Abstract
Background:
Bone morphogenetic protein 2 (BMP-2) and low-intensity pulsed ultrasound (LIPUS) have been used to enhance bone healing in distraction osteogenesis (DO). The aim of this study was to assess the synergistic effect of BMP-2 and LIPUS on bone regeneration in DO and to determine the optimal treatment strategy for enhanced bone regeneration.
Methods:
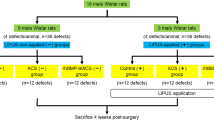
Rat mesenchymal stromal cells were treated with various application protocols of BMP-2 and LIPUS, and cell proliferation, alkaline phosphatase activity, and osteogenesis-related marker expression were evaluated. In vivo experiments were performed in a rabbit DO model according to the application protocols with different timings of BMP-2 and LIPUS application.
Results:
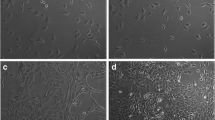
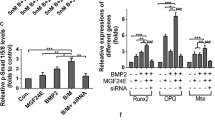
Application of BMP-2 after LIPUS pretreatment (BMP-2 after LIPUS) showed greater cell proliferation than LIPUS treatment alone, and higher ALP activity than all other treatment protocols. BMP-2 after LIPUS also exhibited increased gene expression levels of ALP, Cbfa1, and Osterix compared with LIPUS treatment alone. In vivo experiments revealed no significant differences in bone healing based on the timing of LIPUS treatment in DO. The combination of BMP-2 and LIPUS resulted in increased bone volume and bone mineral density compared with BMP-2 or LIPUS. Regarding the timing of BMP-2 application, the application of BMP-2 after LIPUS pretreatment led to greater bone volume than the application of BMP-2 before LIPUS.
Conclusion:
The results of this study suggest that the combined treatment of BMP-2 and LIPUS can lead to enhanced bone healing in DO and that effective bone healing can be achieved through the application of LIPUS before BMP-2.










Similar content being viewed by others
References
Norholt SE, Jensen J, Schou S, Pedersen TK. Complications after mandibular distraction osteogenesis: a retrospective study of 131 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:420–7.
Swennen G, Schliephake H, Dempf R, Schierle H, Malevez C. Craniofacial distraction osteogenesis: a review of the literature: part 1: clinical studies. Int J Oral Maxillofac Surg. 2001;30:89–103.
Master DL, Hanson PR, Gosain AK. Complications of mandibular distraction osteogenesis. J Craniofac Surg. 2010;21:1565–70.
Hong P, Boyd D, Beyea SD, Bezuhly M. Enhancement of bone consolidation in mandibular distraction osteogenesis: a contemporary review of experimental studies involving adjuvant therapies. J Plast Reconstr Aesthet Surg. 2013;66:883–95.
Makhdom AM, Hamdy RC. The role of growth factors on acceleration of bone regeneration during distraction osteogenesis. Tissue Eng Part B Rev. 2013;19:442–53.
Xie LK, Wangrangsimakul K, Suttapreyasri S, Cheung LK, Nuntanaranont T. A preliminary study of the effect of low intensity pulsed ultrasound on new bone formation during mandibular distraction osteogenesis in rabbits. Int J Oral Maxillofac Surg. 2011;40:730–6.
Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83:259–70.
Hadjiargyrou M, McLeod K, Ryaby JP, Rubin C. Enhancement of fracture healing by low intensity ultrasound. Clin Orthop Relat Res. 1998;355:S216–29.
Parvizi J, Parpura V, Greenleaf JF, Bolander ME. Calcium signaling is required for ultrasound-stimulated aggrecan synthesis by rat chondrocytes. J Orthop Res. 2002;20:51–7.
Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res. 1994;12:40–7.
Yang KH, Parvizi J, Wang SJ, Lewallen DG, Kinnick RR, Greenleaf JF, et al. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. 1996;14:802–9.
El-Bialy TH, Royston TJ, Magin RL, Evans CA, Zaki Ael M, Frizzell LA. The effect of pulsed ultrasound on mandibular distraction. Ann Biomed Eng. 2002;30:1251–61.
Schortinghuis J, Bronckers AL, Stegenga B, Raghoebar GM, de Bont LG. Ultrasound to stimulate early bone formation in a distraction gap: a double blind randomised clinical pilot trial in the edentulous mandible. Arch Oral Biol. 2005;50:411–20.
Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9.
Han JJ, Chang AR, Ahn J, Jung S, Hong J, Oh HK, et al. Efficacy and safety of rhBMP/beta-TCP in alveolar ridge preservation: a multicenter, randomized, open-label, comparative, investigator-blinded clinical trial. Maxillofac Plast Reconstr Surg. 2021;43:42.
Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2). J Oral Maxillofac Surg. 2008;66:616–24.
Park JH, Kim JW, Kim SJ. Does the addition of bone morphogenetic protein 2 to platelet-rich fibrin improve healing after treatment for medication-related osteonecrosis of the jaw? J Oral Maxillofac Surg. 2017;75:1176–84.
