Abstract
BACKGROUND:
Tissue engineering approaches to treat damaged bone include various tissue transplants such as autologous, allogeneic, and xenografts. Artificial materials have been widely introduced to meet the demand for graft materials, but insufficiency in supply is still not resolved. In this study, human adipose tissue, easily obtained from the human body, was harvested, and the tissue was decellularized to fabricate a decellularized human adipose tissue matrix (DM) as an alternative graft material.
METHODS:
Human adipose tissue was obtained via liposuction. The obtained fresh adipose tissue sample was cut into pieces then put into decellularization solution (1% antibiotic–antimycotic solution and 1% phenylmethanesulphonyl fluoride). Lipids were further removed via treatment in isopropanol. The sample was then subjected to another enzymatic digestion and lipid removal processes. The obtained decellularized adipose tissue matrix was lyophilized to form a graft material in disc shape.
RESULTS:
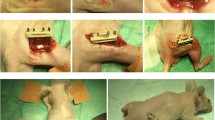
Decellularization was confirmed by nuclear staining methods and detection of RNA and DNA via PCR. Bone morphogenetic protein 2 (BMP2)-loaded DM showed the ability to form new bone tissue when implanted in subcutaneous tissue. In recovery of a mouse calvarial defect model, BMP2-loaded DM exhibited similar levels of bone tissue regeneration efficiency compared with a well-defined commercial product, BMP2-loaded CollaCote®.
CONCLUSION:
The DM developed in this study is expected to address the problem of insufficient supply of graft materials and contribute to the treatment of bone defects of critical size as an alternative bone graft material with preserved extracellular matrix components.





Similar content being viewed by others
References
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408.
Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci. 2009;98:1317–75.
Drosse I, Volkmer E, Capanna R, De Biase P, Mutschler W, Schieker M. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury. 2008;39:S9–20.
Aamodt JM, Grainger DW. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016;86:68–82.
Dang M, Saunders L, Niu X, Fan Y, Ma PX. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018;6:25.
La WG, Kang SW, Yang HS, Bhang SH, Lee SH, Park JH, et al. The efficacy of bone morphogenetic protein-2 depends on its mode of delivery. Artif Organs. 2010;34:1150–3.
Lee TJ, Kang SW, Bhang SH, Kang JM, Kim BS. Apatite-coated porous poly(lactic-co-glycolic acid) microspheres as an injectable bone substitute. J Biomater Sci Polym Ed. 2010;21:635–45.
Das P, Mishra R, Devi B, Rajesh K, Basak P, Roy M, et al. Decellularized xenogenic cartilage extracellular matrix (ECM) scaffolds for the reconstruction of osteochondral defects in rabbits. J Mater Chem B. 2021;9:4873–94.
Chun SY, Lim JO, Lee EH, Han MH, Ha YS, Lee JN, et al. Preparation and characterization of human adipose tissue-derived extracellular matrix, growth factors, and stem cells: a concise review. Tissue Eng Regen Med. 2019;16:385–93.
Lin X, Patil S, Gao YG, Qian A. The bone extracellular matrix in bone formation and regeneration. Front Pharmacol. 2020;11:757.
Migliorini E, Valat A, Picart C, Cavalcanti-Adam EA. Tuning cellular responses to BMP-2 with material surfaces. Cytokine Growth Factor Rev. 2016;27:43–54.
Ruiz-Ojeda FJ, Méndez-Gutiérrez A, Aguilera CM, Plaza-Díaz J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int J Mol Sci. 2019;20:4888.
Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24.
Kim YS, Majid M, Melchiorri AJ, Mikos AG. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng Transl Med. 2019;4:83–95.
Wang L, Johnson JA, Zhang Q, Beahm EK. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 2013;9:8921–31.
Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34.
Han SS, Choi JJ, Lee DE, Jang HS, Chung HM, Moon SH, et al. Histological analysis of in vitro co-culture and in vivo mice co-transplantation of stem cell-derived adipocyte and osteoblast. Tissue Eng Regen Med. 2016;13:227–34.
Panina YA, Yakimov AS, Komleva YK, Morgun AV, Lopatina OL, Malinovskaya NA, et al. Plasticity of adipose tissue-derived stem cells and regulation of angiogenesis. Front Physiol. 2018;9:1656.
Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P, et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci. 2020;21:3242.
Kang SW, Yang HS, Seo SW, Han DK, Kim BS. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J Biomed Mater Res A. 2008;85:747–56.
Vantucci CE, Krishan L, Cheng A, Prather A, Roy K, Guldberg RE. BMP-2 delivery strategy modulates local bone regeneration and systemic immune responses to complex extremity trauma. Biomater Sci. 2021;9:1668–82.
Jung T, Lee JH, Park S, Kim YJ, Seo J, Shim HE, et al. Effect of BMP-2 delivery mode on osteogenic differentiation of stem cells. Stem Cells Int. 2017;2017:7859184.
Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, et al. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013;34:452–9.
Hong JY, Kang SW, Kim JW, Suh SW, Ko YJ, Park JH. Optimal condition of heparin-conjugated fibrin with bone morphogenetic protein-2 for spinal fusion in a rabbit model. Cytotherapy. 2014;16:1441–8.
Khan WS, Longo UG, Adesida A, Denaro V. Stem cell and tissue engineering applications in orthopaedics and musculoskeletal medicine. Stem Cells Int. 2012;2012:403170.
Acknowledgements
This research was supported by the Korea Institute of Toxicology, Republic of Korea (1711133848 and 1711133843).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflict to declare.
Ethical statement
Human adipose tissue was obtained via liposuction in compliance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Korea University Ansan Hospital (Approval Number: AS12194). All animal experiments were approved by the IACUC of Korea University (KUIACUC 2013-21). All animals were managed by following guidelines for the care and use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahn, W.B., Lee, Y.B., Ji, YH. et al. Decellularized Human Adipose Tissue as an Alternative Graft Material for Bone Regeneration. Tissue Eng Regen Med 19, 1089–1098 (2022). https://doi.org/10.1007/s13770-022-00451-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00451-7