S, Khurana N. Ruptured ectopic pregnancy associated with tubal schistosomiasis. J Postgrad Med. 2013;59:315–7. https://doi.org/10.4103/0022-3859.123166.
Schneider D, Steyn DW. Genital schistosomiasis presenting as suspected ectopic pregnancy in the Western Cape. S Afr Med J. 2000;90:609.
Ville Y, Leruez M, Picaud A, Walter P, Fernandez H. Tubal schistosomiasis as a cause of ectopic pregnancy in endemic areas? A report of three cases. Eur J Obstet Gynecol Reprod Biol. 1991;42:77–9. https://doi.org/10.1016/0028-2243(91)90164-g.
Al-Qahtani SM, Droupy SJ. Testicular schistosomiasis. Saudi Med J. 2010;31:325–7.
Kini S, Dayoub N, Raja A, Pickering S, Thong J. Schistosomiasis-induced male infertility. BMJ Case Rep. 2009. https://doi.org/10.1136/bcr.01.2009.1481.
Richter J, Poggensee G, Helling-Giese G, Kjetland E, Chitsulo L, Koumenda N, et al. Transabdominal ultrasound for the diagnosis of Schistosoma haematobium infection of the upper female genital tract: a preliminary report. Trans R Soc Trop Med Hyg. 1995;89:500–1. https://doi.org/10.1016/0035-9203(95)90084-5.
Balasch J, Martínez-Román S, Creus M, Campo E, Fortuny A, Vanrell JA. Schistosomiasis: an unusual cause of tubal infertility. Hum Reprod. 1995;10:1725–7. https://doi.org/10.1093/oxfordjournals.humrep.a136163.
Schanz A, Richter J, Beyer I, Baldus SE, Hess AP, Kruessel JS. Genital schistosomiasis as a cause of female sterility and acute abdomen. Fertil Steril. 2010;93:2075.e7-9. https://doi.org/10.1016/j.fertnstert.2009.05.043.
Nayama M, Garba A, Boulama-Jackou ML, Touré A, Idi N, Garba M, et al. Uro-genital schistosomiasis with S. haematobium and infertility in Niger. Prospective study of 109 cases. Mali Med. 2007;22:15–21.
Krolikowski A, Janowski K, Larsen JV. Asherman syndrome caused by schistosomiasis. Obstet Gynecol. 1995;85:898–9. https://doi.org/10.1016/0029-7844(94)00371-j.
Morice P, Gadonneix P, Van den Akker M, Antoine M, Villet R. Tubal bilharziasis. J Gynecol Obstet Biol Reprod (Paris). 1993;22:848–50.
Owusu-Bempah A, Odoi AT, Dassah ET. Genital schistosomiasis leading to ectopic pregnancy and subfertility: a case for parasitic evaluation of gynaecologic patients in schistosomiasis endemic areas. Case Rep Obstet Gynecol. 2013;2013: 634264. https://doi.org/10.1155/2013/634264.
Darwish AM. Laparoscopic evidence of upper genital schistosomiasis. J Obstet Gynaecol. 1999;19:122–4. https://doi.org/10.1080/01443619965381.
Jones KD, Okaro EO, Sutton C. The laparoscopic appearance of Schistosomiasis may be mistaken for “non-pigmented” endometriosis. Eur J Obstet Gynecol Reprod Biol. 2003;106:227–9. https://doi.org/10.1016/s0301-2115(02)00220-8.
Swai B, Poggensee G, Mtweve S, Krantz I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: a retrospective histopathological study from Tanzania. BMC Infect Dis. 2006;6:134. https://doi.org/10.1186/1471-2334-6-134.
Van Den Broucke S, Potters I, Van Esbroeck M, Cnops L, Siozopoulou V, Hammoud C, et al. A woman with chronic lower abdominal pain, vaginal discharge, and infertility after a stay in Mali. Open Forum Infect Dis. 2020;7:ofaa133.
Kjetland EF, Kurewa EN, Mduluza T, Midzi N, Gomo E, Friis H, et al. The first community-based report on the effect of genital Schistosoma haematobium infection on female fertility. Fertil Steril. 2010;94:1551–3. https://doi.org/10.1016/j.fertnstert.2009.12.050.
