Abstract
Background:
Congenital defects of the urinary bladder (micro- or contracted bladder, bladder exstrophy) remain a challenging problem for pediatric surgeons. Even when conservative treatment options are fully exhausted, irreversible renal dysfunction can be observed in a large number of cases that can even lead to chronic renal failure and the need for kidney transplantation. To protect kidney function bladder augmentation using intestinal tissue is commonly applied as the standard treatment method. However due to the unphysiological nature of intestinal tissue a number of problems and complications such as urinary tract infections or bladder stone formation limit the clinical success of this approach. Moreover a number of substitutes for the implementation of a bladder augmentation have been tested without success to date. Here we used an experimental model to test wether the biocompatible collagen mesh Lyoplant may be a suitable candidate for bladder augmentation.
Methods:
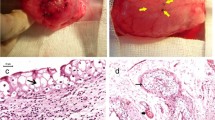
We implanted a biocompatible collagen mesh (Lyoplant®) in a bladder defect rat model for bladder augmentation (Lyoplant®-group: n = 12; sham group n = 4). After 6 weeks the abdomen was reopened and the initial implant as well as the bladder were resected for histological and immunohistochemical examination.
Results:
All but one rat exhibited physiological growth and behaviour after the operation without differences between the Lyoplant®-group (n = 12) and the sham group (n = 3). One rat from the sham group had to be excluded because of a suture leakage. No wound healing complications, wound infections and no herniation were observed. After 5 weeks the implants showed an adequate incorporation in all cases. This was confirmed by immunohistological analyses where a significant cell infiltration and neovascularization was observed.
Conclusion:
In summary, Lyoplant® appears to be a promising tool in experimental bladder augmentation/regeneration in rats.







Similar content being viewed by others
References
Langer S, Radtke C, Györi E, Springer A, Metzelder ML. Bladder augmentation in children: current problems and experimental strategies for reconstruction. Wien Med Wochenschr. 2019;169:61–70.
Ferrer F, Gearhart JP. Bladder exstrophy: considerations and management of the newborn patient. In: Puri P, editor. Newborn Surgery. Oxford: Oxford University Press; 2003. p. 619–27.
Pokrywczynska M, Adamowicz J, Sharma AK, Drewa T. Human urinary bladder regeneration through tissue engineering: an analysis of 131 clinical cases. Exp Biol Med (Maywood). 2014;239:264–71.
Diamond DA, Chan IHY, Holland AJA, Kurtz MP, Nelson C, Estrada CR Jr, et al. Advances in paediatric urology. Lancet. 2017;390:1061–71.
Hoen L’, Ecclestone H, Blok BFM, Karsenty G, Phé V, Bossier R, et al. Long-term effectiveness and complication rates of bladder augmentation in patients with neurogenic bladder dysfunction: a systematic review. Neurourol Urodyn. 2017;36:1685–702.
Smeulders N, Woodhouse CR. Neoplasia in adult exstrophy patients. BJU Int. 2001;87:623–8.
Kollhoff DM, Cheng EY, Sharma AK. Urologic applications of engineered tissue. Regen Med. 2011;6:757–65.
Meyer T, Meyer B, Schwarz K, Höcht B. Immune response to xenogeneic matrix grafts used in pediatric surgery. Eur J Pediatr Surg. 2007;17:420–5.
Meyer T, Schwarz K, Ulrichs K, Höcht B. A new biocompatible material (Lyoplant) for the therapy of congenital abdominal wall defects: first experimental results in rats. Pediatr Surg Int. 2006;22:369–74.
Meyer T, Seifert A, Meyer B, Ulrichs K, Germer CT. PAUL procedure. A new biocompatible concept for the therapy of congenital abdominal wall defects. Chirurg. 2010;81:236–42.
Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol. 2013;9:878–83.
Ayyildiz A, Nuhoglu B, Huri E, Ozer E, Gurdal M, Germiyanoglu C. Using porcine acellular collagen matrix (Pelvicol) in bladder augmentation: experimental study. Int Braz J Urol. 2006;32:88–92.
