Abstract
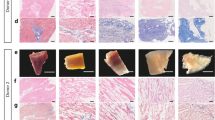
The application of decellularized extracellular matrices to aid tissue regeneration in reconstructive surgery and regenerative medicine has been promising. Several decellularization protocols for removing cellular materials from natural tissues such as heart valves are currently in use. This paper evaluates the feasibility of potential extension of this methodology relative to the desirable properties of load bearing joint tissues such as stiffness, porosity and ability to recover adequately after deformation to facilitate physiological function. Two decellularization protocols, namely: Trypsin and Triton X-100 were evaluated against their effects on bovine articular cartilage, using biomechanical, biochemical and microstructural techniques. These analyses revealed that decellularization with trypsin resulted in severe loss of mechanical stiffness including deleterious collapse of the collagen architecture which in turn significantly compromised the porosity of the construct. In contrast, triton X-100 detergent treatment yielded samples that retain mechanical stiffness relative to that of the normal intact cartilage sample, but the resulting construct contained ruminant cellular constituents. We conclude that both of these common decellularization protocols are inadequate for producing constructs that can serve as effective replacement and scaffolds to regenerate articular joint tissue.
Similar content being viewed by others
References
Metcalf MH, Savoie FH, Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper Tech Orthop 2002;12: 204–208.
Hung KC, Tseng CS, Hsu SH. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv Healthc Mater 2014;3:1578–1587.
Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28:3587–3593.
Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, et al. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int 2013;33:448–458.
Oliveira AC, GarzÓn I, Ionescu AM, Carriel V, Cardona Jde L, González-Andrades M, et al. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One 2013;8:e66538.
Fitzpatrick JC, Clark PM, Capaldi FM. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int J Biomater 2010;2010. pii: 620503.
Xu H, Xu B, Yang Q, Li X, Ma X, Xia Q, et al. Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS One 2014;9:e86723.
Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008;14:213–221.
Tudorache I, Cebotari S, Sturz G, Kirsch L, Hurschler C, Hilfiker A, et al. Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J Heart Valve Dis 2007;16:567–573; discussion 574.
Uchimura E, Sawa Y, Taketani S, Yamanaka Y, Hara M, Matsuda H, et al. Novel method of preparing acellular cardiovascular grafts by decellularization with poly(ethylene glycol). J Biomed Mater Res A 2003;67:834–837.
Schenke-Layland K, Vasilevski O, Opitz F, König K, Riemann I, Halbhuber KJ, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol 2003;143:201–208.
Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg 2005;27:566–571.
Vavken P, Joshi S, Murray MM. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J Orthop Res 2009;27:1612–1618.
Subbotin DV, Larionov PM, Sergeevichev DS, Subbotina OA, Zaitsev GS, Novruzov RB, et al. Morphological evaluation of cytoarchitectonics of aortic graft at the biotechnological stage with analysis of changes in laser-induced fluorescence spectra. Bull Exp Biol Med 2009;148:684–688.
Yang B, Zhang Y, Zhou L, Sun Z, Zheng J, Chen Y, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods 2010;16: 1201–1211.
Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010;31:8626–8633.
Pagoulatou E, Triantaphyllidou IE, Vynios DH, Papachristou DJ, Koletsis E, Deligianni D, et al. Biomechanical and structural changes following the decellularization of bovine pericardial tissues for use as a tissue engineering scaffold. J Mater Sci Mater Med 2012;23:1387–1396.
Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, Zhang L, et al. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater 2014;10:183–193.
Kheir E, Stapleton T, Shaw D, Jin Z, Fisher J, Ingham E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res A 2011;99:283–294.
Heidarkhan Tehrani A, Davari P, Singh S, Oloyede A. Sterilizing tissue-materials using pulsed power plasma. J Mater Sci Mater Med 2014;25:953–964.
Schwarz S, Elsaesser AF, Koerber L, Goldberg-Bockhorn E, Seitz AM, Bermueller C, et al. Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function. J Tissue Eng Regen Med 2012 Nov 29 [Epub]. http://dx.doi.org/10.1002/term.1650.
Tehrani AH, Singh S, Xiao Y, Oloyede A. Fast fourier analysis of structural organization in decellularized cartilage-on-bone laminates. In: Merrell R, Shin DG, Hamza MH, editors. Proceedings of the IASTED International Symposia Imaging and Signal Processing in Health Care and Technology (ISPHT 2012). Baltimore, USA: IEEE Engineering in Medicine and Biology Society; 2012. p.71–77.
