Abstract
Fabrication of functional organs is the holy grail of tissue engineering and the possibilities of repairing a partial or complete liver to treat chronic liver disorders are discussed in this review. Liver is the largest gland in the human body and plays a responsible role in majority of metabolic function and processes. Chronic liver disease is one of the leading causes of death globally and the current treatment strategy of organ transplantation holds its own demerits. Hence there is a need to develop an in vitro liver model that mimics the native microenvironment. The developed model should be a reliable to understand the pathogenesis, screen drugs and assist to repair and replace the damaged liver. The three-dimensional bioprinting is a promising technology that recreates in vivo alike in vitro model for transplantation, which is the goal of tissue engineers. The technology has great potential due to its precise control and its ability to homogeneously distribute cells on all layers in a complex structure. This review gives an overview of liver tissue engineering with a special focus on 3D bioprinting and bioinks for liver disease modelling and drug screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Liver is the largest gland in the human body and is responsible for metabolic functions including production of bile, albumin and cholesterol, regulation of amino acids, filters blood, resists infections and processes glucose. Liver is only organ in the human body that can efficiently regenerate, and hence any damage to the organ leads to various disorders and complications. However, extensive use of drugs can damage the hepatocytes beyond repair and limit its regeneration capabilities leading to liver failure, which in turn gives raise to complications such as neurological impairment, renal dysfunction, and metabolic abnormalities [1]. Chronic liver diseases like fibrosis, cirrhosis, chronic viral hepatitis, fatty liver disease etc. affects people of all age group and race [2] and are on raise globally due to change in lifestyle and contribute to the global healthcare burden (mortality and morbidity) with Cirrhosis, end stage of liver fibrosis ranking the eleventh leading cause of death in the world. According to the latest statistical data, liver diseases are the eighth leading cause of death in India (World Health Organization 2020) [3]. The World Health Organization (WHO) recently estimated that 296 million people were living with chronic hepatitis B infection. The hepatitis B infection caused an estimated 820 000 deaths, mostly from cirrhosis and hepatocellular carcinoma. Globally, there were 36,000 fatal cases due to acute hepatitis B. Hepatitis B virus (HBV) mortality is high but will decline since vaccines for the same are now available [4]. Viral hepatitis has been found to be a leading cause for chronic liver diseases all around the world and two main causes of viral hepatitis is been Alcoholic liver disease (ALD) and Non-alcoholic fatty liver disease (NAFD) [5]. Table 1 summarises the prevalence of liver diseases in India. The current medical intervention available for liver failure is the partial or full liver transplantation. However, organ transplantation has its own shortcomings such as availability of healthy donors, immune response after transplantation and rate of engraftment success. Other tissue engineering options available as treatment includes bioartificial liver system, hepatocyte transplantation, cell therapy approaches with certain shortcomings [6]. Tissue engineering strategies offer alternative solution by fabricating in vitro tissues to repair or replace the damaged part of the liver.
The 3D printing has driven innovations in many areas and has changed the paradigm of “design for manufacturing” to “design for function”. This technology was first introduced by Charles Hull in 1986 where a material was printed in a layer-by-layer fashion and was termed as stereolithography. Since then, the models have been developed in different shapes, sizes and colour for the manufacturing industry using resin, plastic, metal etc. [7]. 3D bioprinting is an additive manufacturing process where the cells, proteins and biomaterials are printed into desired structures. 3D bioprinting helped in automating and scaling up the entire process and provides precision and reproducibility. A lot of research is focused on improving bioinks for shape fidelity, elasticity, cell viability, to mimic the desired native tissue. These innovations have led to fabrication of functional tissues with 3D bioprinting technology while the futuristic approach is their clinical translation [8]. 3D printed patient specific liver models are on the rise as they show great potential in modelling diseases accurately and reduce the time and money for drug screening process [9].
In this review, we focus on 3D bioprinting based liver models for liver regeneration, drug screening, disease models, surgical applications, clinical translations, and commercially available products. Figure 1 represents the overview of 3D bioprinting liver and its applications. Furthermore, a brief introduction on various tissue engineering techniques for in vitro liver models is given in this review which majorly focuses on 3D bioprinting. 3D bioprinting is a promising technique for the development of biocompatible structures that mimic the native organ’s architecture. However, liver tissue engineering has many challenges since the liver is a highly vascular organ. Hence, creating a similar microenvironment could be challenging in order to generate a functional liver tissue. The review also discusses the current challenges and potential solutions along with available cell sources, and suitable bioinks and bioprinting strategies.
2 Liver architecture and functions
The human liver makes up around 1/50th of the total body weight and histological slices of the liver reveal uniform landscape of cells and tissue architecture. There are four lobes present in the adult human liver, namely right lobe, left lobe, caudate and quadrate, which are made up of different cell types in which the hepatocytes make up 80% of the liver cell population [10]. Figure 2 illustrates the different cell populations present in a human liver. The five main systems that make up the liver are—(a) intrahepatic vascular system, (b) stroma, (c) sinusoidal cells, (d) hepatocytes and biliary epithelial cells, and (e) Hepatic lobules and acinus; each of them contributing to total functioning of the liver. The liver is known to have multiple functions that is associated with digestion of food, detoxifying the toxins, haemostasis, balancing the fluids and electrolytes (Fig. 3). These activities are grouped under metabolism, bile secretion and detoxification [12]. The collective functioning of the liver cells helps in understanding the liver functioning mechanism as an organ. The key to the liver's proper operation rests on the ongoing maintenance of a variety of biochemical activities, including the many metabolic processes taking place in the liver's hepatocytes and sinusoidal cells. It is challenging to fully understand all the functions of the liver because of the multiple connections between various metabolic pathways and functional activities. Some of these pathways are represented in Fig. 4. In addition to assisting cellular absorption and excretory activities, metabolic processes entail several distinct and contrasting biochemical paths to enable the synthesis of breakdown and the activation or deactivation of substances [13]. The liver's additional crucial purpose is to supply the right amounts of solutes for the proper operation of all the different organs including the essential organs—the brain, heart, and kidneys. A regulated metabolic system is needed to maintain the complex web of biochemical processes that take place inside the liver cell. Different regulatory metabolism take place at different levels which includes molecular, cellular and organ levels [14,15,16]. The liver capacity for normal functioning fails following the cell death where the damage exceeds the capacity of regeneration and repair. To limit the liver injury, it is an endogenous defence mechanism that operates on cells by recruiting them before it is in an irreversible stage of damage and during this process the cells are condemned to die via apoptosis rather than necrosis. A huge number of factors are involved in the regenerative activities of the cells at various intracellular events which includes extracellular signalling, auxiliary mitogenic signals and complex mitogenic pathways which are summarized in Table 2.
Liver cell types [11]
3 Liver model fabrication techniques
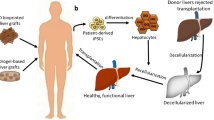
A major rising concern is towards the death of millions annually which are due to the different kinds of liver diseases. One of the reasons for patients dying is the long waiting list due to the lack of availability of donor organs. Scientists are exploring alternate solutions to overcome the rising demand; one such alternative is liver tissue engineering (LTE) principles [63]. The prime aim of liver tissue engineering is to mimic the natural liver architecture and its physiology [64]. Latest trends of liver tissue engineering are the fabrication of micro tissue structures by particularly organizing the liver cells via micro fabrication techniques and nanotechnology [65]. There are two primary uses for LTE; first, bioengineered livers may be utilised as in vitro models for evaluating xenobiotics (such as medications and infections) Barranger et al. developed a 3D model of HepaRG human liver cells using the CometChip technology for investigating the toxicology study as well as a disease model for liver induced toxicity [66]. Vernetti et al. created self-assembly liver model for investigating the physiology, drug safety, and disease models [67]. Second, LTE seeks to create substitutes for donor organs for in vivo transplantations, although being currently distant from practical use. A further advancement in the field of scaffold fabrication for tissue engineering is use of decellularized matrix derived from native organs of cadaveric donors. Uygun et al. first generated a recellularized liver graft which is transplantable in the rats from a decellularized liver biomatrix. The transplanted graft had successful liver-like functions and more efficient when comparable to normal liver in vitro [68].
The main ingredient of tissue engineering is the biocompatible constructs which have features like biochemical, biomechanical, and physicochemical eventually helping in providing the microenvironment for growth of cells and their differentiation which can be applied in vivo for liver regeneration as support devices [13, 69, 70]. These features largely influence the duration of cell survival in vivo, interaction of the constructs with the host post implantation, the toxic and immune responses etc. Hepatic cells are essential and there are already several potential cell sources, which are discussed later in this review paper. Other basic components of liver tissue engineering include various cell sources and supportive materials [64, 71]. The development of such constructs requires a vast knowledge spectrum covering the in-depth anatomy and functioning of organ, tissue engineering aspects, biological sciences, and use of biomaterials. Following common strategies of liver tissue engineering, researchers have started to work on the various scaffold systems, a few being—hydrogels, cell sheets, organ on chip (OOC), scaffolds etc. Many natural biomaterials (gelatin, collagen, fibrin, alginate, cellulose, heparin etc.) or synthetic polymers (polyvinyl alcohol, poly-L-lactic acids, poly-N-isopropylacrylamide, polycaprolactone etc.) are used in order to fabricate the constructs and based on the application of the construct different paracrine factors, cellular components and growth factors are included in the constructs [72,73,74,75]. Latest advancement in LTE has been exploiting the use of decellularized extracellular matrix derived from liver as they pose several advantages [76,77,78,79]. One, they have a range of biochemical constituents that can mimic the native liver tissue [77, 78]. Two, they maintain a variety of growth factors in addition to the bulk of structural proteins and polysaccharides, which may be sufficient to support cellular survival, proliferation, and differentiation with the benefit of obviating the need for extra supplementation [80, 81]. Three, they can maintain the natural liver tissue's structure, which in vivo provides the essential mechanical support. Fourthly, they are pro-inflammatory in nature and have minimal antigenicity, which prevents post-implantation graft rejection [82]. Numerous reviews from the past have covered the various techniques and biomaterials utilised to create tissue engineered constructions and a small attempt has been made in this paper to summarise them. [17, 83, 84]. The liver has a limited supply of cellular components, and its intricate microarchitecture and functions provide a variety of challenges [84]. Various techniques are available in the tissue engineering for fabricating and some studies for the same are summarized in Table 3.
3.1 Electrospinning
Electrospinning helps in fibre fabrication of size range from 100 nm to 0.1 µm using high voltage [126]. The high voltage is being applied on the polymeric solution and based on charge it causes repulsion (opposing the surface tension of the solution) and overcomes the surface tension that leads to formation of the jet. There is a repel created between the ejected solution and the melt, the solvent gets evaporated and the formation of fibres when the jet travels to the collector. Taymour et al. [127] generated an artificial 3D invitro model using alginate and methylcellulose.
3.2 Phase separation
This process can be achieved at another temperature or via non-solvent to fabricate foams and membranes which are porous in nature [128]. To achieve heterogeneous scaffold structures with pores, the phase separation through nonsolvent is used [129]. These scaffolds are usually heat sensitive and not suitable for using at high temperature [130]. Further the thermal phase separation can be subdivided into solid–liquid phase separation and liquid–liquid phase separation [131, 132].
3.3 Freeze-drying
Freeze drying is a popular method to directly convert any solvents into liable materials like solids with stability. A wide application of products derived from these methods are being used in food sciences for packaging and distributing purposes [119, 133, 134]. This process involves three major steps that are—freezing the samples at lower temperature (~ 80 °C), lowering of pressure via vacuum and finally removal of unfrozen water molecules through desorption. The scaffolds derived using this method have been widely used in the field of tissue engineering. Skardal et al. [135] created an in vitro liver construct with PEG based crosslinkers.