Kjetland EF, Poggensee G, Helling-Giese G, Richter J, Sjaastad A, Chitsulo L, et al. Female genital schistosomiasis due to Schistosoma haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Trop. 1996;62:239–55. https://doi.org/10.1016/s0001-706x(96)00026-5.
Dessyn JF, Duquenne S, Hoarau G. Incidental pseudolymphomatous bladder inflammatory polyp revealing urinary schistosomiasis. Int J Infect Dis. 2016;53:39–40. https://doi.org/10.1016/j.ijid.2016.10.022.
López López AI, Cao Avellaneda E, Prieto González A, Ferri Níguez B, Maluff Torres A, Pérez AM. Schistosomiasis: not an uncommon parasitosis in Europe. Actas Urol Esp. 2007;31:915–8. https://doi.org/10.1016/s0210-4806(07)73747-6.
Aytaç B, Sehıtoğlu I. A rare parasitic infection in Turkey: schistosomiasis. Case report. Turk Patoloji Derg. 2012;28:175–7. https://doi.org/10.5146/tjpath.2012.01120.
Ballesta Martínez B, Rodríguez Talavera J, Amador Robayna A, Carrión Valencia A, Orribo Morales N, García García L, et al. Parasitic hematuria: six cases in a row in a single centre in Spain. Urol Int. 2019;102:360–3.
Carrión López P, Pastor Navarro H, Martínez Ruiz J, Martínez Sanchiz C, Donate Moreno MJ, Segura Martín M, et al. Cystoscopy in bladder bilharziasis. Arch Esp Urol. 2010;63:85–6.
Chahdi H, Damiri A, El Ochi MR, Allaoui M, Al Bouzidi A, Oukabli M. Urinary schistosomiasis: report of case diagnosed in bladder biopsy. BMC Clin Pathol. 2018;18:1–3.
Dzeing-Ella A, Mechaï F, Consigny PH, Zerat L, Viard JP, Lecuit M, et al. Cervical schistosomiasis as a risk factor of cervical uterine dysplasia in a traveler. Am J Trop Med Hyg. 2009;81:549–50. https://doi.org/10.4269/ajtmh.2009.08-0498.
Fabiano M, Califano A, Chiancone F, D’Antonio A, Maiorino F, Simeone D, et al. Bladder schistosomiasis in Italy: a case report. Urologia. 2020;87:191–3. https://doi.org/10.1177/0391560320910647.
Hosny K, Luk A. Urinary schistosomiasis presented as bladder malignancy with pulmonary metastases: a case report. Ann R Coll Surg Engl. 2018;100:e145–6. https://doi.org/10.1308/rcsann.2018.0072.
Kameh D, Smith A, Brock MS, Ndubisi B, Masood S. Female genital schistosomiasis: case report and review of the literature. South Med J. 2004;97:525–7. https://doi.org/10.1097/00007611-200405000-00022.
Mascarenhas A, Castro I. A rare case of hematuria. Einstein (Sao Paulo). 2011;9:81–3. https://doi.org/10.1590/s1679-45082011rc1946.
Neal PM. Schistosomiasis–an unusual cause of ureteral obstruction: a case history and perspective. Clin Med Res. 2004;2:216–27. https://doi.org/10.3121/cmr.2.4.216.
Pinto SZ, Friedman R, Van Den Berg EJ. A case of paediatric bladder bilharzioma in Johannesburg, South Africa. Clin Case Rep. 2019;7:1890–4.
Samuel MI, Taylor C. A case of female urogenital schistosomiasis presenting as viral warts. Int J STD AIDS. 2015;26:599–601. https://doi.org/10.1177/0956462414544723.
Scarlata F, Giordano S, Romano A, Marasa L, Lipani G, Infurnari L, et al. Urinary schistosomiasis: remarks on a case. Infez Med. 2005;13:259–64.
Zepeda CM, Coffey KH. Schistosoma haematobium Infection that mimics bladder cancer in a 66-year-old ethnic Egyptian man. Lab Med. 2015;46:338–42. https://doi.org/10.1309/lm96ejppbyadiovc.
