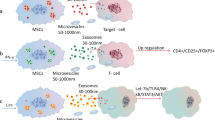
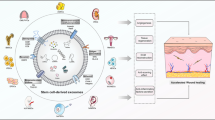
Abstract
Background:
Wound healing is a complicated biological process that leads to the regeneration of damaged skin tissue. Determining the methods to promote wound healing has become a hot topic in medical cosmetology and tissue repair research. Mesenchymal stem cells (MSCs) are a group of stem cells with the potential of self-renewal and multi-differentiation. MSCs transplantation has a broad application prospect in wound healing therapy. Many studies have demonstrated that the therapeutic capacity of MSCs is mainly mediated by paracrine actions. Exosomes (EXOs), which are nanosized vesicles carrying a variety of nucleic acids, proteins and lipids, are an important component of paracrine secretion. It has been demonstrated that exosomal microRNAs (EXO-miRNAs) play a key role in the function of exosomes.
Methods:
In this review, we focus on current research on miRNAs from MSC-derived exosomes (MSC-EXO miRNAs) in terms of sorting, releasing and function and their effects on inflammation regulation, epidermal cell function, fibroblast function, and extracellular matrix formation. At last, we discuss the current attempts to improve the treatment of MSC-EXO-miRNAs.
Results:
Many studies have demonstrated that MSC-EXO miRNAs play a key role in promoting wound healing. They have been shown to regulate inflammation response, enhance epidermal cell proliferation and migration, stimulate fibroblast proliferation and collagen synthesis, and regulate extracellular matrix formation. Besides, there have been a number of strategies developed to promote MSC-EXO and MSC-EXO miRNAs for wound healing treatment.
Conclusion:
Utilizing the association of exosomes from MSCs with miRNAs may be a promising strategy to promote trauma healing. MSC-EXO miRNAs may provide a new approach to promote wound healing and improve the quality of life for patients with skin injuries.


Similar content being viewed by others
References
Dyring-Andersen B, Løvendorf MB, Coscia F, Santos A, Møller LBP, Colaço AR, et al. Spatially and cell-type resolved quantitative proteomic atlas of healthy human skin. Nat Commun. 2020;11:5587.
Hu JC, Zheng CX, Sui BD, Liu WJ, Jin Y. Mesenchymal stem cell-derived exosomes: a novel and potential remedy for cutaneous wound healing and regeneration. World J Stem Cells. 2022;14:318–29.
Sun L, Wang F, Chen H, Liu D, Qu T, Li X, et al. Co-transplantation of human umbilical cord mesenchymal stem cells and human neural stem cells improves the outcome in rats with spinal cord injury. Cell Transplant. 2019;28:893–906.
Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36:165–78.
Yu Y, Luo Y, Fang Z, Teng W, Yu Y, Tian J, et al. Mechanism of sanguinarine in inhibiting macrophages to promote metastasis and proliferation of lung cancer via modulating the exosomes in A549 cells. Onco Targets Ther. 2020;13:8989–9003.
Nair VD, Ge Y, Li S, Pincas H, Jain N, Seenarine N, et al. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol. 2020;11:605.
Imafuku A, Sjoqvist S. Extracellular vesicle therapeutics in regenerative medicine. Adv Exp Med Biol. 2021;1312:131–8.
Matsuzaka Y, Yashiro R. Therapeutic strategy of mesenchymal-stem-cell-derived extracellular vesicles as regenerative medicine. Int J Mol Sci. 2022;23:6480.
Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9:4468.
Fahim SA, Abdullah MS, Espinoza-Sánchez NA, Hassan H, Ibrahim AM, Ahmed SH, et al. Inflammatory breast carcinoma: elevated microRNA miR-181b-5p and reduced miR-200b-3p, miR-200c-3p, and miR-203a-3p expression as potential biomarkers with diagnostic value. Biomolecules. 2020;10:1059.
Brümmer A, Hausser J. MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. Bioessays. 2014;36:617–26.
Pemmari T, Laakso J, Patrikainen MS, Parkkila S, Järvinen TAH. Carbonic anhydrase VI in skin wound healing study on Car6 knockout mice. Int J Mol Sci. 2020;21:5092.