3.4 Three dimensional (3D) bioprinting
This involved the use of biomaterials which is used to print a design 3D based on the computer aided designing. This method is widely used to obtain 3D functional scaffolds with cells for in vivo and in vitro studies [136]. With the evolving technology, the 3D bioprinting of the liver has many applications and one such is the use of 3D bioprinted material in liver transplantation [137]. Our group had recently published the detailed review on advancements in 3D bioprinting for biomedical applications [9]. Wang et al. [115] developed a 3D scaffold for demonstrating hepatocyte like cells derived from iPSC mature on bioprinted matrix.
3.5 Self-assembly
As the name suggests, it is organizing the provided materials into design or structures via technology. It has been widely applied in fabricating various nanofibers [138, 139]. Self-assembly is a process in which nanoscale particles or materials spontaneously organise specified components into ordered superstructures that may be used in a variety of applications. Nanostructures can be created in a stirred medium by static or dynamic self-assembly. Peak et al. created self-assembly liver model for investigating the physiology, drug safety, and disease models [140].
4 Bioprinting techniques used for hepatic engineering
There are various bioprinting techniques that are used to develop 3D bioprinted structures (Fig. 5). The basic components of a 3D printing system contain a filament, extruder motor, hot end, heated bed, and computer logic [141]. The various techniques are classified as extrusion based, inkjet and photocuring based techniques.
4.1 Extrusion- based bioprinting
This technique is one of the most used 3D bioprinting techniques, it generates continuous filaments and forms 3D structures of various size, shape, and resolutions. To print scaffolds with multiple cells and vascular structures, multimaterial and coaxial extrusion bioprinting is used [142]. The parameters like temperature, pressure, velocity, cell density must be optimised to obtain good cell viability, shape fidelity and enhanced cell functionality [143, 144]. Lewis et al., precisely 3D printed gelatin scaffolds having control pore geometry. A pneumatic extrusion-based printer, EnvisionTEC (GMBH) 3D-Bioplotter was used to print the gelatin solution. The parameters such as nozzle diameter, layer slicing, strut spacing were kept constant at 200 µm, 156 µm and 700 µm respectively. The 3D printed scaffolds has 6 layers with dimensions of 15 mm*15 mm and cultured with HUH 7 human hepatocellular carcinoma cell line [108]. Kang et al., created a technique for simultaneously fabricating heterogenous, multicellular, and multi material structures. A precursor cartilage was designed and fabricated that was added to various segments of the printer. The different types of bioinks were added to the cartridge and then placed into the syringe. ECs and HepG2 cells were used to fabricate the co-culture model. The use of heterogeneous cells for developing hepatic lobule increased the cell functionality in comparison to homogenous cell printing [145]. Billiet et al., fabricated 3D printed microporous scaffolds using gelatin methacrylamide using a bioplotter dispensing system to develop the scaffolds. The printer has three axes for pneumatically dispensing the deposit cell laden hydrogel on the platform. To support the temperature of the gelatin, homogenous plotting temperature was adjusted till the dispensing needle and the stationary platform was equipped with cooling elements such as Peltier element and HepG2 cells with seeding density of 1.5*106 cells/ml were seeded. Plotting speed, air pressure and temperature were optimised and a scaffold of dimensions, 1–3 mm thick with 13*13 mm dimensions were fabricated [146].
4.2 Inkjet-based bioprinting
The basic principle of inkjet based bioprinting is the formation of individual droplets to form the construct [147]. There are three main approaches to droplet formation such as thermal, piezoelectric and electrohydrodynamic methods. This method is better than extrusion based in terms of controlling the biological elements but the limitation of this technique is the use of only low viscosity materials [148]. Lee et al., fabricated 3D hepatic block scaffolds using neutralised type 1 atelo collagen solution was bioprinted and it showed differentiated human adipose stem cells to hepatocyte-like cells. The scaffolds were designed in a 3D bioprinter with 29G blunt nozzle and fixed with a moving speed and pressure of 2 cm s−1 and 150 kPa respectively [149]. Parsa et al., improved the reliability of the droplet formation that is inconsistent in inkjet-based 3D printing by stirring and gentle agitation. The researchers worked with type I rat-tail collagen and fabricated a hydrogel and seeded with HepG2 cells. The inkjet printer was piezoelectrically driven and had a nozzle of size 100 µm. The reservoir of the printer was loaded with HepG2 and seeded in concentration of 200,000 droplets or 10,000 cells on the gelled hydrogel [150]. Moya et al., developed an oxygen monitoring system on the liver organ on a chip (OOC) by placing multiple sensors on the thin membrane inside the liver OOC. The sensors were printed using an inkjet printer along with the microfluidic channel. Three kinds of ink formulations were used to print the DO sensors- low-curing gold, silver, and dielectric photoresist SU-8. For the printing process, a primer layer was formed to seal the porosity of the membrane where the sensors will be placed. The steps of printing the DO sensors were first, the printing of SU-8 ink as the primer layer, then printing of two SU-8 layers with spacing between the drops (DS), gold elements and counter elements were printed next also with DS of 15 µm. Silver elements were printed next to develop the electrode structures that form pseudo-reference electrodes with DS 30 µm. Primary human and rat hepatocytes were used to prove the concept and oxygen consumption rate was calculated using carbonyl-cyanide-4-(trifluoromethoxy)phenylhydrazone. The study demonstrated that the inkjet printing technique is a feasible technique that can be used for the integration of the sensors for evaluating the metabolic activity of cells [151].
4.3 Photocuring-based bioprinting
In this technique, the photosensitive hydrogel is cured using light irradiation and creates complex structures in comparison to extrusion and inkjet-based techniques [152]. There are two types of photocuring techniques-stereolithography and digital light processing. Yu et al., used specific decellularized bioink to fabricate patient specific tissues using digital light processing based bioprinter. The bioprinter was an in-house developed DLP based system that had five main components namely-UV light source, computer for sliced image- flow, a digital chip that helps in modulating the UV light and is comprising around 2 million micro-mirrors, projection optics set and a linear stage to position the sample and control the projected image and focal plane. The dimensions for the liver construct were 3 × 3 × 250 µm with a lobular pattern and small regions showcasing central vein and portal triad. hiPSC-hepatocytes were added to the dECM bioink in concentration of 30 million cells/ml and the construct was printed on a 24 well plate which was then maintained further according to the requirement of the cells in the culture. This approach allowed rapid bioprinting with accuracy and precision with maintenance of relevant functional properties [153]. Grix et al., provided with evidence for bioprinting a liver organoid. They utilised the stereolithographic printing approach and used HepaRG and human stellate cells and the liver construct was made up of two materials- PEG for the channels and GelMA for holding HepaRGs and SteCs of 4 mm thickness. Using blue illumination, the layer printed was photopolymerized directly. In one printing process, six tissue models were printed parallelly with each model containing 106 cells [154]. Ma et al. fabricated 3D hydrogel that were having hiPSC-HPCs using stereolithographic based bioprinting system. A customised bioprinting system was used with a DMD system, LED light source and movable stage. The triculture 3D model was printed with first layer of hepatic cells then another layer of complementary cells. The hepatic model was able to provide in vitro maturation of hepatic cells in the microenvironment of hepatic lobule microarchitecture [155].
5 Bioinks for hepatic engineering
Bioinks are the major component of 3D bioprinting and comprises cells, biomaterial, and other biological factors. The materials must be biocompatible, biodegradable, non-toxic, non-immunogenic. Based on the requirement of the construct, the cells and materials are selected, for example, models for drug designing, regeneration, disease modelling, etc. The cells should interact with biomaterials and be able to retain their biological functions. Figure 6 illustrates the types of bioink along with its composition and properties. Hepatocytes are the most abundantly present parenchymal cells in the liver and along with it there are Kupffer cells, endothelial cells, and hepatic stellate cells. For constructing liver tissue models, cell lines are available which utilises the primary human liver cells.
5.1 Natural polymers based bioink
The naturally occurring polymers that are the preferred choice for printing liver tissue owing to their properties such as biocompatibility, biodegradable, non-toxic, support angiogenesis and organogenesis. Gelatin, which has a single chain of polymers that is obtained by partial hydrolysis of collagen is the most sought after natural based polymer for tissue engineering applications. Rapid solubility in biological buffers, adaptability for crosslink, thermosensitivity, cell recognition property, and non-toxin makes gelatin the highly researched compound for 3D bioprinting [154,155,156,157,158]. Roopesh et al., developed a high throughput method that helps in fabrication of liver parenchymal microtissues (LPMT) with use of GelMA and HepG2 cells. The LPMT was fabricated by the technique of hanging drop culture chamber, a novel technique that was developed by the team. HepG2 droplets were suspended on polyethylene terephthalate substrate and this substrate was then kept inside the chamber where LPMTs were obtained. GelMA was used to provide stability to microtissue by sandwiching the LPMT between GelMA hydrogel enhancing the liver functions and response to insulin stimulation. The GelMA sandwich of LPMTs showed better activity of urea synthesis, albumin secretion, P450 cytochrome activity in comparison to only LPMTs in suspension [159]. Alginate, obtained from seaweed algae, and is a negatively charged polysaccharide having made up of two copolymers- guluronic acid and mannuronic acid, that helps in providing the gelation and the flexibility to the material [160]. Crosslinking of alginate is only possible via divalent cations, as physical gelation cannot be supported due the sol–gel temperature being below 0°C [161]. Alginate is a low-cost material with sufficient biocompatibility to help in development of in vitro scaffolds. Xie et al., developed a novel modelling system technique for generating hepatorganoids using HepaRG cells. Patient samples of HCC were obtained and primary HCC cells were isolated. Gelatin and sodium alginate were used as materials to which cells were added. The bioink was added to the 3D cell printer and layer by layer construct was printed and in vitro analysis was performed. The 3D printed- HCC model showed uniformity in cell density and distribution, the biological characteristics were retained, and this model can be used to screen drugs for various HCC patients [162]. Collagen, is one of the most abundant proteins, is highly biocompatible and due to presence of RGD motifs support the cell attachment as well [163]. Collagen is considered for encapsulating hepatocytes. Crosslinking can be done via chemical crosslinkers, changing pH and temperature [164]. The mechanical strength of collagen is very poor, to overcome this, it is added with supporting materials such as polycaprolactone (PCL). Mazzocchi et al., developed a bioink for fabricating 3D printed liver tissue construct using collagen type I and liver cells like hepatocytes and stellate cells. Methacrylated collagen type I and thiolated hyaluronic acid was used to prepare printable bioink and Allevi 2 bioprinter was used for liver model printing. To study the functionality, primary human hepatocytes were bioprinted in a four-spoke structure and cell viability and functional tests were performed by adding acetaminophen. The liver construct was able to maintain urea and albumin production and responded well to APAP [165]. Cellulose is a non-biodegradable and biocompatible natural polymer that does not support cell adhesion [166] and is being used as bioink in two forms, carboxymethylcellulose and nanocellulose [167]. The former properties can be tuned depending on the degree of methylation [168]. Nanocellulose is biocompatible and successfully used to 3D print structures. It is either present in the state of crystals or fibres in a nano-structured form [169]. Wu et al., fabricated a bioink consisting of alginate, cellulose nanocrystal and GelMA to construct liver tissue. A honeycomb scaffold was 3D printed using micro-extrusion based printing and the matrix between the scaffolds were filled with GelMA hydrogels containing HepG2 cells. The construct showed intracellular interactions and alignment along with albumin secretion [170].