Rasendramino MH, Rajaona HR, Ramarokoto CE, Ravaoalimalala VE, Leutscher P, Cordonnier D, et al. Effect of praziquantel on the uro-nephrologic complications of urinary bilharziasis. Nephrologie. 1998;19:347–51.
Garba A, Campagne G, Tassie JM, Barkire A, Vera C, Sellin B, et al. Long-term impact of a mass treatment by praziquantel on morbidity due to Schistosoma haematobium in two hyperendemic villages of Niger. Bull Soc Pathol Exot. 2004;97:7–11.
Ouma JH, King CH, Muchiri EM, Mungai P, Koech DK, Ireri E, et al. Late benefits 10–18 years after drug therapy for infection with Schistosoma haematobium in Kwale District, Coast Province, Kenya. Am J Trop Med Hyg. 2005;73:359–64.
Subramanian AK, Mungai P, Ouma JH, Magak P, King CH, Mahmoud AA, et al. Long-term suppression of adult bladder morbidity and severe hydronephrosis following selective population chemotherapy for Schistosoma haematobium. Am J Trop Med Hyg. 1999;61:476–81. https://doi.org/10.4269/ajtmh.1999.61.476.
Ravi G, Motalib MA. Surgical correction of bilharzial ureteric stricture by Boari flap technique. Br J Urol. 1993;71:535–8. https://doi.org/10.1111/j.1464-410x.1993.tb16021.x.
Mohyelden K, Hussein HA, El Helaly HA, Ibrahem H, Abdelwahab H. Long-term outcomes of two ipsilateral vs single double-j stent after laser endoureterotomy for bilharzial ureteral strictures. J Endourol. 2020. https://doi.org/10.1089/end.2020.0956.
Pieras Ayala E, Salvador J, Vicente J. Bilharziasis, clinical course of the disease: acute and chronic phase. Two clinical cases. Arch Esp Urol. 2000;53:834–9.
Coulibaly Y, Ouattara Z, Togo A, Konate M, Ouattara M, Ouattara K. Bilharziasis and urinary lithiasis: a study of 23 cases at the Gabriel Toure Hospital. Mali Med. 2011;26:26–8.
Salah MA, Holman E, Tóth C. Percutaneous suprapubic cystolithotripsy for pediatric bladder stones in a developing country. Eur Urol. 2001;39:466–70. https://doi.org/10.1159/000052487.
Darko SN, Hanson H, Twumasi-Ankrah S, Baffour-Awuah S, Adjei-Kusi P, Yar D, et al. Three monthly doses of 60 mg/kg praziquantel for Schistosoma haematobium infection is a safe and effective treatment regimen. BMC Infect Dis. 2020;20:323. https://doi.org/10.1186/s12879-020-05053-z.
Barrou B, Bitker MO, Boyer C, Sylla C, Chatelain C. Results of renal transplantation in patients with Schistosoma infection. J Urol. 1997;157:1232–5.
Almeida M, Canas-Marques R, Lopez-Beltran A, Rebola J, Lúcio R, Montironi R, et al. Small cell carcinoma of the bladder associated with schistosomiasis: a case report. Anal Quant Cytopathol Histpathol. 2014;36:339–44.
Ketabchi A, Moshtaghi-Kashanian G. Urinary schistosomiasis with simultaneous bladder squamous cell carcinoma and transitional cell carcinoma. Iran J Parasitol. 2012;7:96–8.
Vieira P, Miranda HP, Cerqueira M, Delgado Mde L, Coelho H, Antunes D, et al. Latent schistosomiasis in Portuguese soldiers. Mil Med. 2007;172:144–6. https://doi.org/10.7205/milmed.172.2.144.
Wishahi MM, Ismail IM, Ruebben H, Otto T. Keyhole-limpet hemocyanin immunotherapy in the bilharzial bladder: a new treatment modality? Phase II trial: superficial bladder cancer. J Urol. 1995;153:926–8.
Wishahi MM, Ismail IM, El-Sherbini M. Immunotherapy with bacille Calmette-Guérin in patients with superficial transitional cell carcinoma of the bladder associated with bilharziasis. Br J Urol. 1994;73:649–54. https://doi.org/10.1111/j.1464-410x.1994.tb07550.x.