Beke Debreceni I, Mezei G, Batár P, Illés Á, Kappelmayer J. Dasatinib Inhibits procoagulant and clot retracting activities of human platelets. Int J Mol Sci. 2019;20:5430.
de Laat-Kremers RMW, Yan Q, Ninivaggi M, de Maat M, de Laat B. Deciphering the coagulation profile through the dynamics of thrombin activity. Sci Rep. 2020;10:12544.
Wada Y, Suzuki A, Ishiguro H, Murakashi E, Numabe Y. Chronological gene expression of human gingival fibroblasts with low reactive level laser (LLL) irradiation. J Clin Med. 2021;10:1952.
Sorkin M, Huber AK, Hwang C, Carson WFt, Menon R, Li J, et al, Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat Commun. 2020;11:722.
Rajendran NK, Dhilip Kumar SS, Houreld NN, Abrahamse H. Understanding the perspectives of forkhead transcription factors in delayed wound healing. J Cell Commun Signal. 2019;13:151–62.
Ding W, Duan Y, Qu Z, Feng J, Zhang R, Li X, et al. Acidic microenvironment aggravates the severity of hepatic ischemia/reperfusion injury by modulating M1-polarization through regulating PPAR-γ signal. Front Immunol. 2021;12:697362.
Hsieh CC, Wang CH. Aspirin disrupts the crosstalk of angiogenic and inflammatory cytokines between 4T1 breast cancer cells and macrophages. Mediators Inflamm. 2018;2018:6380643.
Houseright RA, Rosowski EE, Lam PY, Tauzin SJM, Mulvaney O, Dewey CN, et al. Cell type specific gene expression profiling reveals a role for complement component C3 in neutrophil responses to tissue damage. Sci Rep. 2020;10:15716.
Chen Y, Tian L, Yang F, Tong W, Jia R, Zou Y, et al. Tannic acid accelerates cutaneous wound healing in rats via activation of the ERK 1/2 signaling pathways. Adv Wound Care (New Rochelle). 2019;8:341–54.
Shabestani Monfared G, Ertl P, Rothbauer M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci Rep. 2020;10:16192.
Li M, Hou Q, Zhong L, Zhao Y, Fu X. Macrophage related chronic inflammation in non-healing wounds. Front Immunol. 2021;12:681710.
Soib HH, Ismail HF, Husin F, Abu Bakar MH, Yaakob H, Sarmidi MR. Bioassay-guided different extraction techniques of Carica papaya (Linn.) leaves on in vitro wound-healing activities. Molecules. 2020;25:517.
He M, Halima M, Xie Y, Schaaf MJM, Meijer AH, Wang M. Ginsenoside Rg1 Acts as a selective glucocorticoid receptor agonist with anti-inflammatory action without affecting tissue regeneration in zebrafish larvae. Cells. 2020;9:1107.
Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259.
Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother. 2022;153:113463.
Teng L, Maqsood M, Zhu M, Zhou Y, Kang M, Zhou J, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate diabetic wound healing via promoting M2 macrophage polarization, angiogenesis, and collagen deposition. Int J Mol Sci. 2022;23:10421.
Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68.
Liu J, Yan Z, Yang F, Huang Y, Yu Y, Zhou L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering Angiopoietin-2. Stem Cell Rev Rep. 2021;17:305–17.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46.
Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via microRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–26.
Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H, et al. HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34:2485–500.
Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321.
Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120:10847–54.
Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, Liu QY, et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther. 2019;10:247.
Ren S, Chen J, Duscher D, Liu Y, Guo G, Kang Y, et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther. 2019;10:47.
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993.
Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018;10:eaai8524.
Li J, Li Z, Wang S, Bi J, Huo R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered. 2022;13:8515–25.
Liu J, Li F, Liu B, Yao Z, Li L, Liu G, et al. Adipose-derived mesenchymal stem cell exosomes inhibit transforming growth factor-β1-induced collagen synthesis in oral mucosal fibroblasts. Exp Ther Med. 2021;22:1419.
Hu J, Chen Y, Huang Y, Su Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway. Exp Mol Pathol. 2020;115:104468.
Adamo A, Brandi J, Caligola S, Delfino P, Bazzoni R, Carusone R, et al. Extracellular vesicles mediate mesenchymal stromal cell-dependent regulation of B Cell PI3K-AKT signaling pathway and actin cytoskeleton. Front Immunol. 2019;10:446.