5.2 Synthetic polymers based bioink
The tuneable properties have made the synthesised polymers to be used for 3D printing of liver. The mechanical and physicochemical properties of such materials are tailored and can be controlled by chemical modifications. Most commonly used synthetic polymers for 3D printing of liver are polyethylene glycol (PEG), polycaprolactone (PCL) and poly (lactic- co- glycolic acid) (PLGA) are all FDA approved polymers known for biocompatibility and widely used for various tissue regeneration. Chen et al., fabricated human ectopic artificial livers (HEALS) by using PEG and human hepatocytes and implanted in nude mice. Drug metabolising enzyme functions were tested, HEALS were able to express them and also showed potential in screening of compounds such as Cytochrome P450. This kind of model applications include providing a supportive microenvironment, delivery vehicle and also a barrier for rejection-delaying process [85]. Seng Ng et al., developed a 3D printed hexagonal model using polyethylene glycol (PEG) and human foetal liver cells that was inspired by natural liver architecture to assess antiviral agents, understand specific drug metabolism and also detect specific drug hepatotoxicity. The model showed differentiation of specific factors and also maintained the advanced functions [171]. PCL is a hydrophobic homopolymer that is synthesised by polymerization of ring structure of caprolactone. Grant et al., fabricated a 3D printed scaffold using Electrospun PCL, ECM and HepG2 cells. ECM layer was placed on PCL scaffold layer which was cut from the Electrospun fibres and then onto that was then seeded with HepG2. The construct shows potential in altering the production of ECM and also future in liver tissue engineering [172]. Park et al., fabricated an anastomosis device by the name absorbable vascular anastomosis device using PLGA and consisted of two inner rings and a coupler. The device was able to maintain its shape for 3 weeks, and the device was implanted in mini-pigs and after 4 months autopsy showed that the device was completely absorbed [173].
5.3 Multicomponent bioink
As the name suggests, multicomponent bioink comprises two or more biomaterials having good rheological properties that can be adjusted during and after printing. There are four types of multicomponent bioinks which includes homogenous networks that forms covalent crosslinking with each other, interpenetrating networks forms independent crosslink networks and gets intertwisted with each other, nanocomposite networks has increased stiffness by adding nanocomposites and the supramolecular networks have reversible functional groups [158, 174,175,176,177]. Taymour et al. fabricated a 3D bioprinting based concept to develop a 3D core–shell liver model. Alginate and methylcellulose were used as biomaterials to form this multicomponent bioink along with HepG2 cells and fibroblasts where they acted as supportive cells. There was proper cell attachment, viability, proliferation of the cells in the core shell scaffold [178]. Hiller et al., formulated a bioink that had alginate, gelatin and human extracellular matrix along with HepaRG liver cells and used Pneumatic extrusion printer to fabricate the 3D model. The construct was stable, viable and had desirable properties of metabolic functions of the cells and along with it, the model also showcased the efficient adenoviral replication [179]. Leva et al., used direct laser induced forward transfer technique to develop a 3D printed scaffold for liver regeneration. Collagen-GAG scaffolds were prepared using Huh-7 cells and then analysed quantitatively and qualitatively. The cells were viable for 2 and 24 h in the desired pattern and were properly adhered to the scaffold [180]. Khati et al., used gelatin and polyethylene glycol to develop a 3D printed model for liver. Decellularized liver matrix bioink containing HepG2 cells. To enhance the stability and application, the bioink was cross linked chemically via mushroom tyrosinase and due to this, an increase of 16-fold was observed in the viscosity of the bioink and storage modulus in comparison to bioink without crosslinking. The grid model was able to support cell proliferation and cell adherence [181].
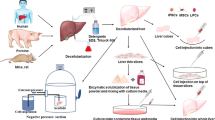
5.4 Decellularized extra cellular matrix based bioinks
The decellularization process removes cellular content on the tissues and organs and preserves the integrity of the native extracellular matrix and its components [182]. There are few criteria that should be fulfilled for a tissue to be considered as fully decellularized firstly, DNA content less than 50 ng/mg, 200 bp or smaller DNA fragment length, second absence of visible cellular content in histological characterizations like H&E staining and DAPI [183, 184]. The physical method of decellularization in which physical parameters are targeted such as temperature, pressure and pH to burst the cells. Some of the techniques for physical methods are freeze–thaw, mechanical agitation, supercritical CO2, high hydrostatic pressure [185, 186]. The chemical method involves use of chemical agents such as detergents, ionic liquids, acids, bases to decellularized the tissues by disrupting protein- protein interactions, disrupting the nucleic acid compositions and solubilising cell membranes [178, 186, 187]. Enzymatic method is another way of decellularizing in which enzymes like proteases, nucleases, chelating agents are used which cleaves the cell matrix adhesions and cleaves proteins and peptidases [178, 186]. To evaluate successful decellularization of any tissue, it is important for qualitative assessments like H&E staining, DAPI and quantitate DNA, sGAGs, collagen, elastin, or other ECM.
Many studies have used dECM bioink to 3D bioprint liver constructs. Skardal et al., developed a 3D bioprinted liver spheroid using hyaluronic acid, gelatin and decellularized extracellular matrix. The dECM tissue was prepared using Triton X-100 and NH4OH and then lyophilised for further use. Hepatocytes, stellate cells and Kupffer cells were combined in a ratio of 80:10:10 and spheroids were formed on 96 well plates. PEGDA and PEG were used as crosslinkers. Multiple crosslinking steps were done including thiol-acrylate and UV light. The 3D liver construct developed showing functional albumin and urea output [187]. Lee et al., fabricated bioink for liver 3D printing applications using decellularized extracellular matrix employing Triton x-100, NaCl, and SDS. Porcine derived collagen was used along with dECM with HepG2 and BMMSCs. Cell behaviour was analysed by cell viability assay. Enhanced liver functions were observed on constructs fabricated using HepG2, dECM and collagen [71]. Mao et al., fabricated a liver microtissue using liver specific bioink having GelMA, dECM and hiHep cells. Tissue was decellularized using triton X-100, 2% SDS and ultrapure water. For solubilising, acetic acid and pepsin was used which completely dissolved the lyophilised decellularized tissue powder and then mixed with GelMA. To the dECM/GelMA ink hiHep cells were added in concentration of 2.5–3.0*106 cells/ml and cell laden bioink was deposited as a small droplet using a DLP based bioprinting system. The cells expressed hepatic function in the 3D bioprinted liver microtissue [188].
5.5 Cell sources for bioinks
Liver tissue engineering has multiple applications, but the main target is to achieve the liver microenvironment on the material being fabricated. Non-parenchymal cells are now thought to be just as crucial for the success of liver tissue engineering as the typical hepatocyte cells. The major downside of using these cell lines is that the isolation and their maintenance in vitro have low yields which also limits their application [189]. Researchers have been attempting to eliminate different liver cells by using stem cells to replace it with other cell types in an effort to overcome this obstacle. Human Umbilical Vein Endothelial Cells (HUVEC) are produced from adult stem cells, induced Pluripotent Stem Cells (iPSCs), and embryonic stem cells are used in a number of research [188, 190]. HSCs have been derived from iPSCs [191], cholangiocytes, the primary building block of the biliary network, are produced in the liver from foetal or progenitor cells [192, 193]. The cell lines are an important source as it contributes majorly in engineering of the liver tissue [194]. In spite of all the findings and advancement in technology, there are multiple complications that arise in the protocol for the differentiation and efficiency of the liver cells that are derived from the stem cells. Another major concern rises as there are ethical concerns and the risk of carcinogenesis [1, 49, 79, 195]. Below are a few of the cell line sources which are potential sources to derive the liver cells.
Human Primary hepatocytes (hPHs) are the leading mature cell lines for the liver and possess the highest proliferation rate in vivo [194, 195] but they tend to lose their function in vitro leading to dedifferentiation. Few studies have proved that the viability and functions can be enhanced if the cells are aggregated in the plates [158]. This led to another challenge of preventing the necrosis taking place in the centre of aggregated cells and to improve the supply of oxygen and nutrition [16, 196, 197]. Another approach that researchers have figured out is to culture the HPHs with other cells like sinusoidal epithelial cells and stellate cells and fibroblasts [198, 199].
Primary hepatocytes are widely used from various animal sources that include rat and porcine. They are famous as they are readily available but their application is very limited due to compress compatibility for which specialised conditions shall be provided (encapsulation). A very good example of the encapsulation is the encapsulation of Fetal liver cell (FLC) and β-Fibroblast Growth Factor (βFGF) which showed improved functioning [200]. Primary hepatocytes from rats are used in few studies which involve bioprinted scaffolds. Another example is the use of hPHs and primary hepatocytes were encapsulated with alginate hydrogel [201]. One of the major drawbacks is that the process of isolation is very difficult and the viability of cells in tissue engineering application is less.
Stem cells (SCs) and Embryonic stem cells (ESC) are increasing interest in the use of stem cells as they have high potential for differentiation, proliferation and self-renewal [202,203,204]. The expression of genes that are specific to the liver were studied by Zhao et al. by the differentiating embryonic stem cells; moreover, the increased functional activities were shown by these cells when they were implanted in mice liver. There was observation of markers which are specific to the liver, absorption of lipoproteins, secretion of albumin and storage of glycogen [205]. The major drawback is that the cells have higher proliferation rate which leads to formation of tumours and hence it’s difficult to maintain them in undifferentiating state [206, 207].
Induced pluripotent stem cells are the undifferentiated pluripotent stem cells that have been reprogrammed via various reprogramming ingredients and are used because of their specificity for the patients [208,209,210,211]. These cells can be isolated from mice, humans, and porcine [212,213,214,215,216,217]. For proper functioning of these cells there is an additional requirement of adipose‐derived stem cells and human umbilical vein endothelial cells for support and structure of the liver. The major drawback is that these cells showcase different phenotypes due to the difference in the epigenetic memory of iPSCs [218].
The multipotent mesenchymal stem cell has multiple sources such as the adipose tissues, bone marrow, umbilical cord blood and tissue, amniotic fluid, Wharton jelly, dental pulp, etc. [83, 219,220,221], they are in the state of undifferentiation until the time the body requires them but at the same time these cells tend to lose their capacity to proliferate when they are cultured [222,223,224,225]. A study proves that the encapsulation helps these cells to have higher levels of cytokines when compared to the in vitro tests as there is a lack of surface receptors [226,227,228,229]. Given a chance to compare the ESCs and iPSCs, iPSCs do not have potential for proliferation [230, 231].
Foetal Liver Cells (FLC) are considered a suitable hepatocyte substitute due to the ability to form a colony unit in cell culture. Suzuki et al. showcased that these cells differentiated into cholangiocytes or hepatocytes which are the parenchymal cells of the liver [125]. Human adult liver stem cells (HLSCs) are in the small branches of bile canaliculi in a matured human liver [232]. They have great potential to differentiate into insulin producer cells, endothelial and bone cells, however they can be differentiated into hepatocytes based on the requirements [233]. Bone Marrow-derived Very Small Embryonic‐Like Stem Cells (BM-VSEL) migrate from the bone to the site of liver injury and express many hematopoietic stem cells. Studies have demonstrated that these cells have capacity to differentiate into hepatocytes. In one of the studies, they attempted to use hepatocellular transplantation from living donors to those in need for liver transplantation [234].
6 Applications of 3D bioprinting for liver diseases
Liver plays a major role in the metabolic process of the body and holds important functions such as protein synthesis, bile secretion, regulates blood clotting, albumin production, regulates amino acids, resists infections and many more. Although the liver has a great capacity to regenerate naturally, an excess level of cell damage leads to irreversible damage to liver cells [235]. Severe liver impairments are observed in liver diseases and hence it is important to develop an in vitro liver disease model to study the diseases and understand the underlying molecular pathways associated. This also helps in performing drug screening and toxicity tests. Also, liver transplantation is an effective treatment in many end-stage liver diseases. In the current scenario, organ shortage is a global problem and tissue engineering techniques are aimed at developing artificial organs to help address this issue [236]. 3D bioprinting shows a huge potential in fabricating an artificial organ with close biological resemblance that can be used for disease modelling, drug screening and transplantation in humans in the near future.
6.1 Pre-clinical and clinical trials
There are currently multiple active research studies that are using 3D bioprinting techniques specifically for the purpose of engineering liver tissue. These clinical trials involve the application of 3D bioprinting technology to create functional liver tissue in a controlled laboratory setting. The trials aim to develop innovative approaches to regenerate or repair damaged liver tissue, potentially leading to advancements in the treatment of liver diseases, transplantation, or drug development. These trials are conducted under rigorous scientific protocols to evaluate the safety, efficacy, and feasibility of 3D bioprinting methods for liver tissue engineering. However, it is still in the early stages of development, and it will take several years before 3D bioprinted liver tissue becomes available for clinical use.