Aminu MB, Abdullahi K, Dattijo LM. Tubal ectopic gestation associated with genital schistosomiasis: a case report. Afr J Reprod Health. 2014;18:144–6.
De Muylder X. Ectopic pregnancy in Zimbabwe. Int J Gynaecol Obstet. 1991;35:55–60. https://doi.org/10.1016/0020-7292(91)90064-c.
Al-Saeed O, Sheikh M, Kehinde EO, Makar R. Seminal vesicle masses detected incidentally during transrectal sonographic examination of the prostate. J Clin Ultrasound. 2003;31:201–6. https://doi.org/10.1002/jcu.10158.
Azami MA, Elalami I, Siati A, Lamalmi N. An unusual presentation of ovarian dermoid cyst: a case report and review of literature. Obstet Gynecol Sci. 2018;61:529–32. https://doi.org/10.5468/ogs.2018.61.4.529.
Badmus TA, Takure AO, Osasan SA, Olajide AO, Sabageh DO. Testicular schistosomiasis: a case report. Niger Postgrad Med J. 2012;19:50–1.
Bailey SL, Price J, Llewelyn M. Fluke infertility: the late cost of a quick swim. J Travel Med. 2011;18:61–2. https://doi.org/10.1111/j.1708-8305.2010.00476.x.
Drew LB, Tang JH, Norris A, Reese PC, Mwale M, Mataya R, et al. Schistosomiasis among obstetric fistula patients in Lilongwe, Malawi. Malawi Med J. 2018;30:225–9. https://doi.org/10.4314/mmj.v30i4.3.
Efared B, Sidibé IS, Erregad F, Hammas N, Chbani L, Fatemi HE. Schistosomiasis mimicking ovarian neoplasm. Trop Doct. 2018;48:238–40. https://doi.org/10.1177/0049475518770574.
Fall I, N’Doye M, Wandaogo A, Sankale AA, Diop A. A case report of epididymo-testicular bilharziasis in a child. Ann Urol (Paris). 1992;26:360–1.
Kato-Hayashi N, Yasuda M, Yuasa J, Isaka S, Haruki K, Ohmae H, et al. Use of cell-free circulating schistosome DNA in serum, urine, semen, and saliva to monitor a case of refractory imported schistosomiasis hematobia. J Clin Microbiol. 2013;51:3435–8. https://doi.org/10.1128/jcm.01219-13.
Oguntunde OA, Ikhisemojie S, Sonusi SE, Oyebode A, Abdulkareem B, Banjo AA. Testicular schistosomiasis mimicking hydrocele in a child: a case report. Pan Afr Med J. 2020;35:56.
Rambau PF, Chandika A, Chalya PL, Jackson K. Scrotal swelling and testicular atrophy due to schistosomiasis in a 9-year-old boy: a case report. Case Rep Infect Dis. 2011;2011: 787961. https://doi.org/10.1155/2011/787961.
Silva IM, Thiengo R, Conceição MJ, Rey L, Lenzi HL, Pereira Filho E, et al. Therapeutic failure of praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Mem Inst Oswaldo Cruz. 2005;100:445–9. https://doi.org/10.1590/s0074-02762005000400018.
Silva IM, Pereira Filho E, Thiengo R, Ribeiro PC, Conceição MJ, Panasco M, et al. Schistosomiasis haematobia: histopathological course determined by cystoscopy in a patient in whom praziquantel treatment failed. Rev Inst Med Trop Sao Paulo. 2008;50:343–6. https://doi.org/10.1590/s0036-46652008000600006.
Soans B, Abel C. Ultrasound appearance of schistosomiasis of the testis. Australas Radiol. 1999;43:385–7. https://doi.org/10.1046/j.1440-1673.1999.433685.x.
Torricelli M, Cerri M, Leva E, Magro P, Roma G, Runza L, et al. An unusual case of macroscopic hematuria in pediatric age. Pediatr Med Chir. 1998;20:81–3.
