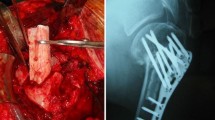
Abstract
Background:
Current therapies to effectively treat long-bone defects and extensive bone tissue loss remains limited. In this study, we created a new bone substitute by integrating advanced technologies such as structure patterning, controlled release of a bone growth factor and conjugation system for clinically effective bone regeneration. This novel bioactive bone substitute was evaluated for its safety and efficacy using a rabbit ulna model.
Methods:
A three dimensional bone patterned cylindrical structure with 1.5 cm in length and 5 mm in diameter was printed using poly(L-lactic acid)(PLLA) as a weight-bearing support and space-filling scaffold. And a bone morphogenetic protein 2 (BMP2) was employed to enhance bone regeneration, and coated to a 3D PLLA using alginate catechol and collagen to prolong the release kinetics. This novel bone substitute (BS)was evaluated for its physico-chemical and biological properties in vitro, and histological analysis and radiographical analysis such as X-ray, CT and micro-CT image analysis were performed to evaluate new bone formation in vivo.
Results:
The BS possesses an ideal shape and mechanically suitable proeperties for clinical use, with an easy-to-grab and break-resistant design at both ends, 80 ± 10 MPa of compression strength, and BMP2 release for two months. Histological analysis demonstrated the biocompability of BS with minimal inflammation and immune response, and X-ray, CT and micro-CT demonstrated effective new bone formation in rabbit ulna defect model.
Conclusion:
The preclinical study of a novel bioactive bone substitute has shown its safe and effective properties in an animal model suggesting its clinical potential.









Similar content being viewed by others
Data availability
The data presented in this study are available on request from all the authors.
References
Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007;19:513–21.
Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25:2445–61.
Jordana F, Le Visage C, Weiss P. Substituts osseux. Médecine/sciences. 2017;33:60–5.
Gotz W, Papageorgiou SN. Molecular, cellular and pharmaceutical aspects of synthetic hydroxyapatite bone substitutes for oral and maxillofacial grafting. Current Pharmaceut Biotechnol. 2017;18:95–106.
Lee YJ, Ryu YH, Lee SJ, Moon SH, Kim KJ, Jin BJ, et al. Bone regeneration with 3D-printed hybrid bone scaffolds in a canine radial bone defect model. Tissue Eng Regen Med. 2022;19:1337–47.
Krishnan L, Priddy LB, Esancy C, Klosterhoff BS, Stevens HY, Tran L, et al. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017;49:101–12.
Wildemann B, Ignatius A, Leung F, Taitsman LA, Smith RM, Pesántez R, et al. Non-union bone fractures. Nat Rev Dis Primers. 2021;7:57.
Ekegren CL, Edwards ER, de Steiger R, Gabbe BJ. Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int J Environ Res Public Health. 2018;15:2845.
Han JJ, Yang HJ, Hwang SJ. Enhanced bone regeneration by bone morphogenetic protein-2 after pretreatment with low-intensity pulsed ultrasound in distraction osteogenesis. Tissue Eng Regen Med. 2022;19:871–86.
Cho SH, Shin KK, Kim SY, Cho MY, Oh DB, Lim YT. In situ-forming collagen/poly-γ-glutamic acid hydrogel system with mesenchymal stem cells and bone morphogenetic protein-2 for bone tissue regeneration in a mouse calvarial bone defect model. Tissue Eng Regen Med. 2022;19:1099–111.
Skovrlj B, Koehler SM, Anderson PA, Qureshi SA, Hecht AC, Iatridis JC, et al. Association between BMP-2 and carcinogenicity. Spine. 2015;40:1862.
Cahill KS, McCormick PC, Levi AD. A comprehensive assessment of the risk of bone morphogenetic protein use in spinal fusion surgery and postoperative cancer diagnosis. J Neurosurg Spine. 2015;23:86–93.
Beachler DC, Yanik EL, Martin BI, Pfeiffer RM, Mirza SK, Deyo RA, et al. Bone morphogenetic protein use and cancer risk among patients undergoing lumbar arthrodesis: a case-cohort study using the SEER-medicare database. J Bone Joint Surg Am. 2016;98:1064–72.
Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62:ONS423–31.
Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–48.
Oshiro JA, Sato MR, Scardueli CR, Lopes de Oliveira GJP, Abucafy MP, Chorilli M. Bioactive molecule-loaded drug delivery systems to optimize bone tissue repair. Curr Protein Pept Sci. 2017;18:850–63.
Howard MT, Wang S, Berger AG, Martin JR, Jalili-Firoozinezhad S, Padera RF, et al. Sustained release of BMP-2 using self-assembled layer-by-layer film-coated implants enhances bone regeneration over burst release. Biomaterials. 2022;288:121721.
Wilson CG, Martín-Saavedra FM, Vilaboa N, Franceschi RT. Advanced BMP gene therapies for temporal and spatial control of bone regeneration. J Dent Res. 2013;92:409–17.
Jordana F, Le Visage C, Weiss P. Bone substitutes. Med Sci (Paris). 2017;33:60–5.
Kang MH, Lee H, Jang TS, Seong YJ, Kim HE, Koh YH. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019;84:453–67.
Feng Y, Zhu S, Mei D, Li J, Zhang J, Yang S, et al. Application of 3D printing technology in bone tissue engineering: a review. Curr Drug Deliv. 2021;18:847–61.