Ernesto et al., evaluated the impact of single cell dispersion, spheroids of iPSc derived parenchymal cells and non-parenchymal cells in both systems tested for liver tissue functionality. Printed spheroid constructs showed greater cell survival and hepatic functions in comparison to single cell dispersion. Their study demonstrated the advantages of using spheroid based bioprinting which can be further used for developing accurate disease models [237]. Two researchers from Wake Forest Institute of Regenerative Medicine have successfully bioprinted a vascularized liver tissue with greater than 85% cell survival rate for 30 days [237]. These two teams have won the vascular tissue challenge by NASA. The implications of their study have applications in disease modelling, drug testing and probable transplantation in the future [238].
A few pre-clinical studies on 3D bioprinting liver are mentioned in Table 4 and clinical trials are listed in Table 5.
6.2 Transplantation
3D printing is a useful tool for anatomical visualisation for surgical planning. It is particularly useful in case of complex structures and surgeries. Zein et al. printed liver models with vascular network and biliary structures mimicking the native livers of six patients—three living donors and three recipients (patients with cirrhosis) for living donor liver transplantation. TangoPlus/VeroClear synthetic material was used for printing. The objective was to generate accurate models to help minimise operative complications by facilitating pre-operative planning to identify vascular networks and biliary anatomy in the patient’s models [247]. Yukio et al., developed a 3D printed liver model for hepatectomy which improved the visibility during the procedure, helped simulate the resection line [248]. Studies showed that these 3D printed liver models have proven to be useful for pre-operative planning [249,250,251,252,253,254,255]. So far, clinicians have used 3D printed models for pre-planning surgeries before transplantation. The development of a 3D bioprinted liver is not at an advanced functional stage that it can be used for human liver transplantation.
The clinical translation of 3D bioprinted liver constructs for transplantation faces several challenges such as biocompatibility and immunogenicity, vascularization, maintaining structural integrity and mechanical strength, long term functionality and integration, scale up manufacturing, regulatory approval and standardization. Ensuring that the 3D bioprinted constructs are biocompatible with the recipient's body and do not trigger an immune response is crucial for successful transplantation. The materials and cells used in the bioprinting process must be carefully selected and engineered to minimize immune reactions and promote integration with the host tissue. Adequate vascularization is essential for the survival and functionality of large, complex 3D bioprinted constructs. Creating a network of blood vessels that can efficiently deliver nutrients and oxygen throughout the construct remains a significant challenge. Without proper vascularization, the transplanted tissue may suffer from insufficient nutrient supply and impaired functionality. 3D bioprinted constructs need to exhibit sufficient structural integrity and mechanical strength to withstand transplantation procedures and function in the recipient's body. Ensuring that the constructs maintain their shape, integrity, and mechanical properties over time is critical for their long-term success. Scaling up the production of 3D bioprinted constructs to meet the demands of clinical transplantation is a challenge. Manufacturing processes need to be optimized to ensure reproducibility, consistency, and cost-effectiveness while maintaining the quality and functionality of the constructs. Meeting the rigorous regulatory requirements for clinical use of 3D bioprinted constructs is a complex and time-consuming process. Establishing standardized protocols, safety assessments, and quality control measures is necessary to obtain regulatory approval for clinical trials and eventual commercialization. Ensuring the long-term functionality and integration of the 3D bioprinted constructs with the recipient's native tissue remains a challenge. Construct durability, cell viability, functional performance, and the ability to integrate into the host tissue are critical factors for successful transplantation outcomes. Addressing these challenges requires ongoing research, technological advancements, collaboration between different disciplines, and rigorous preclinical and clinical evaluations to establish the safety and efficacy of 3D bioprinted constructs for transplantation.
6.3 Disease models and drug screening
Healthy liver tissue models are needed to study the molecular state of a healthy liver and how these change in a diseased liver. Hence, a healthy model is fabricated, then the disease is induced and both the models are compared to study the physiological changes. This helps in understanding the underlying mechanisms and pathways that caused the disease. Liver diseases such as cirrhosis, liver cancer, fatty liver disease, hepatitis, etc. pose a serious threat to human health globally [256]. To reduce the morbidity associated with these diseases, an accurate disease model is required to screen the drugs efficiently. Often, animal models and 2D cultures are used for this purpose but they do not mimic human physiology and greater side effects are observed when translated to humans. These methods do not represent the human metabolic microenvironment either. Also, these methods are time consuming and expensive. Accurate liver disease models can be useful in development of new drugs and screening existing drugs [257,258,259,260]. Disease models help us understand the efficiency of drugs. During the drug development process, most drugs are not successful due to the liver injury caused by them i.e., hepatotoxicity. Since, liver is an essential organ for detoxification and drug metabolism, it is important to have relevant liver models to test for drug toxicity. 3D bioprinting technology helps overcome current challenges of tissue engineering and drug development by providing a personalised tissue/organ model [261]. 3D bioprinting is preferred since it is a more precise way to develop a construct in-vitro. This also helps replace preclinical animal testing in the near future. This technology helps mimic the human liver in-vitro for the purpose of developing disease models to understand the disease progression and the cause of it, to screen and test drugs which are efficient and are not toxic [133, 262, 263].
Xie et al., established a modelling system to fabricate hepatic organoids using 3D bioprinting technology with HepaRG cells for drug screening. Gelatin and sodium alginate were used in the bioink along with patient derived hepatocellular carcinoma cells. Four targeted drugs were tested for efficacy. The established model was designed to retain the natural functions of a liver and prolong mice survival after transplantation [162]. Deborah et al., developed a bioprinted liver model with patient derived hepatocytes and non-parenchymal cells. Two drugs- Trovafloxacin and levofloxacin were tested on the bioprinted model. It was demonstrated that the bioprinted model could model drug induced liver injury and the results showed that they mimicked human drug response at the tissue level [264]. Studies in 3D bioprinting of liver are summarised in Table 6.
6.4 Liver devices
3D printed devices simplify the therapeutic procedures of liver surgeries and also help in diagnosis and detection of diseases. Samar et al., developed an acoustic and electrochemical biosensor for liver cancer cells detection. The developed biosensor recognizes the highly expressed tumour marker CD133 [271, 272]. Shin-Young et al., developed a 3D printed implantable drug delivery device and was evaluated in-vivo for safety and usability. The container in the reservoir was 3D printed which contains a semi-permeable membrane for administration of drugs. The study concluded that the system was safe for acute liver failure treatment and that various drugs could be administered via this 3D printed device [273]. Organ-on chip devices have gained a lot of traction these days for in-vitro studies of pathophysiological processes for drug screening. Micropumps are used to simulate the in-vivo-like fluid flow and they help in transport of nutrients. Amar et al., designed and developed a micropump by 3D printing it for continuous perfusion of nutrients in a liver-on-chip model [274]. Gou et al., designed a detoxification system by 3D printed biomimetic nanocomposite structure in a hydrogel. This device allows the polyacetylene nanoparticles to sense and capture the toxins efficiently [275].
3D printed liver models facilitate pre-surgical planning, medical education, alternate approaches to regenerate liver tissues, evaluation of hepatotoxicity and various drugs; which makes this technology superior over other traditional methods in fabricating scaffolds and tissue models [276]. The same is illustrated in Fig. 7.
6.5 Commercial prospects
While several research groups have made significant progress in developing functional liver tissue using 3D bioprinting, the technology is still in the early stages of development and may take several years for commercial availability of 3D bioprinted liver tissue.
Organovo, a US based company established in 2007 specialises in the design and development of 3D human tissues for research and therapeutic applications. The company synthesised human liver tissue (up to 500 microns in thickness) with their proprietary NovoGen™ bioprinter. The construct was bioprinted using human hepatocytes, liver endothelial cells, hepatic stellate cells, and HUVECs. They showed that the fabricated construct mimicked several biological functions such as albumin, fibrinogen, transferrin, and cholesterol production. They also showed that the fabricated patch can be implanted for up to 90 days in animal disease models [277,278,279]. Their bioprinter human liver tissue patch is termed as ExVive™. This fabricated construct was proved to be reliable for the study of complex, chronic conditions such as steatosis, fibrosis and NASH [117, 122, 280, 281]. Advanced Solutions Life Sciences, a US based company established in 1987 has launched a 3D bioprinting platform called BioAssembly Bot with its early prototype in 2013 and holds patents. BioBots, USA developed a human liver tissue using their commercially available bioink called BioGel and HepG2 cells. The BioBots demonstrated the fabrication of high-resolution liver tissue construct and it remained viable for 2 weeks [282]. Sarah et al. demonstrated the automated fabrication of a vascularized human liver tissue construct using primary human hepatocytes and non-parenchymal cells along with isolated fragments of intact human micro vessels as vascular precursors [283]. Aspect Biosystems, a Canadian company is developing allogeneic tissue therapeutics using their proprietary bioprinting technology. A therapeutic program is dedicated to restore lost or damaged liver functions. A bioprinted cell therapy using human hepatocytes is being developed and is currently at pre-clinical stage. It was demonstrated that the bioprinted tissue was viable for 28 days in mice [284, 285]. RegenHU, a Swiss company founded in 2007 specialises in 3D bioprinting technology and is currently developing a hepatic tissue for the replacement of liver transplantation for end-stage liver failure patients [286, 287]. T&R Biofab, a Korean based company established in 2013 is focused on developing bioresorbable scaffolds using 3D printing technology. The company was successful in patterning spherical tissues into structures (equivalent of liver lobes). These structures were implanted in mice and it showed good viability and structural stability. No cell death or fibrotic tissue was observed [288,289,290,291]. Cyfuse Biomedical, a Japanese company founded in 2010 develops tissue fabrication systems. Their proprietary technology “Bio 3D Printing” is a scaffold free approach to fabricating artificial organs. Spheroids are stacked on an array needle by the Bio 3D Printer and after the cells fuse, the needle is removed, leaving behind a 3D structure made with cells only. This technology was applied in developing a liver tissue for drug discovery and is currently in preclinical stage. The study demonstrated diverse liver metabolic functions for 2 weeks. The study also showed that the printed liver-maintained drug metabolic functions for 50 days [292]. Yanagi et al., reported a transplantation method for growing liver buds in vivo. This study also demonstrated fabrication of scalable liver tissues using Bio 3D Printer, exhibited self-organisation of tissue and successful engraftment on rats [293].
7 Challenges and future directions
3D bioprinted liver models are being developed rapidly over the past few years due to the increasing need of models that can mimic the cell microenvironment accurately. Although 3D bioprinting helps simulate in vivo microenvironment, there is still a long way to go before arriving at a fully functional organ. This is because most research focuses on evaluating only specific liver functions and fails to analyse all the functional capabilities [47]. Another main challenge is vascularization, although few studies reported to model a vascularized liver tissue, it is still in infancy and there remains a huge gap to design improvement. The development of in vitro liver constructs by 3D bioprinting will improve exponentially in the coming years due to advanced ongoing research in biomaterials, bioinks, cell biology and molecular medicine.
8 Conclusion
3D bioprinted in vitro liver structures offer promising results for effective drug screening and disease models, enhance liver regeneration and transplantation. However, its clinical translation depends on how closely the 3D bioprinted model can mimic the functions of a native liver, which requires extensive optimization in the process. 3D bioprinting allows fabrication of macroscopic and microscopic sections in an organ making it a unique technology for precise fabrication of vascular and ductular structures in a liver. This capacity to develop an organ with different cell types and biomaterials was made feasible with 3D bioprinting technology. This field so far has paved the way for research in biomaterials, bioinks, printing techniques, design strategies and a lot of methods were developed to promote cell viability and proliferation on scaffolds.
Data Availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Palakkan AA, Hay DC, Anil Kumar PR, Kumary TV, Ross JA. Liver tissue engineering and cell sources: issues and challenges. Liver Int. 2013;33:666–76.
Sharma A, Nagalli S. Chronic liver disease. InStatPearls 2021. StatPearls Publishing.