Turkistani I, Ghourab S, Al-Rikabi A, Al-Sheikh AE, Al-Orainy I. Large paravaginal solitary fibrous tumor with secondary Schistosoma hematobium infestation. Acta Obstet Gynecol Scand. 2002;81:88–90. https://doi.org/10.1034/j.1600-0412.2002.810116.x.
Ze Ondo C, Sarr A, Sow Y, Thiam I, Fall B, Sow D, et al. Testicular bilharzioma by Schistosomia haematobium: about two cases. Prog Urol. 2014;24:67–9. https://doi.org/10.1016/j.purol.2013.04.016.
Cucchetto G, Buonfrate D, Marchese V, Rodari P, Ferrari A, Zanotti P, et al. High-dose or multi-day praziquantel for imported schistosomiasis? A systematic review. J Travel Med. 2019;26:taz050. https://doi.org/10.1093/jtm/taz050.
King CH, Muchiri EM, Mungai P, Ouma JH, Kadzo H, Magak P, et al. Randomized comparison of low-dose versus standard-dose praziquantel therapy in treatment of urinary tract morbidity due to Schistosoma haema tobium infection. Am J Trop Med Hyg. 2002;66:725–30. https://doi.org/10.4269/ajtmh.2002.66.725.
Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373:1295–306. https://doi.org/10.1056/NEJMoa1507574.
Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, et al. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis. 2009;3: e524. https://doi.org/10.1371/journal.pntd.0000524.
Hatz C, Mayombana C, de Savigny D, MacPherson CN, Koella JC, Degrémont A, et al. Ultrasound scanning for detecting morbidity due to Schistosoma haematobium and its resolution following treatment with different doses of praziquantel. Trans R Soc Trop Med Hyg. 1990;84:84–8. https://doi.org/10.1016/0035-9203(90)90392-r.
King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, et al. Chemotherapy-based control of Schistosomiasis haematobia. II. Metrifonate vs. praziquantel in control of infection-associated morbidity. Am J Trop Med Hyg. 1990;42:587–95. https://doi.org/10.4269/ajtmh.1990.42.587.
Kardorff R, Traoré M, Doehring-Schwerdtfeger E, Vester U, Ehrich JH. Ultrasonography of ureteric abnormalities induced by Schistosoma haematobium infection before and after praziquantel treatment. Br J Urol. 1994;74:703–9. https://doi.org/10.1111/j.1464-410x.1994.tb07110.x.
Alvarez Maestro M, Rios Gonzalez E, Dominguez Garcia P, Vallejo Herrador J, Diez Rodriguez J, Martinez-Piñeiro L. Bladder schistosomiasis: case report and bibliographic review. Arch Esp Urol. 2010;63:554–8. https://doi.org/10.4321/s0004-06142010000700013.
Lee Y, Song HB, Jung BK, Choe G, Choi MH. Case report of urinary schistosomiasis in a returned traveler in Korea. Korean J Parasitol. 2020;58:51–5. https://doi.org/10.3347/kjp.2020.58.1.51.
Tamarozzi F, Ursini T, Hoekstra PT, Silva R, Costa C, Gobbi F, et al. Evaluation of microscopy, serology, circulating anodic antigen (CAA), and eosinophil counts for the follow-up of migrants with chronic schistosomiasis: a prospective cohort study. Parasit Vectors. 2021;14:149. https://doi.org/10.1186/s13071-021-04655-z.
Beltrame A, Guerriero M, Angheben A, Gobbi F, Requena-Mendez A, Zammarchi L, et al. Accuracy of parasitological and immunological tests for the screening of human schistosomiasis in immigrants and refugees from African countries: an approach with latent class analysis. PLoS Negl Trop Dis. 2017;11: e0005593. https://doi.org/10.1371/journal.pntd.0005593.
Zammarchi L, Botta A, Tilli M, Gobbi F, Bartoloni A, Boccalini S. Presumptive treatment or serological screening for schistosomiasis in migrants from Sub-Saharan Africa could save both lives and money for the italian national health system: results of an economic evaluation. J Travel Med. 2022. https://doi.org/10.1093/jtm/taac140.
