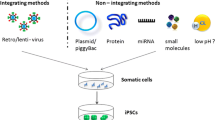
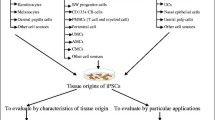
Abstract
Ever since a technology to reprogram somatic cells into pluripotent stem cells was developed by Dr. Shinya Yamanaka’s group in 2006, its therapeutic potential has been extensively discussed. We now call the reprogrammed embryonic stem cell (ESC)-like cell an ‘induced pluripotent stem cell’ (iPSC). The beauty and power of the iPS in human case is that it avoids many ethical issues, in that unlike human ESCs (hESCs), iPSCs do not require destroying a human embryo to establish pluripotent cell lines. The iPSC holds many hopes that many human diseases may be treatable in the near future. On the other hand, there are still several issues that need to be solved prior to the therapeutic use of iPSCs in humans directly. The biggest hurdle is that, so far, there is lack of ways to completely exclude tumorigenic iPSC-derived cells. Additionally, there is an issue that immune rejection may occur, even in autologously grafted iPSCs, as was observed in monkey experiment. Nevertheless, iPSCs, combined with genetic manipulation, hold much promise that iPSCs may be used in cell therapy procedures and as tools for investigating underlying mechanisms of human diseases. This review will discuss recent progress in cellular reprogramming and its potential use in regenerative medicine.
Similar content being viewed by others
References
K Takahashi and S Yamanaka., Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 126(4), 663 (2006).
K Takahashi, K Tanabe, M Ohnuki, et al., Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell, 131(5), 861 (2007).
J Liao, C Cui, S Chen, et al., Generation of induced pluripotent stem cell lines from adult rat cells, Cell Stem Cell, 4(1), 11 (2009).
A Honda, M Hirose, M Hatori, et al., Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine, J Biol Chem, 285(41), 31362 (2010).
H. Liu, F. Zhu, J. Yong, P. Zhang, P Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts, Cell Stem Cell, 3, 587 (2008).
R Fang, K Liu, Y Zhao et al., Generation of naive induced pluripotent stem cells from rhesus monkey fibroblasts, Cell Stem Cell, 15(4), 488 (2014).
MA Esteban, J Xu, J Yang, et al., Generation of induced pluripotent stem cell lines from Tibetan miniature pig, J Biol Chem 284, 26 (2009).
H Sumer, J Liu, LF Malaver Ortega, et al., NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts, J Anim Sci, 89, 2708 (2011).
L Bao, L He, J Chen, et al., Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors, Cell Res 21, 600 (2011).
K Nagy, HK Sung, P Zhang, et al., Induced pluripotent stem cell lines derived from equine fibroblasts, Stem Cell Rev, 7, 693. (2011).
H Shimada, A Nakada, Y Hashimoto, et al., Generation of canine-induced pluripotent stem cells by retroviral transduction and chemical inhibitors, Mol Reprod Dev, 77, 2. (2010).
Y Lu, FD West, BJ Jordan, et al., Induced pluripotency in chicken embryonic fibroblast results in a germ cell fate, Stem Cells Dev, 23(15), 1755 (2014).
X Chen, H Xu, P Yuan, et al., Integration of External Signaling Pathways with the Core Transcriptional Network in Embryonic Stem Cells, Cell, 133, 1106 (2008).
J Kim, J Chu, X Shen, et al., An Extended Transcriptional Network for Pluripotency of Embryonic Stem Cells, Cell, 132, 1049 (2008).
R Schmidt, K Plath. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation, Genome Biol, 13, 251 (2012).
RP Koche, ZD Smith, M Adli, et al., Reprogramming factor expression initiates widespread targeted chromatin remodeling, Cell Stem Cell, 8, 96 (2011).
R Sridharan, J Tchieu, MJ Mason, et al., Role of the murine reprogramming factors in the induction of pluripotency, Cell, 136, 364 (2009).
A Soufi, G Donahue, K Zaret, Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome, Cell, 151, 994 (2012).
MH Kagey, JJ Newman, S Bilodeau, et al., Mediator and Cohesin connect gene expression and chromatin architecture, Nature, 467, 430 (2010).
C Lin, A Garruss, Z Luo, et al., The RNA Pol II elongation factor Ell3 marks enhancers in ES cells and primes future gene activation, Cell, 152, 144 (2012).
T Mikkelsen, J Hanna, X Zhang, et al., Dissecting direct reprogramming thr0ough integrative genomic analysis, Nature, 454, 49 (2008).