Lee C, Han J, Jung Y. Pathological contribution of extracellular vesicles and their micrRNAs to progression of chronic liver disease. Biology (Basel). 2022;11:637.
Jothimani G, Pathak S, Dutta S, Duttaroy AK, Banerjee A. A comprehensive cancer-associated microRNA expression profiling and proteomic analysis of human umbilical cord mesenchymal stem cell-derived exosomes. Tissue Eng Regen Med. 2022;19:1013–31.
Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M, et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11:97.
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127.
Garcia-Martin R, Wang G, Brandão BB, Zanotto TM, Shah S, Kumar Patel S, et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–51.
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980.
Si Y, Liu F, Wang D, Fang C, Tang X, Guo B, et al. Exosomal transfer of miR-185 is controlled by hnRNPA2B1 and impairs re-endothelialization after vascular injury. Front Cell Dev Biol. 2021;9:619444.
Lee H, Li C, Zhang Y, Zhang D, Otterbein LE, Jin Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med. 2019;216:2202–20.
Pérez-Boza J, Boeckx A, Lion M, Dequiedt F, Struman I. hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cell Mol Life Sci. 2020;77:4413–28.
Li Y, Zhang J, Li S, Guo C, Li Q, Zhang X, et al. Heterogeneous nuclear ribonucleoprotein A1 loads batched tumor-promoting MicroRNAs into small extracellular vesicles with the assist of Caveolin-1 in A549 cells. Front Cell Dev Biol. 2021;9: 687912.
Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, et al. The RNA-binding protein SYNCRIP Is a component of the hepatocyte exosomal machinery controlling MicroRNA sorting. Cell Rep. 2016;17:799–808.
McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, et al. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 2016;15:978–87.
Lu P, Li H, Li N, Singh RN, Bishop CE, Chen X, et al. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. PLoS One. 2017;12:e0185992.
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52.
Robinson H, Ruelcke JE, Lewis A, Bond CS, Fox AH, Bharti V, et al. Caveolin-1-driven membrane remodelling regulates hnRNPK-mediated exosomal microRNA sorting in cancer. Clin Transl Med. 2021;11:e381.
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–32.
Albanese M, Chen YA, Hüls C, Gärtner K, Tagawa T, Mejias-Perez E, et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 2021;17:e1009951.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X, et al. miRNA-126-3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo. Stem Cell Res Ther. 2020;11:464.
Tan H, Huang S, Zhang Z, Qian X, Sun P, Zhou X. Pan-cancer analysis on microRNA-associated gene activation. EBioMedicine. 2019;43:82–97.
Liang Y, Lu Q, Li W, Zhang D, Zhang F, Zou Q, et al. Reactivation of tumour suppressor in breast cancer by enhancer switching through NamiRNA network. Nucleic Acids Res. 2021;49:8556–72.
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–6.
Wallach T, Wetzel M, Dembny P, Staszewski O, Krüger C, Buonfiglioli A, et al, Identification of CNS injury-related microRNAs as novel toll-like receptor 7/8 signaling activators by small RNA sequencing. Cells. 2020;9:186.
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308.
Tian J, Chen W, Xiong Y, Li Q, Kong S, Li M, et al. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact Mater. 2023;22:326–42.
Ti D, Hao H, Fu X, Han W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci. 2016;59:1305–12.
Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82.
Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264:118658.
Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–21.
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019;2019:7132708.
Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human adipose mesenchymal stem cell-derived exosomes: A key player in wound healing. Tissue Eng Regen Med. 2021;18:537–48.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–9.
Pi L, Yang L, Fang BR, Meng XX, Qian L. Exosomal microRNA-125a-3p from human adipose-derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN. Mol Cell Biochem. 2022;477:115–27.
Xu F, Xiang Q, Huang J, Chen Q, Yu N, Long X, et al. Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10:106.
Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:9742765.
Zhang L, Ouyang P, He G, Wang X, Song D, Yang Y, et al. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med. 2021;25:2148–62.
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–84.
Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, et al. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472–87.
Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. 2018;27:1170–2.
Gao S, Chen T, Hao Y, Zhang F, Tang X, Wang D, et al. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res Ther. 2020;11:56.