Liver Disease in India. World Life Expectancy. Available from: https://www.worldlifeexpectancy.com/india-liver-disease
World Health Statistics 2022. Available from: https://www.who.int/news/item/20-05-2022-world-health-statistics-2022
Cheemerla S, Balakrishnan M. Global epidemiology of chronic liver disease. Clin Liver Disease. 2021;17:365.
Mondal D, Das K, Chowdhury A. Epidemiology of liver Diseases in india. Clin Liver Disease. 2022;19:114.
Heydari Z, Pooyan P, Bikmulina P, Pozdnyakov A, Fomin V, Seydi H, et al. Mimicking the liver function in micro-patterned units: challenges and perspectives in 3D-Bioprinting. Bioprinting. 2022;27:e00208.
Shahrubudin N, Lee TC, Ramlan RJ. An overview on 3D printing technology: technological, materials, and applications. Proc Manuf. 2019;35:1286–96.
Mota C, Camarero-Espinosa S, Baker MB, Wieringa P, Moroni L. Bioprinting: from tissue and organ development to in vitro models. Chem Rev. 2020;120:10547–607.
Kasturi M, Mathur V, Agarwal P, Srinivasan V, Vasanthan KS. 3D printing for tissue regeneration. InAdvances in 3D Printing 2022. IntechOpen.
Ishibashi H, Nakamura M, Komori A, Migita K, Shimoda S. Liver architecture, cell function, and disease. InSeminars in immunopathology Springer. 2009;31:399–409.
Grisham JW. Organizational principles of the liver. The liver: biology and pathobiology. 2009:1–5.
Kuntz E, Kuntz HD. Biochemistry and functions of the liver. Hepatology Textbook and Atlas: History·Morphology Biochemistry·Diagnostics Clinic Therapy. 2008:35–76.
Alamri ZZ. The role of liver in metabolism: an updated review with physiological emphasis. 2018.
Jungermann K. Metabolic zonation of liver parenchyma. InSeminars in liver disease. Thieme Medical Publishers, Inc. 1988;8:329–41.
Kuntz E, Kuntz HD. Hepatology: textbook and atlas. Berlin: Springer; 2009.
Köhler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–65.
Kim T, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904.
Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743–50.
Saegusa S, Isaji S, Kawarada Y. Changes in serum hyaluronic acid levels and expression of CD44 and CD44 mRNA in hepatic sinusoidal endothelial cells after major hepatectomy in cirrhotic rats. World J Surg. 2002;26:694–9.
Bhave VS, Paranjpe S, Bowen WC, Donthamsetty S, Bell AW, Khillan JS, et al. Genes inducing iPS phenotype play a role in hepatocyte survival and proliferation in vitro and liver regeneration in vivo. Hepatology. 2011;54:1360–70.
Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82.
Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-α-targeted mice. Mol Carcinog Publ Cooper Univ Texas MD Anderson Cancer Center. 1996;15:183–9.
Greenbaum LE, Cressman DE, Haber BA, Taub R. Coexistence of C/EBP alpha, beta, growth-induced proteins and DNA synthesis in hepatocytes during liver regeneration. Implications for maintenance of the differentiated state during liver growth. J Clin Investig. 1995;96:1351–65.
Paranjpe S, Bowen WC, Mars WM, Orr A, Haynes MM, DeFrances MC, et al. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology. 2016;64:1711–24.
Mullany LK, White P, Hanse EA, Nelsen CJ, Goggin MM, Mullany JE, et al. Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle. 2008;7:2215–24.
Michalopoulos G, Weidner M, Gebhardt R. Different proliferative responses of periportal and pericentral rat hepatocytes to hepatocyte growth factor. Biochem Biophys Res Commun. 1995;207:578–84.
Biondo-Simões MD, Matias JE, Montibeller GR, Siqueira LC, Nunes ED, Grassi CA. Effect of aging on liver regeneration in rats. Acta Cirurgica Brasileira. 2006;21:197–202.
Matsumoto T, Wakefield L, Tarlow BD, Grompe M. In vivo lineage tracing of polyploid hepatocytes reveals extensive proliferation during liver regeneration. Cell Stem Cell. 2020;26:34–47.
Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48.
Zarnegar R, DeFrances MC, Kost DP, Lindroos P, Michalopoulos GK. Expression of hepatocyte growth factor mRNA in regenerating rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1991;177:559–65.
Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27:85–94.
Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Uptake and distribution of hepatocyte growth factor in normal and regenerating adult rat liver. Am J Pathol. 1994;144:129.
Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;15:638–54.
Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55.
Kono S, Nagaike M, Matsumoto K, Nakamura T. Marked induction of hepatocyte growth factor mRNA in intact kidney and spleen in response to injury of distant organs. Biochem Biophys Res Commun. 1992;186:991–8.
Yanagita K, Nagaike M, Ishibashi H, Niho Y, Matsumoto K, Nakamura T. Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem Biophys Res Commun. 1992;182:802–9.
Broten J, Michalopoulos G, Petersen B, Cruise J. Adrenergic stimulation of hepatocyte growth factor expression. Biochem Biophys Res Commun. 1999;262:76–9.
Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–6.
Carver RS, Stevenson MC, Scheving LA, Russell WE. Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology. 2002;123:2017–27.
Rudolph KL, Trautwein C, Kubicka S, Rakemann T, Bahr MJ, Sedlaczek N, et al. Differential regulation of extracellular matrix synthesis during liver regeneration after partial hepatectomy in rats. Hepatology. 1999;30:1159–66.
Norris CA, He M, Kang LI, Ding MQ, Radder JE, Haynes MM, et al. Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS ONE. 2014;9:e96053.
Gallai M, Sebestyén A, Nagy P, Kovalszky I, Ónody T, Thorgeirsson SS. Proteoglycan gene expression in rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1996;228:690–4.
Paranjpe S, Bowen WC, Tseng GC, Luo JH, Orr A, Michalopoulos GK. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am J Pathol. 2010;1:2669–81.
Taylor SR, Markesbery MG, Harding PA. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) and proteolytic processing by a disintegrin and metalloproteinases (ADAM): a regulator of several pathways. In: Seminars in cell and developmental biology. Academic Press. 2014;28:22–30.
Olsen PS, Poulsen SS, Kirkegaard P. Adrenergic effects on secretion of epidermal growth factor from Brunner’s glands. Gut. 1985;26:920–7.
Dao DT, Anez-Bustillos L, Adam RM, Puder M, Bielenberg DR. Heparin-binding epidermal growth factor–like growth factor as a critical mediator of tissue repair and regeneration. Am J Pathol. 2018;188:2446–56.
Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Marsh JW, Tom K, et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986–93.
Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, et al. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562–8.
Maretti-Mira AC, Wang X, Wang L, DeLeve LD. Incomplete differentiation of engrafted bone marrow endothelial progenitor cells initiates hepatic fibrosis in the rat. Hepatology. 2019;69:1259–72.
Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci. 2007;23:17081–6.
Webber EM, Fitzgerald MJ, Brown PI, Bartlett MH, Fausto N. Transforming growth factor-α expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between transforming growth factor-α and hepatocyte growth factor. Hepatology. 1993;18:1422–31.
Scheving LA, Zhang X, Stevenson MC, Threadgill DW, Russell WE. Loss of hepatocyte EGFR has no effect alone but exacerbates carbon tetrachloride-induced liver injury and impairs regeneration in hepatocyte Met-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2015;308:G364-77.
Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16.
Tsagianni A, Mars WM, Bhushan B, Bowen WC, Orr A, Stoops J, et al. Combined systemic disruption of MET and epidermal growth factor receptor signaling causes liver failure in normal mice. Am J Pathol. 2018;188:2223–35.
Bhushan B, Stoops JW, Mars WM, Orr A, Bowen WC, Paranjpe S, et al. TCPOBOP-induced hepatomegaly and hepatocyte proliferation are attenuated by combined disruption of MET and EGFR signaling. Hepatology. 2019;69:1702–18.
Huang X, Yu C, Jin C, Kobayashi M, Bowles CA, Wang F, et al. Ectopic activity of fibroblast growth factor receptor 1 in hepatocytes accelerates hepatocarcinogenesis by driving proliferation and vascular endothelial growth factor-induced angiogenesis. Can Res. 2006;66:1481–90.
Luo Y, Yang C, Lu W, Xie R, Jin C, Huang P, et al. Metabolic regulator βKlotho interacts with fibroblast growth factor receptor 4 (FGFR4) to induce apoptosis and inhibit tumor cell proliferation. J Biol Chem. 2010;285:30069–78.
Padrissa-Altés S, Bachofner M, Bogorad RL, Pohlmeier L, Rossolini T, Böhm F, et al. Control of hepatocyte proliferation and survival by Fgf receptors is essential for liver regeneration in mice. Gut. 2015;64:1444–53.
Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–70.
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83.
Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31.
Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, et al. Control of liver cell fate decision by a gradient of TGFβ signaling modulated by Onecut transcription factors. Genes Dev. 2005;19:1849–54.
Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, et al. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip. 2007;7:302–9.
Jain E, Damania A, Kumar A. Biomaterials for liver tissue engineering. Hep Intl. 2014;8:185–97.
Barranger A, Le Hégarat L. Towards better prediction of xenobiotic genotoxicity: CometChip technology coupled with a 3D model of HepaRG human liver cells. Arch Toxicol. 2022;96:2087–95.
Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Ying Shun T, Gough A, et al. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med. 2016;241:101–14.
Uygun BE, Yarmush ML, Uygun K. Application of whole-organ tissue engineering in hepatology. Nat Rev Gastroenterol Hepatol. 2012;9:738–44.
Ananthanarayanan A, Narmada BC, Mo X, McMillian M, Yu H. Purpose-driven biomaterials research in liver-tissue engineering. Trends Biotechnol. 2011;1:110–8.
Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;1(7):211–24.
Lee JS, Cho SW. Liver tissue engineering: recent advances in the development of a bio-artificial liver. Biotechnol Bioprocess Eng. 2012;17:427–38.
Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci U S A. 2009;106:15714–9.
Oe S, Fukunaka Y, Hirose T, Yamaoka Y, Tabata Y. A trial on regeneration therapy of rat liver cirrhosis by controlled release of hepatocyte growth factor. J Control Release. 2003;7:193–200.
Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21:3269–85.
Vasanthan KS, Subramanian A, Krishnan UM, Sethuraman S. Role of biomaterials, therapeutic molecules and cells for hepatic tissue engineering. Biotechnol Adv. 2012;30:742–52.
Agarwal T, Maiti TK, Ghosh SK. Decellularized caprine liver-derived biomimetic and pro-angiogenic scaffolds for liver tissue engineering. Mater Sci Eng C. 2019;98:939–48.
Mazza G, Al-Akkad W, Rombouts K, Pinzani M. Liver tissue engineering: from implantable tissue to whole organ engineering. Hepatol Commun. 2018;2:131–41.
Ogiso S, Yasuchika K, Fukumitsu K, Ishii T, Kojima H, Miyauchi Y, et al. Efficient recellularisation of decellularised whole-liver grafts using biliary tree and foetal hepatocytes. Sci Rep. 2016;6:35887.
Agarwal T, Narayan R, Maji S, Ghosh SK, Maiti TK. Decellularized caprine liver extracellular matrix as a 2D substrate coating and 3D hydrogel platform for vascularized liver tissue engineering. J Tissue Eng Regen Med. 2018;12:e1678–90.
Damania A, Kumar A, Teotia AK, Kimura H, Kamihira M, Ijima H, et al. Decellularized liver matrix-modified cryogel scaffolds as potential hepatocyte carriers in bioartificial liver support systems and implantable liver constructs. ACS Appl Mater Interfaces. 2018;10:114–26.
Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, et al. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 2011;17:418–27.
Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17.
Agarwal T, Subramanian B, Maiti TK. Liver tissue engineering: challenges and opportunities. ACS Biomater Sci Eng. 2019;5:4167–82.