R Li, J Liang, S Ni, et al., A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts, Cell Stem Cell, 7, 51 (2010).
A Golipour, L David, Y Liu, et al., A late transition in somatic cell reprogramming requires regulators distinct from the pluripotency network, Cell Stem Cell, 11, 769 (2012).
J Polo, E Anderssen, R Walsh, et al., A molecular roadmap of reprogramming somatic cells into iPS cells, Cell, 151, 1617 (2012).
Y Buganim, D Faddah, A Cheng, et al., Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase, Cell, 150, 1209 (2012).
J Hanna, K Saha, B Pando, et al. Direct cell reprogramming is a stochastic process amenable to acceleration, Nature, 462, 595 (2009).
EP Papapetrou, MJ Tomishima, SM Chambers, et al., Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation, Proc Natl Acad Sci U S A, 106(31), 12759 (2009).
EP Papapetrou and M Sadelain. Generation of transgene-free human induced pluripotent stem cells with an excisable single polycistronic vector, Nat Protoc, 6(9), 1251 (2011).
CS Swindle, HG Kim and CA Klug. Mutation of CpGs in the murine stem cell virus retroviral vector long terminal repeat represses silencing in embryonic stem cells, J Biol Chem, 279(1), 34 (2004).
M Stadtfeld, M Nagaya, J Utikal, et al., Induced pluripotent stem cells generated without viral integration, Science, 322, 945 (2008).
T Seki, S Yuasa, M Oda, et al., Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells, Cell Stem Cell, 7, 11 (2010).
N Fusaki, H Ban, A Nishiyama, et al., Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome, Proc Jpn Acad Ser B Phys Biol Sci, 85, 348 (2009).
F Gonzalez, M Barragan Monasterio, G Tiscornia, et al., Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector, Proc Natl Acad Sci USA, 106, 8918 (2009).
K Okita, M Nakagawa, H Hyenjong, et al., Generation of mouse induced pluripotent stem cells without viral vectors, Science, 322, 949 (2008).
J Yu, K Hu, K Smuga-Otto, et al., Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences, Science, 324, 797 (2009)
K Okita, Y Matsumura, Y Sato, et al., A more efficient method to generate integration-free human iPS cells. Nature Methods, 8, 409 (2011)
L Warren, PD Manos, T Ahfeldt, et al., Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA, Cell Stem Cell, 7, 618. (2010).
H Zhou, S Wu, JY Joo, et al. Generation of induced pluripotent stem cells using recombinant proteins, Cell Stem Cell, 4, 381 (2009).
JH Moon, JS Heo, JS Kim, et al., Reprogramming fibroblasts into induced pluripotent stem cells with Bmi1, Cell Research, 21, 1305 (2011).
RL Judson, JE Babiarz, M Venere, et al., Embryonic stem cellspecific microRNAs promote induced pluripotency, Nat Biotechnol, 27, 459 (2009).
SL Lin, DC Chang, CH Lin, et al., Regulation of somatic cell reprogramming through inducible mir-302 expression, Nucleic Acids Res, 39, 1054 (2010).
N Pfaff, J Fiedler, A Holzmann, et al., miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2, EMBO reports, 12, 1153 (2011).
F Anokye-Danso, CM Trivedi, D Juhr, et al., Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency, Cell Stem Cell, 8, 376 (2011).
M Tahiliani, KP Koh, Y Shen, et al., Conversion of 5- methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1, Science. 324, 930 (2009).
S Ito, AC D’Alessio, OV Taranova, et al., Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification, Nature, 466, 1129 (2010).
S Ito, L Shen, Q Dai, et al., Tet proteins can convert 5- methylcytosine to 5-formylcytosine and 5-carboxylcytosine, Science. 333, 1300 (2011).
CA Doege, K Inoue, T Yamashita, et al., Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2, Nature, 488, 652 (2012).
X Hu, L Zhang, SQ Mao, et al., Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming, Cell Stem Cell, 14(4), 512 (2014).
J Chen, L Guo, L Zhang, et al., Vitamin C modulates TET1 function during somatic cell reprogramming, Nat Genet, 45, 1504 (2013).
J He, EM Kallin, Y Tsukada, et al., The H3K36 demethylase Jhdm1b/Kdm2b regulates cell proliferation and senescence through p15(Ink4b), Nat Struct Mol Biol, 15, 1169 (2008).
G Liang, J He, Y Zhang, Kdm2b promotes induced pluripotent stem cell generation by facilitating gene activation early in reprogramming, Nat Cell Bio, 14, 457 (2012).