Bocanegra C, Pintar Z, Mendioroz J, Serres X, Gallego S, Nindia A, et al. Ultrasound evolution of pediatric urinary schistosomiasis after treatment with praziquantel in a highly endemic area. Am J Trop Med Hyg. 2018;99:1011–7. https://doi.org/10.4269/ajtmh.18-0343.
Barda B, Coulibaly JT, Hatz C, Keiser J. Ultrasonographic evaluation of urinary tract morbidity in school-aged and preschool-aged children infected with Schistosoma haematobium and its evolution after praziquantel treatment: a randomized controlled trial. PLoS Negl Trop Dis. 2017;11: e0005400. https://doi.org/10.1371/journal.pntd.0005400.
Campagne G, Garba A, Barkiré H, Vera C, Sidiki A, Chippaux JP. Continued ultrasonic follow-up of children infected with Schistosoma haematobium after treatment with praziquantel. Trop Med Int Health. 2001;6:24–30. https://doi.org/10.1046/j.1365-3156.2001.00660.x.
Keita AD, Dembélé M, Kané M, Fongoro S, Traoré M, Sacko M, et al. Ultrasonographic aspects of urinary schistosomiasis in children of the Dogon plateau and the Niger office; impact of praziquantel treatment. Bull Soc Pathol Exot. 2001;94:335–8.
Santos J, Gouveia MJ, Vale N, Delgado Mde L, Gonçalves A, da Silva JM, et al. Urinary estrogen metabolites and self-reported infertility in women infected with Schistosoma haematobium. PLoS One. 2014;9: e96774. https://doi.org/10.1371/journal.pone.0096774.
Ahmed NS, Mahmoud SF, Mohamed ER, Khalifa RM. Histopathological analysis of Schistosoma haematobium metaplasia of the urinary bladder. J Egypt Soc Parasitol. 2017;47:211–8.
Al-Samawi AS, Aulaqi SM. Urinary bladder cancer in Yemen. Oman Med J. 2013;28:337–40. https://doi.org/10.5001/omj.2013.97.
Amin HAA, Kobaisi MH, Samir RM. Schistosomiasis and bladder cancer in Egypt: truths and myths. Open Access Maced J Med Sci. 2019;7:4023–9. https://doi.org/10.3889/oamjms.2019.857.
Bedwani R, Renganathan E, El Kwhsky F, Braga C, Abu Seif HH, Abul Azm T, et al. Schistosomiasis and the risk of bladder cancer in Alexandria, Egypt. Br J Cancer. 1998;77:1186–9. https://doi.org/10.1038/bjc.1998.197.
Darré T, Kpatcha M, Tchaou M, Amégbor K, Sonhaye L, N’Timon B, et al. Histological aspect of urinary schistosomiasis in Togo: results of a cohort of 192 cases. Bull Soc Pathol Exot. 2015;108:124–5. https://doi.org/10.1007/s13149-015-0427-4.
Gaye AM, Doh K, Thiam I, Bentefouet L, Woto-Gaye G. Schistosomiasis and cancer: a fortuitous association or relationships cause and effect. Bull Cancer. 2016;103:806–7. https://doi.org/10.1016/j.bulcan.2016.07.002.
Groeneveld AE, Marszalek WW, Heyns CF. Bladder cancer in various population groups in the greater Durban area of KwaZulu-Natal, South Africa. Br J Urol. 1996;78:205–8. https://doi.org/10.1046/j.1464-410x.1996.09310.x.
Martin JW, Vernez SL, Lotan Y, Abdelhalim A, Dutta R, Shokeir A, et al. Pathological characteristics and prognostic indicators of different histopathological types of urinary bladder cancer following radical cystectomy in a large single-center Egyptian cohort. World J Urol. 2018;36:1835–43. https://doi.org/10.1007/s00345-018-2331-6.
Mungadi IA, Malami SA. Urinary bladder cancer and schistosomiasis in North-Western Nigeria. West Afr J Med. 2007;26:226–9. https://doi.org/10.4314/wajm.v26i3.28315.
