Abstract
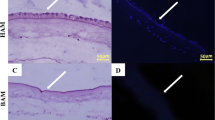
Combination between tissue engineering and other fields has brought an innovation in the area of regenerative medicine which ultimate aims are to repair, improve, and produce a good tissue construct. The availability of many types of scaffold, both synthetically and naturally have developed into many outstanding end products that have achieved the general objective in tissue engineering. Interestingly, most of this scaffold emulates extracellular matrix (ECM) characteristics. Therefore, ECM component sparks an interest to be explored and manipulated. The ECM featured in human amniotic membrane (HAM) provides a suitable niche for the cells to adhere, grow, proliferate, migrate and differentiate, and could possibly contribute to the production of angiogenic micro-environment indirectly. Previously, HAM scaffold has been widely used to accelerate wound healing, treat bone related and ocular diseases, and involved in cardiovascular repair. Also, it has been used in the angiogenicity study, but with a different technical approach. In addition, both side of HAM could be used in cellularised and decellularised conditions depending on the objectives of a particular research. Therefore, it is of paramount importance to investigate the behavior of ECM components especially on the stromal side of HAM and further explore the angiogenic potential exhibited by this scaffold.
Similar content being viewed by others
References
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011 [Epub]. DOI:10.1155/2011/290602.
Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 2007;105:215–228.
Barbara Z, Eriberto B, Stefano S, Giulia B, Chiara G, Ferrarese N, et al. Dental Pulp Stem Cells and Tissue Engineering Strategies for Clinical Application on Odontoiatric Field. In: Pignatello R, editor. Biomaterials Science and Engineering. Croatia: InTech; 2011. p.339–348.
Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev 2009;20:435–440.
Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol 2003;12:295–310.
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engi neering. Eur Cell Mater 2008;15:88–99.
Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod 2007;33:377–390.
Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 2008;15:100–114.
Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 2006;324:225–236.
Plant AL, Bhadriraju K, Spurlin TA, Elliott JT. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta 2009;1793:893–902.
Sieminski AL, Gooch KJ. Biomaterial-microvasculature interactions. Biomaterials 2000;21:2232–2241.
Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 2006; 10:45–55.
Carmeliet P. Angiogenesis in health and disease. Nat Med 2003;9:653–660.
Djonov V, Makanya AN. New insights into intussusceptive angiogenesis. EXS 2005;(94):17–33.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008;34:962–969.
Demarco FF, Conde MC, Cavalcanti BN, Casagrande L, Sakai VT, Nör JE. Dental pulp tissue engineering. Braz Dent J 2011;22:3–13.
Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, et al. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci U S A 2008;105:11099–11104.
Arkudas A, Balzer A, Buehrer G, Arnold I, Hoppe A, Detsch R, et al. Evaluation of angiogenesis of bioactive glass in the arteriovenous loop model. Tissue Eng Part C Methods 2013;19:479–486.
Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006;27:3249–3255.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249–257.
Sun J, Wang Y, Qian Z, Hu C. An approach to architecture 3D scaffold with interconnective microchannel networks inducing angiogenesis for tissue engineering. J Mater Sci Mater Med 2011;22:2565–2571.
Li Z, Qu T, Ding C, Ma C, Sun H, Li S, et al. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater 2015;13:88–100.
Mittermayr R, Morton T, Hofmann M, Helgerson S, van Griensven M, Redl H. Sustained (rh)VEGF(165) release from a sprayed fibrin biomatrix induces angiogenesis, up-regulation of endogenous VEGF-R2, and reduces ischemic flap necrosis. Wound Repair Regen 2008;16:542–550.
Moon JJ, West JL. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr Top Med Chem 2008;8: 300–310.
Brennan JA, Arrizabalaga JH, Nollert MU. Development of a small diameter vascular graft using the human amniotic membrane. Cardiovasc Eng Techno 2014;5:96–109.
Caruso M, Silini A, Parolini O. The human amniotic membrane: A tissue with multifaceted properties and different potential clinical applications. In: Kyle JC, Curtis L, Cetrulo Jr, Rouzbeh R, Taghizadeh A, editors. Perinatal Stem Cells. 2nd ed. New Jersey: John Wiley & Sons; 2013. p.177–195.
Chopra A, Thomas BS. Amniotic membrane: a novel material for regeneration and repair. J Biomim Biomater Tissue Eng 2013;18:106.
