Abstract
BACKGROUND:
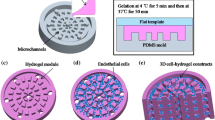
The fabrication of microchannels in hydrogel can facilitate the perfusion of nutrients and oxygen, which leads to guidance cues for vasculogenesis. Microchannel patterning in biomimetic hydrogels is a challenging issue for tissue regeneration because of the inherent low formability of hydrogels in a complex configuration. We fabricated microchannels using wire network molding and immobilized the angiogenic factors in the hydrogel and evaluated the vasculogenesis in vitro and in vivo.
METHODS:
Microchannels were fabricated in a hyaluronic acid-based biomimetic hydrogel by using “wire network molding” technology. Substance P was immobilized in acrylated hyaluronic acid for angiogenic cues using Michael type addition reaction. In vitro and in vivo angiogenic activities of hydrogel with microchannels were evaluated.
RESULTS:
In vitro cell culture experiment shows that cell viability in two experimental biomimetic hydrogels (with microchannels and microchannels + SP) was higher than that of a biomimetic hydrogel without microchannels (bulk group). Evaluation on differentiation of human mesenchymal stem cells (hMSCs) in biomimetic hydrogels with fabricated microchannels shows that the differentiation of hMSC into endothelial cells was significantly increased compared with that of the bulk group. In vivo angiogenesis analysis shows that thin blood vessels of approximately 25–30 μm in diameter were observed in the microchannel group and microchannel + SP group, whereas not seen in the bulk group.
CONCLUSION:
The strategy of fabricating microchannels in a biomimetic hydrogel and simultaneously providing a chemical cue for angiogenesis is a promising formula for large-scale tissue regeneration.







Similar content being viewed by others
References
Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–41.
Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–3.
Hall H. Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr Pharm Des. 2007;13:3597–607.
Tabata Y, Miyao M, Ozeki M, Ikada Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J Biomater Sci Polym Ed. 2000;11:915–30.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Zieris A, Chwalek K, Prokoph S, Levental KR, Welzel PB, Freudenberg U, et al. Dual independent delivery of pro-angiogenic growth factors from starpeg-heparin hydrogels. J Control Release. 2011;156:28–36.
Kim SK, Cho TH, Han JJ, Kim IK, Park Y, Hwang SJ. Comparative study of BMP-2 alone and combined with VEGF carried by hydrogel for maxillary alveolar bone regeneration. Tissue Eng Regen Med. 2016;13:171–81.
Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B–PDGFRbeta signaling. J Cell Sci. 2005;118:3759–68.
Chwalek K, Tsurkan MV, Freudenberg U, Werner C. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci Rep. 2014;4:4414.
Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb). 2014;6:555–63.
Kim SK, Lee J, Song M, Kim M, Hwang SJ, Jang H, et al. Combination of three angiogenic growth factors has synergistic effects on sprouting of endothelial cell/mesenchymal stem cell-based spheroids in a 3D matrix. J Biomed Mater Res B Appl Biomater. 2016;104:1535–43.
Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, et al. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation. 2004;109:777–83.
Song M, Jang H, Lee J, Kim JH, Kim SH, Sun K, et al. Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor SDF-1 and angiogenic peptide Ac-SDKP. Biomaterials. 2014;35:2436–45.
Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010;31:8617–25.
Kim JH, Jung Y, Kim BS, Kim SH. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013;34:1657–68.
Charles PT, Goldman ER, Rangasammy JG, Schauer CL, Chen MS, Taitt CR. Fabrication and characterization of 3d hydrogel microarrays to measure antigenicity and antibody functionality for biosensor applications. Biosens Bioelectron. 2004;20:753–64.
Kim G, Park K. Alginate-nanofibers fabricated by an electrohydrodynamic process. Polym Eng Sci. 2009;49:2242–8.
Liu C, Kray J, Chan C. Schwann cells enhance penetration of regenerated axons into three-dimensional microchannels. Tissue Eng Regen Med. 2018;15:351–61.
Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905–25.
Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep. 2016;6:24474.
Fedorovich NE, de Wijn JR, Verbout AJ, Alblas J, Dhert WJ. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng Part A. 2008;14:127–33.
Muehleder S, Ovsianikov A, Zipperle J, Redl H, Holnthoner W. Connections matter: channeled hydrogels to improve vascularization. Front Bioeng Biotechnol. 2014;2:52.
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–11.
Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–74.
Tseng TC, Hsieh FY, Theato P, Wei Y, Hsu SH. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials. 2017;133:20–8.
Lee SH, Jo AR, Choi GP, Woo CH, Lee SJ, Kim B, et al. Fabrication of 3d alginate scaffold with interconnected pores using wire-network molding technique. Tissue Eng Regen Med. 2013;10:53–9.
Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830–7.
Kim J, Park Y, Tae G, Lee KB, Hwang SJ, Kim IS, et al. Synthesis and characterization of matrix metalloprotease sensitive-low molecular weight hyaluronic acid based hydrogels. J Mater Sci Mater Med. 2008;19:3311–8.
Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–89.
Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–70.
Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–24.
Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–78.
Hribar KC, Meggs K, Liu J, Zhu W, Qu X, Chen S. Three-dimensional direct cell patterning in collagen hydrogels with near-infrared femtosecond laser. Sci Rep. 2015;5:17203.
Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28:677–84.
Kinstlinger IS, Miller JS. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip. 2016;16:2025–43.
Richards D, Jia J, Yost M, Markwald R, Mei Y. 3D bioprinting for vascularized tissue fabrication. Ann Biomed Eng. 2017;45:132–47.
Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, et al. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7:e46842.
Rnjak-Kovacina J, Wray LS, Golinski JM, Kaplan DL. Arrayed hollow channels in silk-based scaffolds provide functional outcomes for engineering critically-sized tissue constructs. Adv Funct Mater. 2014;24:2188–96.
Zhang W, Wray LS, Rnjak-Kovacina J, Xu L, Zou D, Wang S, et al. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials. 2015;56:68–77.
Kim J, Kim IS, Cho TH, Kim HC, Yoon SJ, Choi J, et al. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A. 2010;95:673–81.
Acknowledgements
This study was supported by a grant from the Ministry of Health and Welfare in the Republic of Korea (HI14C2143).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal experiment procedures were approved by the institutional animal care and use committee of Korea University College of Medicine (KUIACUC-2015-165).
Additional information
Jaeyeon Lee and Se-Hwan Lee contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J., Lee, SH., Lee, BK. et al. Fabrication of Microchannels and Evaluation of Guided Vascularization in Biomimetic Hydrogels. Tissue Eng Regen Med 15, 403–413 (2018). https://doi.org/10.1007/s13770-018-0130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-0130-1