Abdel Mohsen MA, Hassan AA, El-Sewedy SM, Aboul-Azm T, Magagnotti C, Fanelli R, et al. Biomonitoring of n-nitroso compounds, nitrite and nitrate in the urine of Egyptian bladder cancer patients with or without Schistosoma haematobium infection. Int J Cancer. 1999;82:789–94. https://doi.org/10.1002/(sici)1097-0215(19990909)82:6%3c789::aid-ijc3%3e3.0.co;2-c.
Gaber DA, Wassef RM, El-Ayat WM, El-Moazen MI, Montasser KA, Swar SA, et al. Role of a Schistosoma haematobium specific microRNA as a predictive and prognostic tool for bilharzial bladder cancer in Egypt. Sci Rep. 2020;10:18844. https://doi.org/10.1038/s41598-020-74807-1.
Khaled HM, Abdel-Salam I, Abdel-Gawad M, Metwally A, El-Demerdash S, El-Didi M, et al. Evaluation of the BTA tests for the detection of bilharzial related bladder cancer: the Cairo experience. Eur Urol. 2001;39:91–4. https://doi.org/10.1159/000052418.
El-Sharkawi F, El Sabah M, Hassan Z, Khaled H. The biochemical value of urinary metalloproteinases 3 and 9 in diagnosis and prognosis of bladder cancer in Egypt. J Biomed Sci. 2014;21:72. https://doi.org/10.1186/s12929-014-0072-4.
Mohammed MA, Seleim MF, Abdalla MS, Sharada HM, Abdel Wahab AH. Urinary high molecular weight matrix metalloproteinases as non-invasive biomarker for detection of bladder cancer. BMC Urol. 2013;13:25. https://doi.org/10.1186/1471-2490-13-25.
Eissa S, Ali-Labib R, Swellam M, Bassiony M, Tash F, El-Zayat TM. Noninvasive diagnosis of bladder cancer by detection of matrix metalloproteinases (MMP-2 and MMP-9) and their inhibitor (TIMP-2) in urine. Eur Urol. 2007;52:1388–96. https://doi.org/10.1016/j.eururo.2007.04.006.
Eissa S, Labib RA, Mourad MS, Kamel K, El-Ahmady O. Comparison of telomerase activity and matrix metalloproteinase-9 in voided urine and bladder wash samples as a useful diagnostic tool for bladder cancer. Eur Urol. 2003;44:687–94. https://doi.org/10.1016/s0302-2838(03)00417-2.
Eissa S, Swellam M, Sadek M, Mourad MS, El Ahmady O, Khalifa A. Comparative evaluation of the nuclear matrix protein, fibronectin, urinary bladder cancer antigen and voided urine cytology in the detection of bladder tumors. J Urol. 2002;168:465–9.
Eissa S, Swellam M, Ali-Labib R, Mansour A, El-Malt O, Tash FM. Detection of telomerase in urine by 3 methods: evaluation of diagnostic accuracy for bladder cancer. J Urol. 2007;178:1068–72. https://doi.org/10.1016/j.juro.2007.05.006.
Eissa S, Motawi T, Badr S, Zaghlool A, Maher A. Evaluation of urinary human telomerase reverse transcriptase mRNA and scatter factor protein as urine markers for diagnosis of bladder cancer. Clin Lab. 2013;59:317–23. https://doi.org/10.7754/clin.lab.2012.120507.
Eissa S, Matboli M, Essawy NO, Shehta M, Kotb YM. Rapid detection of urinary long non-coding RNA urothelial carcinoma associated one using a PCR-free nanoparticle-based assay. Biomarkers. 2015;20:212–7. https://doi.org/10.3109/1354750x.2015.1062918.
Eissa S, Matboli M, Essawy NO, Kotb YM. Integrative functional genetic-epigenetic approach for selecting genes as urine biomarkers for bladder cancer diagnosis. Tumour Biol. 2015;36:9545–52. https://doi.org/10.1007/s13277-015-3722-6.
Eissa S, Habib H, Ali E, Kotb Y. Evaluation of urinary miRNA-96 as a potential biomarker for bladder cancer diagnosis. Med Oncol. 2015;32:413. https://doi.org/10.1007/s12032-014-0413-x.