Yang M, Chen CZ, Wang XN, Zhu YB, Gu YJ. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J Biomed Mater Res B Appl Biomater 2009;91:354–361.
Ficklin T, Thomas G, Barthel JC, Asanbaeva A, Thonar EJ, Masuda K, et al. Articular cartilage mechanical and biochemical property relations before and after in vitro growth. J Biomech 2007;40:3607–3614.
Afara IO, Singh S, Oloyede A. Load-unloading response of intact and artificially degraded articular cartilage correlated with near infrared (NIR) absorption spectra. J Mech Behav Biomed Mater 2013;20:249–258.
Afara I, Singh S, Oloyede A. Application of near infrared (NIR) spectroscopy for determining the thickness of articular cartilage. Med Eng Phys 2013;35:88–95.
Heidarkhan Tehrani A, Zadhoush A, Karbasi S, Sadeghi-Aliabadi H. Scaffold percolative efficiency: in vitro evaluation of the structural criterion for electrospun mats. J Mater Sci Mater Med 2010;21:2989–2998.
Tehrani AH, Zadhoush A, Karbasi S, Khorasani SN. Experimental investigation of the governing parameters in the electrospinning of poly(3-hydroxybutyrate) scaffolds: structural characteristics of the pores. J Appl Polym Sci 2010;118:2682–2689.
Tehrani AH, Singh S, Xiao Y, Oloyede A. Anisotropy of articular cartilage reflects the ECM gradient architecture: Hough-Radon Transform Analysis. In: Merrell R, editor. Proceedings of the IASTED International Symposia Imaging and Signal Processing in Health Care and Technology (ISPHT 2012). Baltimore: ACTA Press; 2012. p.64–70.
Tehrani AH, Singh S, Xiao Y, Oloyede A. Local stress-strain distribution and load transfer across cartilage matrix at micro-scale using combined microscopy-based finite element method. In 23rd Annual Conference of the Australasian Society of Biomaterials and Tissue Engineering, 22-24 April 2014, Mantra Resort Lorne, VIC; 2014.
Jeffery AK, Blunn GW, Archer CW, Bentley G. Three-dimensional collagen architecture in bovine articular cartilage. J Bone Joint Surg Br 1991;73:795–801.
Somers P, De Somer F, Cornelissen M, Thierens H, Van Nooten G. Decellularization of heart valve matrices: search for the ideal balance. Artif Cells Blood Substit Immobil Biotechnol 2012;40:151–162.
Dainese L, Guarino A, Burba I, Esposito G, Pompilio G, Polvani G, et al. Heart valve engineering: decellularized aortic homograft seeded with human cardiac stromal cells. J Heart Valve Dis 2012;21:125–134.
Schwarz S, Koerber L, Elsaesser AF, Goldberg-Bockhorn E, Seitz AM, Dürselen L, et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A 2012;18:2195–2209.
Keech MK. The effect of collagenase and trypsin on collagen; an electron microscopic study. Anat Rec 1954;119:139–159.
Gratzer PF, Harrison RD, Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng 2006;12:2975–2983.
Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med 2005;24:1–12.
von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kästner SB, et al. Mosaicplasty with photooxidized, mushroom shaped, bovine, osteochondral xenografts in experimental sheep. Vet Comp Orthop Traumatol 2006;19:147–156.
Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med 2005;54:1087–1093.
Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology 1997;205:546–550.
Tameem HZ, Sinha US. Automated image processing and analysis of cartilage MRI: enabling technology for data mining applied to osteoarthritis. AIP Conf Proc 2007;953:262–276.
Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 2001;7:679–689.
Scanlon CM. Cartilage Tissue Engineering Using a Modified Decellularized Porcine Cartilage Scaffold and a Novel Centrifugation Cell Seeding Technique. Florida: University of Miami; 2012.
Ingram JH, Korossis S, Howling G, Fisher J, Ingham E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng 2007;13: 1561–1572.
Thevenot P, Nair A, Dey J, Yang J, Tang L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng Part C Methods 2008;14:319–331.
Pridgen BC, Woon CY, Kim M, Thorfinn J, Lindsey D, Pham H, et al. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C Methods 2011;17:819–828.
Kryger ZB, Sisco M, Roy NK, Lu L, Rosenberg D, Mustoe TA. Temporal expression of the transforming growth factor-Beta pathway in the rabbit ear model of wound healing and scarring. J Am Coll Surg 2007;205:78–88.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Afara, I.O., Tehrani, A.H. et al. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng Regen Med 12, 294–305 (2015). https://doi.org/10.1007/s13770-014-0083-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-014-0083-y