J He, L Shen, M Wan, et al., Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes, Nat Cell Bio, 15, 373 (2013).
Y Rais, A Zviran, S Geula, et al., Deterministic direct reprogramming of somatic cells to pluripotency, Nature, 502, 65 (2013).
R Santos, L Tosti, A Radzisheuskaya, et al., MBD3/NuRD Facilitates Induction of Pluripotency in a Context-Dependent Manner, Cell Stem Cell, 15, 102 (2014).
P Hou, Y Li, X Zhang, et al., Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds, Science, 341, 651 (2013).
C Waddington. The Strategy of the Genes. London, Allen & Unwin, 262 (1957).
N Heins, P Malatesta, F Cecconi, et al., Glial cells generate neurons: the role of the transcription factor Pax6, Nat Neurosci, 5, 308 (2002).
B Berninger, M Costa, U Koch, et al., Functional Properties of Neurons Derived from In Vitro Reprogrammed Postnatal Astroglia, Journal of Neuroscience, 27, 8654 (2007).
C Heinrich, R Blum, S Gascón, et al., Directing Astroglia from the Cerebral Cortex into Subtype Specific Functional Neurons, PLoS Biol, 8, e1000373 (2010).
Heinrich C, Gascón S, Masserdotti G, et al., Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex, Nat Protoc, 6, 214 (2011).
A Buffo, Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair, Proc of the Nat Aca of Sci, 102, 18183, (2005).
T Vierbuchen, A Ostermeier, ZP Pang, et al., Direct conversion of fibroblasts to functional neurons by defined factors, Nature, 463, 1035 (2010).
R Ambasudhan, M Talantova, R Coleman, et al., Direct Reprogramming of Adult Human Fibroblasts to Functional Neurons under Defined Conditions, Cell Stem Cell, 9, 113 (2011).
SM Kim, H Flaßkamp, A Hermann, et al., Direct conversion of mouse fibroblasts into induced neural stem cells, Nature Protocols, 9, 871 (2014).
OL Wapinski, T Vierbuchen, K Qu, et al., Hierarchical Mechanisms for Direct Reprogramming of Fibroblasts to Neurons, Cell, 155, 621 (2013).
JH Kim, JA Efe, S Zhu, et al., Direct reprogramming of mouse fibroblasts to neural progenitors, PNAS, 108, 7838 (2011).
HS Kim, JH Kim, YJ Jo, et al., Direct lineage reprogramming of mouse fibroblasts to functional midbrain dopaminergic neuronal progenitors, Stem Cell Research, 12, 60 (2014).
DW Han, N Tapia, A Hermann, et al., Direct Reprogramming of Fibroblasts into Neural Stem Cells by Defined Factors, Cell Stem Cell, 10, 465 (2012).
M Ieda, JD Fu, P Delgado-Olguin, et al., Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors, Cell, 142, 375 (2010).
L Qian, Y Huang, CI Spencer, et al., In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes, Nature, 485, 593 (2012).
J Fu, N Stone, L Liu, et al., Direct Reprogramming of Human Fibroblasts toward a Cardiomyocyte-like State, Stem Cell Reports, 1, 235 (2013).
S Sekiya, A Suzuki, Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors, Nature, 475, 390 (2011).
P Huang, Z He, S Ji, et al., Induction of functional hepatocytelike cells from mouse fibroblasts by defined factors, Nature, 475, 386 (2011).
B Yu, ZY He, P You, et al., Reprogramming Fibroblasts into Bipotential Hepatic Stem Cells by Defined Factors, Cell Stem Cell, 13, 328 (2013).
P Huang, L Zhang, Y Gao, et al., Direct Reprogramming of Human Fibroblasts to Functional and Expandable Hepatocytes, Cell Stem Cell, 14, 370 (2014).
A Margaritia, B Winklera, E Karamaritia, et al., Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels, Proc. Natl. Acad. Sci. U.S.A., 109, 13793 (2012).
J Li, N Huang, J Zou, et al., Conversion of Human Fibroblasts to Functional Endothelial Cells by Defined Factors, Arterioscler Thromb Vasc Biol, 33, 1366 (2013).
L Kurian, I Sancho-Martinez, E Nivet, et al., Conversion of human fibroblasts into angioblast-like multipotent progenitor cells, Nat Methods, 10, 77 (2013).
A Morizane, D Doi, T Kikuchi, et al., Direct Comparison of Autologous and Allogeneic Transplantation of iPSC-Derived Neural Cells in the Brain of a Nonhuman Primate, Stem Cell Reports, 1, 283 (2013).