Eissa S, Matboli M, Hegazy MG, Kotb YM, Essawy NO. Evaluation of urinary microRNA panel in bladder cancer diagnosis: relation to bilharziasis. Transl Res. 2015;165:731–9. https://doi.org/10.1016/j.trsl.2014.12.008.
Eissa S, Kassim SK, Labib RA, El-Khouly IM, Ghaffer TM, Sadek M, et al. Detection of bladder carcinoma by combined testing of urine for hyaluronidase and cytokeratin 20 RNAs. Cancer. 2005;103:1356–62. https://doi.org/10.1002/cncr.20902.
Eissa S, Badr S, Barakat M, Zaghloul AS, Mohanad M. The diagnostic efficacy of urinary survivin and hyaluronidase mRNA as urine markers in patients with bladder cancer. Clin Lab. 2013;59:893–900. https://doi.org/10.7754/clin.lab.2012.120623.
Eissa S, Kenawy G, Swellam M, El-Fadle AA, Abd El-Aal AA, El-Ahmady O. Comparison of cytokeratin 20 RNA and angiogenin in voided urine samples as diagnostic tools for bladder carcinoma. Clin Biochem. 2004;37:803–10. https://doi.org/10.1016/j.clinbiochem.2004.05.027.
Eissa S, Zohny SF, Swellam M, Mahmoud MH, El-Zayat TM, Salem AM. Comparison of CD44 and cytokeratin 20 mRNA in voided urine samples as diagnostic tools for bladder cancer. Clin Biochem. 2008;41:1335–41. https://doi.org/10.1016/j.clinbiochem.2008.08.085.
Eissa S, Matboli M, Mansour A, Mohamed S, Awad N, Kotb YM. Evaluation of urinary HURP mRNA as a marker for detection of bladder cancer: relation to bilharziasis. Med Oncol. 2014;31:804. https://doi.org/10.1007/s12032-013-0804-4.
Eissa S, Matboli M, Awad N, Kotb Y. Identification and validation of a novel autophagy gene expression signature for human bladder cancer patients. Tumour Biol. 2017;39:1010428317698360. https://doi.org/10.1177/1010428317698360.
El-Ahmady O, Halim AB, El-Din AG. The clinical value of CYFRA21–1 in bladder cancer patients: Egyptian experience. Anticancer Res. 1999;19:2603–8.
Abdou A, Tligui M, Le Loup G, Raynal G. A western cohort of urinary schistosomiasis. Prog Urol. 2012;22:598–601. https://doi.org/10.1016/j.purol.2012.03.004.
Aly MS, Khaled HM, Emara M, Hussein TD. Cytogenetic profile of locally advanced and metastatic Schistosoma-related bladder cancer and response to chemotherapy. Cancer Genet. 2012;205:156–62. https://doi.org/10.1016/j.cancergen.2012.01.011.
Acknowledgements
We thank the TropNet coordinator (Zeno Bisoffi, Negrar, Italy) and the TropNet Research committee (Thomas Zoller, Berlin-Charité, Germany; Philipp Zanger, Heidelberg, Germany; Juan Cuadros Gonzales, Madrid-HUPA, Spain; Emmanuel Bottieau, Antwerp, Belgium; Daniel Camprubí, Barcelona-UHC) for reviewing the study protocol. Davide Marangoni was awarded the “Rosario Russo” prize for the best Tropical Medicine MD thesis work by the Italian Society for Tropical Medicine and Global Health (SIMET). Federico Gobbi and Leonardo Motta were supported by the Italian Ministry of Health “Fondi Ricerca Corrente” to IRCCS Sacro Cuore Don Calabria Hospital – Linea 2.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the research conception and design. Literature search and data analysis were performed by DM, TM and LZ. The first draft of the manuscript was written by TM and DM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interests in any material discussed in this review. Outside of this research work, Prof. Alessandro Bartoloni reports institutional grants from MSD, ViiV and personal honoraria from GSK and Gilead.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manciulli, T., Marangoni, D., Salas-Coronas, J. et al. Diagnosis and management of complicated urogenital schistosomiasis: a systematic review of the literature. Infection 51, 1185–1221 (2023). https://doi.org/10.1007/s15010-023-02060-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02060-5