Sauer V, Roy-Chowdhury N, Guha C, Roy-Chowdhury J. Induced pluripotent stem cells as a source of hepatocytes. Curr Pathobiol Rep. 2014;2:11–20.
Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011;108:11842–7.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20.
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4.
Shaer A, Azarpira N, Aghdaie MH, Esfandiari E. Isolation and characterization of human mesenchymal stromal cells derived from placental decidua basalis; umbilical cord Wharton’s jelly and amniotic membrane. Pak J Med Sci. 2014;30:1022.
Jitraruch S, Dhawan A, Hughes RD, Filippi C, Soong D, Philippeos C, et al. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS ONE. 2014;9:e113609.
Lee JS, Shin J, Park HM, Kim YG, Kim BG, Oh JW, et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206–18.
Stevens KR, Miller JS, Blakely BL, Chen CS, Bhatia SN. Degradable hydrogels derived from PEG-diacrylamide for hepatic tissue engineering. J Biomed Mater Res Part A. 2015;103:3331–8.
Song W, Lu YC, Frankel AS, An D, Schwartz RE, Ma M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci Rep. 2015;5:1–3.
Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–9.
Toprakhisar B, Verfaillie CM, Kumar M. Advances in recellularization of decellularized liver grafts with different liver (stem) cells: towards clinical applications. Cells. 2023;12:301.
Hussein KH, Park KM, Kang KS, Woo HM. Heparin–gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016;38:82–93.
Chang SH, Huang HH, Kang PL, Wu YC, Chang MH, Kuo SM. In vitro and in vivo study of the application of volvox spheres to co-culture vehicles in liver tissue engineering. Acta Biomater. 2017;63:261–73.
Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017;21:2661–70.
Xu L, Wang S, Sui X, Wang Y, Su Y, Huang L, et al. Mesenchymal stem cell-seeded regenerated silk fibroin complex matrices for liver regeneration in an animal model of acute liver failure. ACS Appl Mater Interfaces. 2017;9:14716–23.
Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci Transl Med. 2017;9:eaah5505.
Ng S, March S, Galstian A, Gural N, Stevens KR, Mota MM, et al. Towards a humanized mouse model of liver stage malaria using ectopic artificial livers. Sci Rep. 2017;31:1–3.
Mavila N, Trecartin A, Spurrier R, Xiao Y, Hou X, James D, et al. Functional human and murine tissue-engineered liver is generated from adult stem/progenitor cells. Stem Cells Transl Med. 2017;6:238–48.
Li J, Xing F, Chen F, He L, So KF, Liu Y, et al. Functional 3D human liver bud assembled from MSC-derived multiple liver cell lineages. Cell Transplant. 2019;28:510–21.
Nie YZ, Zheng YW, Ogawa M, Miyagi E, Taniguchi H. Human liver organoids generated with single donor-derived multiple cells rescue mice from acute liver failure. Stem Cell Res Ther. 2018;9:1–2.
Ng SS, Saeb-Parsy K, Blackford SJ, Segal JM, Serra MP, Horcas-Lopez M, et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018;182:299–311.
Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, et al. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190–2.
Goren A, Dahan N, Goren E, Baruch L, Machluf M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 2010;24:22–31.
De Bartolo L, Jarosch-Von Schweder G, Haverich A, Bader A. A novel full-scale flat membrane bioreactor utilizing porcine hepatocytes: cell viability and tissue-specific functions. Biotechnol Prog. 2000;16:102–8.
Lewis PL, Green RM, Shah RN. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018;15:63–70.
German CL, Madihally SV. Type of endothelial cells affects HepaRG cell acetaminophen metabolism in both 2D and 3D porous scaffold cultures. J Appl Toxicol. 2019;39:461–72.
Li J, Tao R, Wu W, Cao H, Xin J, Li J, et al. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell–derived hepatocytes. Stem Cells Dev. 2010;19:1427–36.
Azarpira N, Kaviani M, Salehi S. The role of mesenchymal stem cells in diabetes mellitus. Int J Stem Cell Res Ther. 2015;2:1–5.
Kang K, Kim Y, Jeon H, Lee SB, Kim JS, Park SA, et al. Three-dimensional bioprinting of hepatic structures with directly converted hepatocyte-like cells. Tissue Eng Part A. 2018;24:576–83.
Cho CS, Seo SJ, Park IK, Kim SH, Kim TH, Hoshiba T, et al. Galactose-carrying polymers as extracellular matrices for liver tissue engineering. Biomaterials. 2006;27:576–85.
Nakatsuka R, Iwaki R, Matsuoka Y, Sumide K, Kawamura H, Fujioka T, et al. Identification and characterization of lineage− CD45− Sca-1+ VSEL phenotypic cells residing in adult mouse bone tissue. Stem Cells Dev. 2016;25:27–42.
Wang B, Jakus AE, Baptista PM, Soker S, Soto-Gutierrez A, Abecassis MM, et al. Functional maturation of induced pluripotent stem cell hepatocytes in extracellular matrix—a comparative analysis of bioartificial liver microenvironments. Stem Cells Transl Med. 2016;5:1257–67.
Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305.
Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;1:1075–82.
Lee H, Han W, Kim H, Ha DH, Jang J, Kim BS, et al. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017;18:1229–37.
Török É, Vogel C, Lü Tgehetmann M, Ma PX, Dandri M, Petersen J, et al. Morphological and functional analysis of rat hepatocyte spheroids generated on poly (L-lactic acid) polymer in a pulsatile flow bioreactor. Tissue Eng. 2006;12:1881–90.
Ogawa K, Asonuma K, Inomata Y, Kim I, Ikada Y, Tabata Y, et al. The efficacy of prevascularization by basic FGF for hepatocyte transplantation using polymer devices in rats. Cell Transplant. 2001;10:723–9.
Jiang J, Kojima N, Kinoshita T, Miyajima A, Yan W, Sakai Y. Cultivation of fetal liver cells in a three-dimensional Poly-L-lactic acid scaffold in the presence of oncostatin M. Cell Transplant. 2002;11:403–6.
Wang X, Yan Y, Pan Y, Xiong Z, Liu H, Cheng J, et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006;12:83–90.
Xu W, Wang X, Yan Y, Zhang R. A polyurethane-gelatin hybrid construct for manufacturing implantable bioartificial livers. J Bioact Compat Polym. 2008;23:409–22.
Ratajczak MZ, Ratajczak J, Suszynska M, Miller DM, Kucia M, Shin DM. A novel view of the adult stem cell compartment from the perspective of a quiescent population of very small embryonic-like stem cells. Circ Res. 2017;120:166–78.
Suzuki H, Watabe T, Kato M, Miyazawa K, Miyazono K. Roles of vascular endothelial growth factor receptor 3 signaling in differentiation of mouse embryonic stem cell–derived vascular progenitor cells into endothelial cells. Blood. 2005;15:2372–9.
Xu W, Wang X, Yan Y, Zheng W, Xiong Z, Lin F, et al. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J Bioact Compat Polym. 2007;22:363–77.
Taymour R, Chicaiza-Cabezas NA, Gelinsky M, Lode A. Core–shell bioprinting of vascularized in vitro liver sinusoid models. Biofabrication. 2022;14:045019.
Alberti S, Saha S, Woodruff JB, Franzmann TM, Wang J, Hyman AA. A user’s guide for phase separation assays with purified proteins. J Mol Biol. 2018;430:4806–20.
Glicklis R, Shapiro L, Agbaria R, Merchuk JC, Cohen S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol Bioeng. 2000;67:344–53.
Seo SJ, Kim IY, Choi YJ, Akaike T, Cho CS. Enhanced liver functions of hepatocytes cocultured with NIH 3T3 in the alginate/galactosylated chitosan scaffold. Biomaterials. 2006;1:1487–95.
Jeon H, Kang K, Park SA, Kim WD, Paik SS, Lee SH, et al. Generation of multilayered 3D structures of HepG2 cells using a bio-printing technique. Gut Liver. 2017;11:121.
Nugraha B, Hong X, Mo X, Tan L, Zhang W, Chan PM, et al. Galactosylated cellulosic sponge for multi-well drug safety testing. Biomaterials. 2011;32:6982–94.
Takei T, Ijima H, Sakai S, Ono T, Kawakami K. Enhanced angiogenesis in bFGF-containing scaffold promoted viability of enclosed hepatocytes and maintained hepatospecific glycogen storage capacity. J Chem Eng Jpn. 2005;38:913–7.
Lee JW, Choi YJ, Yong WJ, Pati F, Shim JH, Kang KS, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8:015007.
Skardal A, Devarasetty M, Kang HW, Seol YJ, Forsythe SD, Bishop C, et al. Bioprinting cellularized constructs using a tissue-specific hydrogel bioink. JoVE. 2016;110:e53606.
Koçak E, Yıldız A, Acartürk F. Three dimensional bioprinting technology: applications in pharmaceutical and biomedical area. Colloids Surf B. 2021;197:111396.
Liu JP, Lerut J, Yang Z, Li ZK, Zheng SS. Three-dimensional modeling in complex liver surgery and liver transplantation. Hepatobiliary Pancreat Dis Int. 2022;21:318–24.
Shim JH, Kim AJ, Park JY, Yi N, Kang I, Park J, et al. Effect of solid freeform fabrication-based polycaprolactone/poly (lactic-co-glycolic acid)/collagen scaffolds on cellular activities of human adipose-derived stem cells and rat primary hepatocytes. J Mater Sci Mater Med. 2013;24:1053–65.
Vozzi G, Previti A, De Rossi D, Ahluwalia AR. Microsyringe-based deposition of two-dimensional and three-dimensional polymer scaffolds with a well-defined geometry for application to tissue engineering. Tissue Eng. 2002;8:1089–98.
Peak CW, Cross L, Singh A, Gaharwar AK. Microscale technologies for engineering complex tissue structures. Microscale Technol Cell Eng. 2016:3–25.
Sgroi A, Mai G, Morel P, Baertschiger RM, Gonelle-Gispert C, Serre-Beinier V, et al. Transplantation of encapsulated hepatocytes during acute liver failure improves survival without stimulating native liver regeneration. Cell Transpl. 2011;20:1791–803.
Sarkar J, Kamble SC, Kashikar NC. Polymeric bioinks for 3D hepatic printing. Chemistry. 2021;3:164–81.
Zhang Y, Yu Y, Akkouch A, Dababneh A, Dolati F, Ozbolat IT. In vitro study of directly bioprinted perfusable vasculature conduits. Biomater Sci. 2015;3:134–43.
You F, Eames BF, Chen X. Application of extrusion-based hydrogel bioprinting for cartilage tissue engineering. Int J Mol Sci. 2017;18:1597.
Kang D, Ahn G, Kim D, Kang HW, Yun S, Yun WS, et al. Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication. 2018;6:035008.
Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35:49–62.
Gu Z, Fu J, Lin H, He Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J Pharm Sci. 2020;15:529–57.
Nie J, Gao Q, Fu J, He Y. Grafting of 3D bioprinting to in vitro drug screening: a review. Adv Healthc Mater. 2020;9:1901773.
Lee JS, Yoon H, Yoon D, Kim GH, Yang HT, Chun W. Development of hepatic blocks using human adipose tissue-derived stem cells through three-dimensional cell printing techniques. J Mater Chem B. 2017;5:1098–107.
Parsa S, Gupta M, Loizeau F, Cheung KC. Effects of surfactant and gentle agitation on inkjet dispensing of living cells. Biofabrication. 2010;2:025003.
Moya A, Ortega-Ribera M, Guimerà X, Sowade E, Zea M, Illa X, et al. Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system. Lab Chip. 2018;18:2023–35.
Skoog SA, Goering PL, Narayan RJ. Stereolithography in tissue engineering. J Mater Sci Mater Med. 2014;25:845–56.
Yu C, Ma X, Zhu W, Wang P, Miller KL, Stupin J, et al. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–3.
Grix T, Ruppelt A, Thomas A, Amler AK, Noichl BP, Lauster R, et al. Bioprinting perfusion-enabled liver equivalents for advanced organ-on-a-chip applications. Genes. 2018;22:176.