Hong YR, Kim TH, Park KH, Kang J, Lee K, Park EK, et al. rhBMP-2-conjugated three-dimensional-printed poly (L-lactide) scaffold is an effective bone substitute. Tissue Eng Regen Med. 2023;20:69–81.
Thangaraju P, Varthya SB. ISO 10993: biological evaluation of medical devices. in Medical Device Guidelines and Regulations Handbook. 2022, Springer. p 163–187.
Upman PJ. ISO 10993–6: Test for local effects after implantation. BONEZone. 2006;5:50–2.
Zhang Q, Mochalin VN, Neitzel I, Hazeli K, Niu J, Kontsos A, et al. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials. 2012;33:5067–75.
Yuan B, Zhou SY, Chen XS. Rapid prototyping technology and its application in bone tissue engineering. J Zhejiang Univ Sci B. 2017;18:303–15.
Guda T, Walker JA, Singleton BM, Hernandez JW, Son JS, Kim SG, et al. Guided bone regeneration in long-bone defects with a structural hydroxyapatite graft and collagen membrane. Tissue Eng Part A. 2013;19:1879–88.
Lee H, Rho J, Messersmith PB. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 2009;21:431–4. .
Wang F, Zheng L, Theopold J, Schleifenbaum S, Heyde CE, Osterhoff G. Methods for bone quality assessment in human bone tissue: a systematic review. J Orthop Surg Res. 2022;17:174.
Lobb DC, DeGeorge BR Jr, Chhabra AB. Bone graft substitutes: current concepts and future expectations. J Hand Surg. 2019;44:497–505.
Zhang H, Yang L, Yang XG, Wang F, Feng JT, Hua KC, et al. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg. 2019;11:725–37.
West TA, Williams ML. Orthobiologics. Clin Podiatr Med Surg. 2019;36:609–26.
Lin S, Yin S, Shi J, Yang G, Wen X, Zhang W, et al. Orchestration of energy metabolism and osteogenesis by Mg2+ facilitates low-dose BMP-2-driven regeneration. Bioact Mater. 2022;18:116–27.
Ruehle MA, Krishnan L, Vantucci CE, Wang Y, Stevens HY, Roy K, et al. Effects of BMP-2 dose and delivery of microvascular fragments on healing of bone defects with concomitant volumetric muscle loss. J Orthopaed Res. 2019;37:553–61.
Decambron A, Fournet A, Bensidhoum M, Manassero M, Sailhan F, Petite H, et al. Low-dose BMP-2 and MSC dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J Orthop Res. 2017;35:2637–45.
Efraim Y, Sarig H, Cohen Anavy N, Sarig U, de Berardinis E, Chaw SY, et al. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater. 2017;50:220–33.
Hu T, Liu L, Lam RWM, Toh SY, Abbah SA, Wang M, et al. Bone marrow mesenchymal stem cells with low dose bone morphogenetic protein 2 enhances scaffold-based spinal fusion in a porcine model. J Tissue Eng Regen Med. 2022;16:63–75.
Girón J, Kerstner E, Medeiros T, Oliveira L, Machado GM, Malfatti CF, et al. Biomaterials for bone regeneration: an orthopedic and dentistry overview. Braz J Med Biol Res. 2021;54:e11055.
Fung SL, Wu X, Maceren JP, Mao Y, Kohn J. In vitro evaluation of recombinant bone morphogenetic protein-2 bioactivity for regenerative medicine. Tissue Eng Part C Methods. 2019;25:553–9.
Lafuente-Gracia L, Borgiani E, Nasello G, Geris L. Towards in silico models of the inflammatory response in bone fracture healing. Front Bioeng Biotechnol. 2021;9:703725.
Schell H, Duda GN, Peters A, Tsitsilonis S, Johnson KA, Schmidt-Bleek K. The haematoma and its role in bone healing. J Exp Orthopaed. 2017;4:5.
Hankenson K, Zimmerman G, Marcucio R. Biological perspectives of delayed fracture healing. Injury. 2014;45:S8–15.
Wang W, Yeung KW. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioactive materials. 2017;2:224–47.
Zhu G, Zhang T, Chen M, Yao K, Huang X, Zhang B, et al. Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact Mater. 2021;6:4110–40.
Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–43.
Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389–99.
Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–62.
Gambari L, Grigolo B, Grassi F. Hydrogen sulfide in bone tissue regeneration and repair: state of the art and new perspectives. Int J Mol Sci. 2019;20:5231.
Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–8.
Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66.
Nakase T, Yoshikawa H. Potential roles of bone morphogenetic proteins (BMPs) in skeletal repair and regeneration. J Bone Miner Metab. 2006;24:425–33.
Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51–65.
Acknowledgements
This research was supported by the Korean Fund for Regenerative Medicine funded by the Ministry of Science and ICT and by the Ministry of Health and Welfare (21C0705L1-11). We thank Enago for professional English language editing.
Author information
Authors and Affiliations
Contributions
JOL and C-WO conceived of the study. YRH, T-HK, KL, JOL, and C-WO wrote the manuscript. YRH and T-HK performed experiments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical statement
Animal experiments were performed in accordance with the guidelines approved by KMEDI-hub (IACUC Approval no. KMEDI-22052302-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, Y.R., Kim, TH., Lee, K. et al. Bioactive Bone Substitute in a Rabbit Ulna Model: Preclinical Study. Tissue Eng Regen Med 20, 1205–1217 (2023). https://doi.org/10.1007/s13770-023-00591-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00591-4