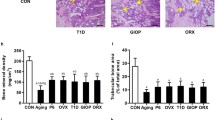
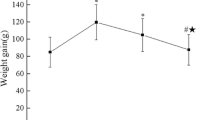
Abstract
BACKGROUND:
Estrogen deficiency decreases bone density and increases the risk of osteoporosis and fracture, thereby necessitating reconstruction of bone regeneration. As bone marrow mesenchymal stem cell (BMSCs) lose viability and differentiation potential under osteoporotic conditions, it is impossible to use autologous BMSCs for osteoporosis treatment. As an alternative, adipose-derived stem cells (ADSCs) may serve as the source of therapeutic cells.
METHOD:
We evaluated the effects of osteoporosis on the functional characteristics of ADSCs. Osteoporosis was induced in ovariectomy (OVX) rat model, and the ADSCs from Sham and OVX groups were cultured and analyzed comparatively.
RESULTS:
As a result, the viability was higher for the ADSCs from Sham group than those from OVX group. The analysis of the paracrine potential of ADSCs revealed the elevated levels of inflammatory and cellular senescence factors in the ADSCs from OVX group. The ADSCs from OVX group had much higher differentiation potential into adipocytes than those from the Sham group. Osteoporotic environment had no effect on the osteogenic potential of ADSCs.
CONCLUSION:
Osteoporosis may reduce the activity and influence immune response of ADSCs by modulating paracrine action and adipogenic potential. These characteristics of ADSCs should be given consideration for therapeutic purpose.





Similar content being viewed by others
References
Ji MX, Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med. 2015;1:9–13.
Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–94.
Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-y-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100:10405–10.
Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–37.
Zheng SX, Vrindts Y, Lopez M, De Groote D, Zangerle PF, Collette J, et al. Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71.
D’Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100.
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complication of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–9.
Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg. 2001;9:210–8.
Calabrese G, Giuffrida R, Forte S, Fabbi C, Figallo E, Salvatorelli L, et al. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci Rep. 2017;7:7110.
Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–65.
Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70.
Gallagher JC. Role of estrogens in the management of postmenopausal bone loss. Rheum Dis Clin North Am. 2001;27:143–62.
Hadji P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric. 2012;15:513–23.
Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019;129:3214–23.
Shen L, Xie X, Su Y, Luo C, Zhang C, Zeng B. Parathyroid hormone versus bisphosphonate treatment on bone mineral density in osteoporosis therapy: a meta-analysis of randomized controlled trials. PLoS One. 2011;6:e26267.
Miller PD. Optimizing the management of postmenopausal osteoporosis with bisphosphonates: the emerging role of intermittent therapy. Clin Ther. 2005;27:361–76.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
Jódar-Gimeno E. Full length parathyroid hormone in the treatment of osteoporosis in postmenopausal women. Clin Interv Aging. 2007;2:163–74.
Ponnapakkam T, Katikaneni R, Sakon J, Stratford R, Gensure RC. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov Today. 2014;19:204–8.
Baroncelli GI, Bertelloni S. The use of Bisphosphonates in Pediatrics. Horm Res Paediatr. 2014;82:290–302.
Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009;24:98–103.
Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. Tissue Eng Regen Med. 2008;2:169–83.
Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, et al. Strategies to optimize adult stem cell therapy for tissue regeneration. Int J Mol Sci. 2016;17:982.
Burdon TJ, Paul A, Noiseux N, Prakash S, ShumTim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326.
Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–8.
Cortes Y, Ojeda M, Araya D, Dueñas F, Fernández MS, Peralta OA. Isolation and multilineage differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet Res. 2013;9:133.
Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–75.
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–16.
Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother. 2016;43:268–74.
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–30.
Sophocleous A, Idris AI. Rodent models of osteoporosis. Bonekey Rep. 2014;3:614.
Khajuria DK, Razdan R, Mahapatra DR. Description of a new method of ovariectomy in female rats. Rev Bras Reumatol. 2012;52:462–70.
Boichuck M, Zorea J, Elkabets M, Wolfson M, Fraifeld VE. c-Met as a new marker of cellular senescence. Aging (Albany NY). 2019;11:2889–97.
Zhao R. Immune regulation of bone loss by Th17 cells in estrogen-deficient osteoporosis. Eur J Clin Invest. 2013;43:1195–202.
Fliefel R, Tröltzsch M, Kühnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44:568–85.
Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–63.
Li J, Liu X, Zuo B, Zhang L. The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging Dis. 2015;7:514–25.
Acknowledgement
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B0704104813).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Ethical statement
Animal experiments were approved by the Ethical Committees for Experimental Animals at Kyung Hee University (KHMC-IACUC 2018-40).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, J.S., Piao, J., Park, G. et al. Osteoporotic Conditions Influence the Activity of Adipose-Derived Stem Cells. Tissue Eng Regen Med 17, 875–885 (2020). https://doi.org/10.1007/s13770-020-00289-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00289-x