O Iglesias-García, B Pelacho, F Prósper, Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling, Journal of Molecular and Cellular Cardiology, 62, 43 (2013).
P Liu, S Chen, X Li, et al., Low Immunogenicity of Neural Progenitor Cells Differentiated from Induced Pluripotent Stem Cells Derived from Less Immunogenic Somatic Cells, PLoS One, 8, e69617 (2013).
Z Guo, L Zhang, Z Wu, et al., In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model, Cell Stem Cell, 14, 188 (2014).
O Mohamad, D Drury-Stewart, Mingke Song, et al., Vector-Free and Transgene-Free Human iPS Cells Differentiate into Functional Neurons and Enhance Functional Recovery after Ischemic Stroke in Mice, PLoS One, 8, e64160 (2013).
J Polentes, P Jendelova, M Cailleret, et al., Human induced pluripotent stem cells improve stroke outcome and reduce secondary degeneration in the recipient brain, Cell Transplant., 21, 2587 (2012).
K Oki, J Tatarishvili, J Wood, et al., Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain, Stem Cells, 30, 1120 (2012).
MB Jensen, H Yan, R Krishnaney-Davison, et al., Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model, J. Stroke Cerebrovasc. Dis., 22, 304 (2013).
J Qin, B Song, H Zhang, et al., Transplantation of human neuroepithelial- like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage, Neurosci. Lett., 548, 95 (2013).
F Soldner, D Hockemeyer, C Beard, et al., Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors, Cell, 136, 964 (2009).
G Hargus, O Cooper, M Deleidi, et al., Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats, PNAS, 107, 15921 (2010).
M. Wernig, J.P. Zhao, J. Pruszak, et al., Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease, Proc. Natl. Acad. Sci. U.S.A., 105, 5856 (2008).
YH Rhee, JY Ko, MY Chang, et al., Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease, J. Clin. Invest., 121, 2326 (2011).
A Swistowski, J Peng, Q Liu, et al., Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions, Stem Cells, 28, 1893 (2010).
MF Burkhardt, FJ Martinez, S Wright, et al., A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells, Molecular and Cellular Neuroscience, 56, 355 (2013).
S Nori, Y Okada, A Yasuda, et al., Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice, Proc. Natl. Acad. Sci. U.S.A., 108, 16825 (2011).
ST Rashid, S Corbineau, N Hannan, et al., Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells, The Journal of Clinical Investigation, 120, 3127 (2010).
H Liu, Y Kim, S Sharkis, et al., In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins, Sci. Transl. Med., 3, 82ra39 (2011).
J Hanna, M Wernig, S Markoulaki, et al., Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin, Science, 318, 1920 (2007).
M Bershteyn, Y Hayashi, G Desachy, et al., Cell-autonomous correction of ring chromosomes in human induced pluripotent stem cells, Nature, 507, 99 (2014).
A Filareto, S Parker, R Darabi, et al., An ex vivo gene therapy approach to treat muscular dystrophy using inducible pluripotent stem cells, Nat. Commun., 4, 1549 (2013).
H Suzuki, R Shibata, T Kito, et al., Therapeutic angiogenesis by transplantation of induced pluripotent stem cell-derived Flk- 1 positive cells, BMC Cell Biol., 11, 72 (2010).
CH Yoo, HJ Na, DS Lee, et al., Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases, Biomaterials, 34, 8149 (2013).
Q Lian, Y Zhang, J Zhang, et al., Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice, Circulation, 121, 1113 (2010).
TJ Nelson, A Martinez-Fernandez, S Yamada, et al., Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells, Circulation, 120, 408 (2009).
Y Li, YT Tsai, CW Hsu, et al., Long-term safety and efficacy of human induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa, Mol. Med., 18, 1312 (2012).
K Homma, S Okamoto, M Mandai, et al., Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors, Stem Cells, 31, 1149 (2013).
ZB Jin, S Okamoto, F Osakada, et al., Modeling Retinal Degeneration Using Patient-Specific Induced Pluripotent Stem Cells, PLoS One, 6, e17084 (2011).
K Kulangara, A Adler, H Wang, et al., The effect of substrate topography on direct reprogramming of fibroblasts to induced neurons, Biomaterials, 35, 5327 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, K. Cellular reprogramming and its application in regenerative medicine. Tissue Eng Regen Med 12, 80–89 (2015). https://doi.org/10.1007/s13770-014-0099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-014-0099-3