Cheung LK, Zheng LW. Effect of recombinant human bone morphogenetic protein-2 on mandibular distraction at different rates in an experimental model. J Craniofac Surg. 2006;17:100–8.
Li G, Bouxsein ML, Luppen C, Li XJ, Wood M, Seeherman HJ, et al. Bone consolidation is enhanced by rhBMP-2 in a rabbit model of distraction osteogenesis. J Orthop Res. 2002;20:779–88.
Sailhan F, Gleyzolle B, Parot R, Guerini H, Viguier E. Rh-BMP-2 in distraction osteogenesis: dose effect and premature consolidation. Injury. 2010;41:680–6.
Angle SR, Sena K, Sumner DR, Virkus WW, Virdi AS. Combined use of low-intensity pulsed ultrasound and rhBMP-2 to enhance bone formation in a rat model of critical size defect. J Orthop Trauma. 2014;28:605–11.
Sant’Anna EF, Leven RM, Virdi AS, Sumner DR. Effect of low intensity pulsed ultrasound and BMP-2 on rat bone marrow stromal cell gene expression. J Orthop Res. 2005;23:646–52.
Wijdicks CA, Virdi AS, Sena K, Sumner DR, Leven RM. Ultrasound enhances recombinant human BMP-2 induced ectopic bone formation in a rat model. Ultrasound Med Biol. 2009;35:1629–37.
Sakurakichi K, Tsuchiya H, Uehara K, Yamashiro T, Tomita K, Azuma Y. Effects of timing of low-intensity pulsed ultrasound on distraction osteogenesis. J Orthop Res. 2004;22:395–403.
Mayr E, Laule A, Suger G, Ruter A, Claes L. Radiographic results of callus distraction aided by pulsed low-intensity ultrasound. J Orthop Trauma. 2001;15:407–14.
Shimazaki A, Inui K, Azuma Y, Nishimura N, Yamano Y. Low-intensity pulsed ultrasound accelerates bone maturation in distraction osteogenesis in rabbits. J Bone Joint Surg Br. 2000;82:1077–82.
Lai CH, Chen SC, Chiu LH, Yang CB, Tsai YH, Zuo CS, et al. Effects of low-intensity pulsed ultrasound, dexamethasone/TGF-beta1 and/or BMP-2 on the transcriptional expression of genes in human mesenchymal stem cells: chondrogenic vs. osteogenic differentiation. Ultrasound Med Biol. 2010;36:1022–33.
Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43–50.
Selvamurugan N, Kwok S, Vasilov A, Jefcoat SC, Partridge NC. Effects of BMP-2 and pulsed electromagnetic field (PEMF) on rat primary osteoblastic cell proliferation and gene expression. J Orthop Res. 2007;25:1213–20.
Imafuji T, Shirakata Y, Shinohara Y, Nakamura T, Noguchi K. Enhanced bone formation of calvarial bone defects by low-intensity pulsed ultrasound and recombinant human bone morphogenetic protein-9: a preliminary experimental study in rats. Clin Oral Investig. 2021;25:5917–27.
Yonezawa H, Harada K, Ikebe T, Shinohara M, Enomoto S. Effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on bone consolidation on distraction osteogenesis: a preliminary study in rabbit mandibles. J Craniomaxillofac Surg. 2006;34:270–6.
Egermann M, Lill CA, Griesbeck K, Evans CH, Robbins PD, Schneider E, et al. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006;13:1290–9.
Freitas RM, Spin-Neto R, Marcantonio Junior E, Pereira LA, Wikesjo UM, Susin C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: a systematic review. Clin Implant Dent Relat Res. 2015;17:e192-201.
Gao TJ, Lindholm TS, Kommonen B, Ragni P, Paronzini A, Lindholm TC, et al. The use of a coral composite implant containing bone morphogenetic protein to repair a segmental tibial defect in sheep. Int Orthop. 1997;21:194–200.
Viljanen VV, Gao TJ, Lindholm TC, Lindholm TS, Kommonen B. Xenogeneic moose (Alces alces) bone morphogenetic protein (mBMP)-induced repair of critical-size skull defects in sheep. Int J Oral Maxillofac Surg. 1996;25:217–22.
Acknowledgements
This study was supported by a grant (No. 10045651) from the Advanced Technology Center R&D Project, Ministry of Trade, Industry and Energy, Republic of Korea and National Research Foundation of Korea (Grant No. NRF-2018R1A5A2024418). We thank Tae-Hyung Cho, and Beom Seok Lee for providing technical assistance with animal experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal studies were performed after receiving approval from the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (IACUC no. SNU-141120-2).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, J.J., Yang, H.J. & Hwang, S.J. Enhanced Bone Regeneration by Bone Morphogenetic Protein-2 after Pretreatment with Low-Intensity Pulsed Ultrasound in Distraction Osteogenesis. Tissue Eng Regen Med 19, 871–886 (2022). https://doi.org/10.1007/s13770-022-00457-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00457-1