Chen T, Gao S, Hao Y, Zhang F, Tang X, Wei Z, et al. Experimental study of human amniotic mesenchymal stem cell exosome promoting fibroblasts migration through microRNA-135a. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34:234–9.
Zhao W, Zhang R, Zang C, Zhang L, Zhao R, Li Q, et al. Exosome derived from mesenchymal stem cells alleviates pathological scars by inhibiting the proliferation, migration and protein expression of fibroblasts via delivering miR-138-5p to Target SIRT1. Int J Nanomedicine. 2022;17:4023–38.
Ma J, Zhang Z, Wang Y, Shen H. Investigation of miR-126-3p loaded on adipose stem cell-derived exosomes for wound healing of full-thickness skin defects. Exp Dermatol. 2022;31:362–74.
Yang C, Luo L, Bai X, Shen K, Liu K, Wang J, et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020;681:108259.
Wang J, Qiu Y, Shi NW, Zhao JN, Wang YC, Jiang H, et al. microRNA-21 mediates the TGF-β1-induced migration of keratinocytes via targeting PTEN. Eur Rev Med Pharmacol Sci. 2016;20:3748–59.
Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021;24:758.
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425–39.
Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12:221.
Duan M, Zhang Y, Zhang H, Meng Y, Qian M, Zhang G. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res Ther. 2020;11:452.
Zhang Y, Yan J, Liu Y, Chen Z, Li X, Tang L, et al. Human amniotic fluid stem cell-derived exosomes as a novel cell-free therapy for cutaneous regeneration. Front Cell Dev Biol. 2021;9: 685873.
Chen R, Yuan W, Zheng Y, Zhu X, Jin B, Yang T, et al. Delivery of engineered extracellular vesicles with miR-29b editing system for muscle atrophy therapy. J Nanobiotechnol. 2022;20:304.
Park DJ, Seo YJ. Engineering of extracellular vesicles based on payload changes for tissue regeneration. Tissue Eng Regen Med. 2021;18:485–97.
Chen Z, Xie Y, Chen W, Li T, Chen X, Liu B. microRNA-6785-5p-loaded human umbilical cord mesenchymal stem cells-derived exosomes suppress angiogenesis and metastasis in gastric cancer via INHBA. Life Sci. 2021;284: 119222.
Naseri Z, Oskuee RK, Forouzandeh-Moghadam M, Jaafari MR. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev Rep. 2020;16:541–56.
Bai Y, Han YD, Yan XL, Ren J, Zeng Q, Li XD, et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun. 2018;500:310–7.
Heo JS. Selenium-stimulated exosomes enhance wound healing by modulating inflammation and angiogenesis. Int J Mol Sci. 2022;23:11543.
Zhou Y, Zhang XL, Lu ST, Zhang NY, Zhang HJ, Zhang J, et al. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13:407.
Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021;120:111671.
Xu H, Wang J, Wu D, Qin D. A hybrid hydrogel encapsulating human umbilical cord mesenchymal stem cells enhances diabetic wound healing. J Mater Sci Mater Med. 2022;33:60.
Hahm J, Kim J, Park J. Strategies to enhance extracellular vesicle production. Tissue Eng Regen Med. 2021;18:513–24.
Jiang B, Yan L, Wang X, Li E, Murphy K, Vaccaro K, et al. Concise review: mesenchymal stem cells derived from human pluripotent cells, an unlimited and quality-controllable source for therapeutic applications. Stem Cells. 2019;37:572–81.
Wang X, Jiang B, Sun H, Zheng D, Zhang Z, Yan L, et al. Noninvasive application of mesenchymal stem cell spheres derived from hESC accelerates wound healing in a CXCL12-CXCR4 axis-dependent manner. Theranostics. 2019;9:6112–28.
Acknowledgement
This work was supported by the National Natural Science Foundation of China under Grant Number 81460293.
Author information
Authors and Affiliations
Contributions
JCC contributed to the investigation, data curation, and manuscript writing; LDW supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical statement
There were no animal or human subject experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ju, C., Liu, D. Exosomal microRNAs from Mesenchymal Stem Cells: Novel Therapeutic Effect in Wound Healing. Tissue Eng Regen Med 20, 647–660 (2023). https://doi.org/10.1007/s13770-023-00542-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00542-z