Ma X, Qu X, Zhu W, Li YS, Yuan S, Zhang H, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci. 2016;113:2206–11.
Wang X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines. 2019;10:814.
Liu F, Chen Q, Liu C, Ao Q, Tian X, Fan J, et al. Natural polymers for organ 3D bioprinting. Polymers. 2018;10:1278.
Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28:677–84.
Roopesh RP, Muthusamy S, Velayudhan S, Sabareeswaran A, Anil Kumar PR. High-throughput production of liver parenchymal microtissues and enrichment of organ-specific functions in gelatin methacrylamide microenvironment. Biotechnol Bioeng. 2022;119:1018–32.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85.
Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:1–5.
Xie F, Sun L, Pang Y, Xu G, Jin B, Xu H, et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials. 2021;265:120416.
Elvevold K, Smedsrød B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol. 2008;294:391–400.
Rodriguez-Pascual F, Slatter DA. Collagen cross-linking: insights on the evolution of metazoan extracellular matrix. Sci Rep. 2016;6:1–7.
Mazzocchi A, Devarasetty M, Huntwork R, Soker S, Skardal A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication. 2018;11:015003.
Law N, Doney B, Glover H, Qin Y, Aman ZM, Sercombe TB, et al. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J Mech Behav Biomed Mater. 2018;77:389–99.
Ávila HM, Schwarz S, Rotter N, Gatenholm P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting. 2016;1:22–35.
Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng. 2017;45:210–23.
Markstedt K, Escalante A, Toriz G, Gatenholm P. Biomimetic inks based on cellulose nanofibrils and cross-linkable xylans for 3D printing. ACS Appl Mater Interfaces. 2017;9:40878–86.
Wu Y, Wenger A, Golzar H, Tang X. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci Rep. 2020;10:20648.
Ng SS, Xiong A, Nguyen K, Masek M, Elazar M, Shteyer E, et al. Long-term culture of human liver tissue with advanced hepatic functions. Jci Insight. 2017;6:2.
Grant R, Hallett J, Forbes S, Hay D, Callanan A. Drug induced hybrid electrospun PLA: cell derived extracellular matrix scaffolds support the survival and function of human primary hepatocytes.
Park UJ, Jeong W, Kwon SY, Kim Y, Choi K, Kim HT, et al. Fabrication of a novel absorbable vascular anastomosis device and testing in a pig liver transplantation model. Ann Biomed Eng. 2019;47:1063–77.
Ashammakhi N, Ahadian S, Xu C, Montazerian H, Ko H, Nasiri R, et al. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater Today Bio. 2019;1:100008.
Cui X, Li J, Hartanto Y, Durham M, Tang J, Zhang H, et al. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9:1901648.
Chimene D, Kaunas R, Gaharwar AK. Hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv Mater. 2020;32:1902026.
Jang J, Park JY, Gao G, Cho DW. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials. 2018;156:88–106.
Taymour R, Kilian D, Ahlfeld T, Gelinsky M, Lode A. 3D bioprinting of hepatocytes: Core–shell structured co-cultures with fibroblasts for enhanced functionality. Sci Rep. 2021;4:1–8.
Hiller T, Berg J, Elomaa L, Röhrs V, Ullah I, Schaar K, et al. Generation of a 3D liver model comprising human extracellular matrix in an alginate/gelatin-based bioink by extrusion bioprinting for infection and transduction studies. Int J Mol Sci. 2018;19:3129.
Leva V, Chatzipetrou M, Alexopoulos L, Tzeranis DS, Zergioti I. Direct laser printing of liver cells on porous collagen scaffolds. J Laser Micro Nanoeng. 2018;13:234–7.
Khati V, Ramachandraiah H, Pati F, Svahn HA, Gaudenzi G, Russom A. 3D bioprinting of multi-material decellularized liver matrix hydrogel at physiological temperatures. Biosensors. 2022;12:521.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. In: Seminars in immunology. Academic Press. 2008;20:109–16.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34.
Kim BS, Kim H, Gao G, Jang J, Cho DW. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication. 2017;9:034104.
Sawada K, Terada D, Yamaoka T, Kitamura S, Fujisato T. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 2008;83:943–9.
Nazari M, Kurdi M, Heerklotz H. Classifying surfactants with respect to their effect on lipid membrane order. Biophys J. 2012;102:498–506.
Mao Q, Wang Y, Li Y, Juengpanich S, Li W, Chen M, et al. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C Mater Biol Appl. 2020;109:110625.
Koui Y, Kido T, Ito T, Oyama H, Chen SW, Katou Y, et al. An in vitro human liver model by iPSC-derived parenchymal and non-parenchymal cells. Stem Cell Rep. 2017;9:490–8.
Coll M, Perea L, Boon R, Leite SB, Vallverdú J, Mannaerts I, et al. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell. 2018;23:101–13.
Tanimizu N, Mitaka T. Re-evaluation of liver stem/progenitor cells. Organogenesis. 2014;10:208–15.
Sampaziotis F, de Brito MC, Geti I, Bertero A, Hannan NR, Vallier L. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc. 2017;12:814–27.
Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, Yarmush ML. Hepatic tissue engineering for adjunct and temporary liver support: critical technologies. Liver Transpl. 2004;10:1331–42.
Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr2.
Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–530.
Tahmasbi Rad A, Ali N, Kotturi HS, Yazdimamaghani M, Smay J, Vashaee D, et al. Conducting scaffolds for liver tissue engineering. J Biomed Mater Res Part A. 2014;102:4169–81.
Aghdaie MH, Geramizadeh B, Azarpira N, Esfandiari E, Darai M, Rahsaz M, et al. Hepatocyte isolation from unused/rejected livers for transplantation: Initial step toward hepatocyte transplantation, the first experience from Iran. Hepat Mon. 2013;13:e10397.
Sakai Y, Yamanouchi K, Ohashi K, Koike M, Utoh R, Hasegawa H, et al. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials. 2015;65:66–75.
Ware BR, Durham MJ, Monckton CP, Khetani SR. A cell culture platform to maintain long-term phenotype of primary human hepatocytes and endothelial cells. Cell Mol Gastroenterol Hepatol. 2018;5:187–207.
Teng Y, Wang Y, Li S, Wang W, Gu R, Guo X, et al. Treatment of acute hepatic failure in mice by transplantation of mixed microencapsulation of rat hepatocytes and transgenic human fetal liver stromal cells. Tissue Eng Part C Methods. 2010;16:1125–34.
Mei J, Sgroi A, Mai G, Baertschiger R, Gonelle-Gispert C, Serre-Beinier V, et al. Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transpl. 2009;18:101–10.
Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–6.
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401.
Soto-Gutiérrez A, Navarro-Alvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N, et al. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat Protoc. 2007;2:347–56.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Ewart L, Apostolou A, Briggs SA, Carman CV, Chaff JT, Heng AR, et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun Med. 2022;2:154.
Heydari Z, Najimi M, Mirzaei H, Shpichka A, Ruoss M, Farzaneh Z, et al. Tissue engineering in liver regenerative medicine: insights into novel translational technologies. Cells. 2020;9:304.
Shaer A, Azarpira N, Karimi MH. Differentiation of human induced pluripotent stem cells into insulin-like cell clusters with miR-186 and miR-375 by using chemical transfection. Appl Biochem Biotechnol. 2014;174:242–58.
Shaer A, Azarpira N, Vahdati A, Karimi MH, Shariati M. Differentiation of human-induced pluripotent stem cells into insulin-producing clusters. Exp Clin Transplant. 2014;13:68–75.
Shaer A, Azarpira N, Karimi MH, Soleimani M, Dehghan S. Differentiation of human-induced pluripotent stem cells into insulin-producing clusters by microRNA-7. Exp Clin Transplant. 2016;14:555–63.
Sancho-Bru P, Roelandt P, Narain N, Pauwelyn K, Notelaers T, et al. Directed differentiation of murine-induced pluripotent stem cells to functional hepatocyte-like cells. J Hepatol. 2011;54:98–107.
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–42.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Aravalli RN, Cressman EN, Steer CJ. Hepatic differentiation of porcine induced pluripotent stem cells in vitro. Vet J. 2012;194:369–74.
Debnath T, Mallarpu CS, Chelluri LK. Development of bioengineered organ using biological acellular rat liver scaffold and hepatocytes. Organogenesis. 2020;16:61–72.
Shaer A, Azarpira N, Vahdati A, Karimi M, Shariati M. miR-375 induces human decidua basalis-derived stromal cells to become insulin-producing cells. Cell Mol Biol Lett. 2014;19:483–99.
Pirjali T, Azarpira N, Ayatollahi M, Aghdaie MH, Geramizadeh B, Talai T. Isolation and characterization of human mesenchymal stem cells derived from human umbilical cord Wharton’s jelly and amniotic membrane. Int J Organ Transplant Med. 2013;4:111–6.
Khosravi M, Azarpira N, Shamdani S, Hojjat-Assari S, Naserian S, Karimi MH. Differentiation of umbilical cord derived mesenchymal stem cells to hepatocyte cells by transfection of miR-106a, miR-574-3p, and miR-451. Gene. 2018;667:1–9.
Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabetic Stud. 2010;7:168.
Ataie M, Solouk A, Bagheri F, Seyed JE. Regeneration of musculoskeletal injuries using mesenchymal stem cells loaded scaffolds. Tehran Univ Med J TUMS Publs. 2017;75:241–50.
Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:1–9.
Nekoei SM, Azarpira N, Sadeghi L, Kamalifar S. In vitro differentiation of human umbilical cord Wharton’s jelly mesenchymal stromal cells to insulin producing clusters. World J Clin Cases. 2015;3:640.
GLiu Y, Liu T, Ma X, Fan X, Bao C, Cui Z. Effects of encapsulated rabbit mesenchymal stem cells on ex vivo expansion of human umbilical cord blood hematopoietic stem/progenitor cells. J Microencapsul. 2009;26:130–42.
Moravej A, Karimi MH, Geramizadeh B, Azarpira N, Zarnani AH, Yaghobi R, Khosravi M, Kalani M, Gharesi-Fard B. Mesenchymal stem cells upregulate the expression of PD-L1 but not VDR in dendritic cells. Immunol Invest. 2017;46:80–96.
Moravej A, Geramizadeh B, Azarpira N, Zarnani AH, Yaghobi R, Kalani M, et al. Mesenchymal stem cells increase skin graft survival time and up-regulate PD-L1 expression in splenocytes of mice. Immunol Lett. 2017;182:39–49.
Zare MA, Zare A, Azarpira N, Pakbaz S. The protective effect of bone marrow-derived mesenchymal stem cells in liver ischemia/reperfusion injury via down-regulation of miR-370. Iran J Basic Med Sci. 2019;22:683.
Didar G, Delpazir F, Kaviani M, Azarpira N, Sepehrara L, Ebadi P, et al. Influence of mesenchymal stem cells and royal jelly on kidney damage triggered by ischemia-reperfusion injury: comparison with ischemic preconditioning in an animal model. Comp Clin Pathol. 2019;28:311–20.
Hueck IS, Frimodig J, Itkin-Ansari P, Gough DA. Encapsulation of Stem Cells in Research and Therapy. Biol Phys Tech Basics Cell Eng. 2018:29–69.
Wilson JL, McDevitt TC. Stem cell microencapsulation for phenotypic control, bioprocessing, and transplantation. Biotechnol Bioeng. 2013;110:667–82.
Pan G, Mu Y, Hou L, Liu J. Examining the therapeutic potential of various stem cell sources for differentiation into insulin-producing cells to treat diabetes. In: Annales D’endocrinologie. Elsevier Masson. 2019;80:47–53.
Nakatsuka R, Matsuoka Y, Sumide K, Kawamura H, Fujioka T, Sasaki Y, et al. Evaluation of stem cell characteristics of adult mouse bone-derived small cells. Exp Hematol. 2015;43:S83.