AG, B.B.M. Lyoplant. 2015; Available from: https://www.bbraun.de/de/products/b0/lyoplant-onlay.html.
Kouame BD, Kouame GS, Sounkere M, Koffi M, Yaokreh JB, Odehouri-Koudou T, et al. Aesthetic, urological, orthopaedic and functional outcomes in complex bladder exstrophy-epispadias’s management. Afr J Paediatr Surg. 2015;12:56–60.
Bertin KD, Serge KY, Moufidath S, Maxime K, Hervé OK, Baptiste YJ, et al. Complex bladder-exstrophy-epispadias management: causes of failure of initial bladder closure. Afr J Paediatr Surg. 2014;11:334–40.
Inouye BM, Tourchi A, Di Carlo HN, Young EE, Gearhart JP. Modern management of the exstrophy-epispadias complex. Surg Res Pract. 2014;2014:587064.
Woodhouse CR, North AC, Gearhart JP. Standing the test of time: long-term outcome of reconstruction of the exstrophy bladder. World J Urol. 2006;24:244–9.
Gearhart JP, Ben-Chaim J, Sciortino C, Sponseller PD, Jeffs RD. The multiple reoperative bladder exstrophy closure: what affects the potential of the bladder? Urology. 1996;47:240–3.
Hesh CA, Young E, Intihar P, Gearhart JP. The cost of failure: the economic impact of failed primary closure in classic bladder exstrophy. J Pediatr Surg. 2016;51:1312–6.
Stein R, Hohenfellner M, Pahernik S, Roth S, Thüroff JW, Rübben H. Übersichtsarbeit-Therapiekonzepte und Konsequenzen der Harnableitung. Dtsch Arztebl Ausg A. 2012;109:617–22.
El-Taji OM, Khattak AQ, Hussain SA. Bladder reconstruction: the past, present and future. Oncol Lett. 2015;10:3–10.
Wünsch L, Ehlers EM, Russlies M. Matrix testing for urothelial tissue engineering. Eur J Pediatr Surg. 2005;15:164–9.
Cranidis A, Nestoridis G, Delakas D, Lumbakis P, Kanavaros P. Bladder autoaugmentation in the rabbit using de-epithelialized segments of small intestine, stomach and lyophilized human dura mater. Br J Urol. 1998;81:62–7.
Bolland F, Korossis S, Wilshaw SP, Ingham E, Fisher J, Kearney JN, et al. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28:1061–70.
Yang B, Zhang Y, Zhou L, Sun Z, Zheng J, Chen Y, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods. 2010;16:1201–11.
Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–40.
Kaufmann SHE. T-Zellen. In: Kaufmann SHE, editor. Basiswissen immunologie. Berlin: Springer; 2014. p. 63–83.
Gulbins E, Lang KS. Immunsystem. In: Schmidt RF, Lang F, Heckmann M, editors. Physiologie des menschen. Berlin: Springer; 2007. p. 550–62.
Roelofs LA, Kortmann BB, Oosterwijk E, Eggink AJ, Tiemessen DM, Crevels AJ, et al. Tissue engineering of diseased bladder using a collagen scaffold in a bladder exstrophy model. BJU Int. 2014;114:447–57.
Leonhäuser D, Stollenwerk K, Seifarth V, Zraik IM, Vogt M, Srinivasan PK, et al. Two differentially structured collagen scaffolds for potential urinary bladder augmentation: proof of concept study in a Göttingen minipig model. J Transl Med. 2017;15:3.
Acknowledgement
The author’s thanks Mrs. Chodnesvska for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study was performed according to a protocol approved by the local committee for animal use and care (Regierung von Unterfranken: 55.2-2531.01-01/12).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Winde, F., Backhaus, K., Zeitler, J.A. et al. Bladder Augmentation Using Lyoplant®: First Experimental Results in Rats. Tissue Eng Regen Med 16, 645–652 (2019). https://doi.org/10.1007/s13770-019-00209-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00209-8