Riau AK, Beuerman RW, Lim LS, Mehta JS. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010;31:216–225.
Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 2000;20:173–177.
Nor Kamalia Z, Suzina SAH, Yusof N. Changes in biophysical properties of human amniotic membranes after different preservation methods and radiation sterilization. Regen Res 2014;3:64–70.
Ab Hamid SS, Zahari NK, Yusof N, Hassan A. Scanning electron microscopic assessment on surface morphology of preserved human amniotic membrane after gamma sterilisation. Cell Tissue Bank 2014;15:15–24.
Taghiabadi E, Nasri S, Shafieyan S, Jalili Firoozinezhad S, Aghdami N. Fabrication and characterization of spongy denuded amniotic membrane based scaffold for tissue engineering. Cell J 2015;16:476–487.
Madri JA, Williams SK. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol 1983;97:153–165.
Wolbank S, Hildner F, Redl H, van Griensven M, Gabriel C, Hennerbichler S. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med 2009;3:651–654.
Ma DH, Yao JY, Yeh LK, Liang ST, See LC, Chen HT, et al. In vitro antiangiogenic activity in ex vivo expanded human limbocorneal epithelial cells cultivated on human amniotic membrane. Invest Ophthalmol Vis Sci 2004;45:2586–2595.
Tsai SH, Liu YW, Tang WC, Zhou ZW, Hwang CY, Hwang GY, et al. Characterization of porcine arterial endothelial cells cultured on amniotic membrane, a potential matrix for vascular tissue engineering. Biochem Biophys Res Commun 2007;357:984–990.
Maral T, Borman H, Arslan H, Demirhan B, Akinbingol G, Haberal M. Effectiveness of human amnion preserved long-term in glycerol as a temporary biological dressing. Burns 1999;25:625–635.
Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg Engl 1979;61:444–447.
Yang L, Shirakata Y, Tokumaru S, Xiuju D, Tohyama M, Hanakawa Y, et al. Living skin equivalents constructed using human amnions as a matrix. J Dermatol Sci 2009;56:188–195.
Starecki M, Razzano P, Schwartz JA, Grande DA. Evaluation of amniotic derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Adv Orthop Surg 2014 ID 572586.
Díaz-Prado S, Rendal-Vázquez ME, Muiños-López E, Hermida-Gómez T, Rodríguez-Cabarcos M, Fuentes-Boquete I, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank 2010;11:183–195.
Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng 2007;13:693–702.
Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol 2003;48:631–646.
Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol 2001;85:444–449.
Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci 2004;45:800–806.
Lo V, Pope E. Amniotic membrane use in dermatology. Int J Dermatol 2009;48:935–940.
Hopkinson A, Shanmuganathan VA, Gray T, Yeung AM, Lowe J, James DK, et al. Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng Part C Methods 2008;14:371–381.
Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol 2010;5:645–661.
Burgos H. Angiogenic factor from human term placenta. Purification and partial characterization. Eur J Clin Invest 1986;16:486–493.
Langer R, Tirrell DA. Designing materials for biology and medicine. Nature 2004;428:487–492.
Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane cross talk between the extracellular matrix—cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2001;2:793–805.
Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 2010;48:474–482.
Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 2002;14:633–639.
Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in threedimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5) beta(1) integrins. Am J Pathol 2000;156:1673–1683.
Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol 1996;135(6 Pt 1):1633–1642.
Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis 2009;12:165–175.
Zhang YQ, Ji SZ, Fang H, Zheng YJ, Luo PF, Wu HB, et al. Use of amniotic microparticles coated with fibroblasts overexpressing SDF-1a to create an environment conducive to neovascularization for repair of fullthickness skin defects. Cell Transplant 2015;25:365–376.
Brauer R, Beck IM, Roderfeld M, Roeb E, Sedlacek R. Matrix metalloproteinase-19 inhibits growth of endothelial cells by generating angiostatin-like fragments from plasminogen. BMC Biochem 2011;12:38.
Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, et al. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006;9:33–44.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashim, S.N.M., Yusof, M.F.H., Zahari, W. et al. Angiogenic potential of extracellular matrix of human amniotic membrane. Tissue Eng Regen Med 13, 211–217 (2016). https://doi.org/10.1007/s13770-016-9057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-016-9057-6