Chen ZH, Lv X, Dai H, Liu C, Lou D, Chen R, et al. Hepatic regenerative potential of mouse bone marrow very small embryonic-like stem cells. J Cell Physiol. 2015;230:1852–61.
Virant-Klun I, Skerl P, Novakovic S, Vrtacnik-Bokal E, Smrkolj S. Similar population of CD133+ and DDX4+ VSEL-like stem cells sorted from human embryonic stem cell, ovarian, and ovarian cancer ascites cell cultures: the real embryonic stem cells? Cells. 2019;8:706.
Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43:1009–17.
Ishikawa M, Sekine K, Okamura A, Zheng YW, Ueno Y, Koike N, et al. Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor. J Biosci Bioeng. 2011;1:711–8.
Jin B, Liu Y, Du S, Sang X, Yang H, Mao Y. Current trends and research topics regarding liver 3D bioprinting: a bibliometric analysis research. Front Cell Dev Biol. 2022;10:1047524.
Goulart E, de Caires-Junior LC, Telles-Silva KA, Araujo BH, Rocco SA, Sforca M, et al. 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication. 2019;12:015010.
Han JJ. Teams successfully 3D print vascularized liver tissue to win NASA’s vascular tissue challenge. Artif Organs. 2021;45:802–3.
Potter S. Teams engineer complex human tissues, win top prizes in NASA challenge. NASA. 2021. http://www.nasa.gov/press-release/teams-engineer-complex-human-tissues-win-top-prizes-in-nasa-challenge
Yang H, Sun L, Pang Y, Hu D, Xu H, Mao S, et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut. 2021;1:567–74.
Sun L, Yang H, Wang Y, Zhang X, Jin B, Xie F, et al. Application of a 3D bioprinted hepatocellular carcinoma cell model in antitumor drug research. Front Oncol. 2020;3:878.
Zhong C, Xie HY, Zhou L, Xu X, Zheng SS. Human hepatocytes loaded in 3D bioprinting generate mini-liver. Hepatobiliary Pancreat Dis Int. 2016;15:512–8.
Ma X, Yu C, Wang P, Xu W, Wan X, Lai CS, et al. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018;185:310–21.
Khati V, Turkki JA, Ramachandraiah H, Pati F, Gaudenzi G, Russom A. Indirect 3D bioprinting of a robust trilobular hepatic construct with decellularized liver matrix hydrogel. Bioengineering. 2022;9:603.
Arai K, Yoshida T, Okabe M, Goto M, Mir TA, Soko C, et al. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J Biomed Mater Res Part A. 2017;105:1583–92.
Janani G, Priya S, Dey S, Mandal BB. Mimicking native liver lobule microarchitecture in vitro with parenchymal and non-parenchymal cells using 3D bioprinting for drug toxicity and drug screening applications. ACS Appl Mater Interfaces. 2022;14:10167–86.
Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304–10.
Oshiro Y, Mitani J, Okada T, Ohkohchi N. A novel three-dimensional print of liver vessels and tumors in hepatectomy. Surg Today. 2017;47:521–4.
Ziogas IA, Zein NN, Quintini C, Miller CM, Tsoulfas G. Three-dimensional (3D) printing and liver transplantation. In: 3D printing: applications in medicine and surgery. Elsevier. 2020. pp. 97–116.
Igami T, Nakamura Y, Hirose T, Ebata T, Yokoyama Y, Sugawara G, et al. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38:3163–6.
Souzaki R, Kinoshita Y, Ieiri S, Hayashida M, Koga Y, Shirabe K, et al. Three-dimensional liver model based on preoperative CT images as a tool to assist in surgical planning for hepatoblastoma in a child. Pediatr Surg Int. 2015;31:593–6.
Takagi K, Nanashima A, Abo T, Arai J, Matsuo N, Fukuda T, et al. Three-dimensional printing model of liver for operative simulation in perihilar cholangiocarcinoma. Hepatogastroenterology. 2014;61:2315–6.
Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7:668.
Xiang N, Fang C, Fan Y, Yang J, Zeng N, Liu J, et al. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int J Clin Exp Med. 2015;8:18873.
Madurska MJ, Poyade M, Eason D, Rea P, Watson AJ. Development of a patient-specific 3D-printed liver model for preoperative planning. Surg Innov. 2017;24:145–50.
Soejima Y, Taguchi T, Sugimoto M, Hayashida M, Yoshizumi T, Ikegami T, et al. Three-dimensional printing and biotexture modeling for preoperative simulation in living donor liver transplantation for small infants. Liver Transpl. 2016;22:1610–4.
Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SM, et al. Liver diseases in the Asia-Pacific region: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2020;5:167–228.
Drews J, Ryser S. Drug development: the role of innovation in drug development. Nat Biotechnol. 1997;15:1318–9.
Kaitin KI. Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther. 2010;87:356–61.
Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA, et al. Model-based drug development. Clin Pharmacol Ther. 2007;82:21–32.
Morgan S, Grootendorst P, Lexchin J, Cunningham C, Greyson D. The cost of drug development: a systematic review. Health Policy. 2011;100:4–17.
Satpathy A, Datta P, Wu Y, Ayan B, Bayram E, Ozbolat IT. Developments with 3D bioprinting for novel drug discovery. Expert Opin Drug Discov. 2018;13:1115–29.
Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017;57:26–46.
Yi HG, Kim H, Kwon J, Choi YJ, Jang J, Cho DW. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct Target Ther. 2021;6:177.
Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE. 2016;11:e0158674.
Kim Y, Kang K, Jeong J, Paik SS, Kim JS, Park SA, et al. Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure. Ann Surg Treat Res. 2017;92:67–72.
Kang D, Hong G, An S, Jang I, Yun WS, Shim JH, et al. Bioprinting of multiscaled hepatic lobules within a highly vascularized construct. Small. 2020;16:1905505.
Norona LM, Nguyen DG, Gerber DA, Presnell SC, LeCluyse EL. Editor’s highlight: modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci. 2016;154:354–67.
Blidisel A, Marcovici I, Coricovac D, Hut F, Dehelean CA, Cretu OM. Experimental models of hepatocellular carcinoma—a preclinical perspective. Cancers. 2021;13:3651.
Lee H, Cho DW. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip. 2016;16:2618–25.
Damiati S, Küpcü S, Peacock M, Eilenberger C, Zamzami M, Qadri I, et al. Acoustic and hybrid 3D-printed electrochemical biosensors for the real-time immunodetection of liver cancer cells (HepG2). Biosens Bioelectron. 2017;94:500–6.
Damiati S, Peacock M, Leonhardt S, Damiati L, Baghdadi MA, Becker H, et al. Embedded disposable functionalized electrochemical biosensor with a 3D-printed flow cell for detection of hepatic oval cells (HOCs). Genes. 2018;9:89.
Kim SY, Han G, Hwang DB, Won DH, Shin YS, Kim C, et al. Design and usability evaluations of a 3d-printed implantable drug delivery device for acute liver failure in preclinical settings. Adv Healthcare Mater. 2021;10:2100497.
Dhwaj A, Roy N, Jaiswar A, Prabhakar A, Verma D. 3D-printed impedance micropump for continuous perfusion of the sample and nutrient medium integrated with a liver-on-chip prototype. ACS Omega. 2022;7:40900–10.
Gou M, Qu X, Zhu W, Xiang M, Yang J, Zhang K, et al. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat Commun. 2014;5:3774.
Wang JZ, Xiong NY, Zhao LZ, Hu JT, Kong DC, Yuan JY. Review fantastic medical implications of 3D-printing in liver surgeries, liver regeneration, liver transplantation and drug hepatotoxicity testing: a review. Int J Surg. 2018;56:1–6.
Root C. Organovo synthesizes human liver tissue with 3D bioprinting. Bioprocess Online. 2014. https://www.bioprocessonline.com/doc/organovo-synthesizes-human-liver-tissue-with-d-bioprinting-0001
Shepherd B. 3D bioprinted therapeutic liver tissue. Organovo. 2017. https://organovo.com/3d-bioprinted-therapeutic-liver-tissue/
Scott C. University of California San Diego’s 3D printed liver tissue may be the closest we’ve gotten to a real printed Liver. 3DPrint.com. The Voice of 3D Printing. Additive Manufacturing. 2016. https://3dprint.com/118932/uc-san-diego-3d-printed-liver/
3D bioprinted human liver tissue for modeling progressive liver disease. World Preclinical Congress. Organovo. 2017. https://organovo.com/3d-bioprinted-human-liver-tissue-modeling-progressive-liver-disease/
Vaidya M. Startups tout commercially 3D-printed tissue for drug screening. Biomedicine. 2015;7:3.
Timothy O, Margaret P, Eza K, Madeline W, Nicholas M, Daniel C, et al. Using BioBots for standardizing the 3D bioprinting of liver bioink. Front Bioeng Biotechnol. 2016. https://doi.org/10.3389/conf.FBIOE.2016.01.02327/event_abstract.
Moss SM, Schilp J, Yaakov M, Cook M, Schuschke E, Hanke B, et al. Point-of-use, automated fabrication of a 3D human liver model supplemented with human adipose microvessels. SLAS Discov. 2022;27:358–68.
Pipeline of bioprinted tissue therapeutics. Aspect Biosystems. https://www.aspectbiosystems.com/programs
Aspect biosystems to present new data on bioprinted liver tissues at AASLD liver meeting 2021. Aspect Biosystems. 2021. https://www.aspectbiosystems.com/news-resources/present-new-data-on-bioprinted-liver-tissues-at-aasld-liver-meeting-2021
Aspect biosystems to present data from liver tissue therapeutic Program at the international liver congress 2022. Aspect Biosystems. 2022. https://aspectbiosystems.com/news-resources/to-present-data-from-liver-tissue-therapeutic-program-ilc-2022
Listek V. regenHU CEO: Bioprinting will strengthen organtrans project to 3D print liver organoid. 3DPrint.com | The Voice of 3D Printing / Additive Manufacturing. https://3dprint.com/270845/regenhu-ceo-bioprinting-will-strengthen-eus-organtrans-project-to-3d-print-a-liver-organoid/
Marketing R. REGENHU bioprinters to help develop liver tissue for OrganTrans. REGENHU. 2020. https://www.regenhu.com/community/news/biofabricated-liver-tissue-2/
Hanaphy P. T&R Biofab pulls-off breakthrough 3D bioprinted liver tissue transplant. 3D Printing Industry. 2021. https://3dprintingindustry.com/news/tr-biofab-pulls-off-breakthrough-3d-bioprinted-liver-tissue-transplant-192682/
T&R Biofab wins patent for liver organoid tech. KBR. 2020. https://www.koreabiomed.com/news/articleView.html?idxno=9890
Hong G, Kim J, Oh H, Yun S, Kim CM, Jeong YM, et al. Production of multiple cell-laden microtissue spheroids with a biomimetic hepatic-lobule-like structure. Adv Mater. 2021;33:2102624.
Kizawa H, Nagao E, Shimamura M, Zhang G, Torii H. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem Biophys Rep. 2017;10:186–91.
Yanagi Y, Nakayama K, Taguchi T, Enosawa S, Tamura T, Yoshimaru K, et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci Rep. 2017;7:14085.
Acknowledgements
The authors wish to acknowledge the Department of Science and Technology- Science Engineering Research Board, Early Career Research Award (ECR/2018/000709) and Indian Council for Medical Research (ICMR Adhoc -5/3/8/53/2020 – ITR) for funding the research. Open access funding provided by Manipal Academy of Higher Education, Manipal.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Contributions
Ms. MK—contributed to major writing, original draft preparation, data curation, tables and figures, proof reading and corrections. Ms. VM—contributed to writing and figures. Ms. MG—contributed to writing and figures & tables. Dr. VS—designed, edited and proof read the manuscript. Dr. KSV—conceptualized, designed and edited, proof read the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kasturi, M., Mathur, V., Gadre, M. et al. Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications. Tissue Eng Regen Med 21, 21–52 (2024). https://doi.org/10.1007/s13770-023-00576-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00576-3