Abstract
Poly-3-hydroxybutyrate (P3HB) is a biodegradable polymer with a potential extensive use in agriculture. However, while P3HB biodegradation boosts microbial enzyme activity, it significantly reduces plant biomass due to nutrient competition. In this study, we test the hypothesis that these detrimental effects can be mitigated though the co-application of nutrient-rich organic amendments, such as compost and digestate. A pot experiment with lettuce (Lactuca sativa), grown in soil amended with P3HB lone or combined with either compost or digestate. Six variants were tested: Control, Compost, Compost + P3HB, Digestate, Digestate + P3HB, and P3HB alone. We evaluated degradation of the P3HB polymer, biological soil properties, and both the dry and fresh biomass of the lettuce. We observed that adding P3HB alone enhanced dehydrogenase and urease activities, as well as all types of respiration, except for L-arginine-induced respiration. However, it strongly and negatively affected the biomass of lettuce (both aboveground and root). The strong adverse effects of P3HB on plant growth were also observed when compost was co-applied, although this combination enhanced all enzyme activities except for suppressed β-glucosidase. Conversely, co-applying digestate with P3HB alleviated the negative effect of P3HB on both the dry and fresh biomass together lettuce. Additionally, this combination increased the activity of several enzymes (dehydrogenase, arylsulfatase, N-acetyl-β-D-glucosaminidase, urease), and enhanced all types of respiration, except for L-arginine-induced respiration. The use of biodegradable plastics in agriculture is on rise, but it may be compromised, because their biodegradation my negatively impact plant growth. The results showed that co-application of digestate is an effective solution to alleviate these effects, while co-application of compost failed. Generally, organic amendments seem to be an option to alleviate the negative effects of bioplastics biodegradation, and offers options how to handle the treatment of waste bioplastics or their residues, but further investigation is needed to understand the underlaying mechanisms involved.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing production of biodegradable polymers provides a broad range of materials, including the biopolyesters belonging to the group of polyhydroxyalkanoates (PHAs) (Sudesh et al. 2000). PHAs are produced by numerous prokaryotic species during fermentation of carbohydrates, usually under growth-limited conditions such as nutrient deficiencies (Grousseau et al. 2013; Lee 1996). Their physical and chemical properties make them suitable substitutes for conventional nonbiodegradable plastics (Bonartseva et al. 2003). PHAs are biodegradable, biocompatible, and thermoprocessible (Medeiros Garcia Alcantara et al. 2020). In agriculture, they can be used for mulching (as cover films), in pots and containers (as bands for sowing), and for the controlled release of agricultural chemicals (Touchaleaume et al. 2016; Vroman and Tighzert 2009).
Nowadays, among the most notable PHAs belongs poly-3-hydroxybutyrate (P3HB), which serves as a bacterial intracellular carbon (C) and energy storage polymer. The complete degradability of PHAs, especially P3HB, is a characteristic that has made them widely used and environmentally friendly polymers (Vroman and Tighzert 2009; Luckachan and Pillai 2011).
P3HB has been found to biodegrade under both anaerobic and aerobic conditions (Nishida and Tokiwa 1993; Sharifzadeh et al. 2009) in soil, compost, and water bodies (Deroiné et al. 2015; Savenkova et al. 2000; Altaee et al. 2016; Bonartsev et al. 2018). Enzyme activities of P3HB-depolymerases and hydrolases have been identified in several microbial taxa (Kadouri et al. 2003; Shah et al. 2007; Panayotidou et al. 2014; Roohi and Kuddus 2018). The soil´s capacity for the P3HB degradation is largely determined by the biota structure, which includes macrodegraders, mostly earthworms (Sanchez-Hernandez et al. 2020) and microbial community (Abou-Zeid et al. 2004; Rychter et al.2006).
Indeed, the microbial community structure is often referred as the key factor in the rapid and efficient biodegradation of P3HB materials in soil (Guo et al. 2010; Vogel et al. 2021). However, the activity of P3HB-degrading microbes is influenced by balances and nutritional state at various levels of soil metabolism. The rate and the soils ‘ microbiome capacity to degrade P3HB depend on the availability of limiting nutrients (Nishide et al. 1999; Sang et al. 2004; Muneer et al. 2020; Zhou et al. 2021a)). Nitrogen, a crucial nutrient in the decomposition medium (Corrêa et al. 2008), must be supplied during soil P3HB degradation, either via co-application with compost (Eya et al. 1997) or through pre-degradation of P3HB in composted organic matter (Eya et al. 1997; Rosa et al. 2023).
Due to the origin of P3HB, the non-toxicity of its particles and degradation products is assumed. Nevertheless, this assumption is increasingly being challenged by available studies. Only a few studies have assessed the effect of PHA-amended soil on plant growth, and they reported negative effects (Zhou et al. 2021a; Dahal et al. 2020). For example, in our recent work (Brtnicky et al. 2022) we tested the impact of P3HB addition on biological properties of a wide range of nutrient-restricted soil substrates and lettuce growth. It was observed that P3HB addition increased dehydrogenase and urease activities, and basal and substrate-induced respiration in nutrient-restricted soils. Furthermore, it appeared that in those soils, P3HB can temporarily replace the SOM as a C source for microbial communities. Nevertheless, the addition of P3HB to all soils, tested in cited work, showed a negative impact of biodegradation on lettuce growth.
On the contrary, the potential negative effects of P3HB degradation products on terrestrial or freshwater organisms are well recognized (González-Pleiter et al. 2019). In addition, the P3HB degradation product such as 3-hydroxybutyric acid (3-HB) has been reported to play a significant signaling role in the global regulation processes of eukaryotic cells (Puchalska and Crawford 2017). In a previous study, the amendment of exogenous 3-HB to flax plants (Linum usitatissimum L.) resulted in changes to the DNA de-/methylation pattern, which potentially impacts the expression of genes involved in the phenylpropanoid pathway (Mierziak et al. 2020). Changes in the biosynthesis of phenylpropanoids, which help to inactivate reactive oxygen species, may alter plant responses to abiotic stresses conditions (Sharma et al. 2019). Malik et al. (Malik et al. 2015) further reported that in transgenic plant Camelina sativa, where the P3HB constituted up to 15% of mature seed weight, there were negative effects on germination, emergence, and survival of seedlings. Therefore, there are compelling reasons to evaluate and investigate the factors, strategies, and management practices for controlled biodegradation of P3HB-based plastics in the soil.
In addition, soil hydrophysical properties are among the traits known to be affected by poly-3-hydroxybutyrate and other plastic residues. Wan et al. (Wan et al. 2019) referred that the addition of plastic to soil creates channels and alters water movement, leading to negative impacts on water evaporation. De Souza Machado, on the contrary, observed an increased water holding capacity for polyester, and no effects for polyacrylic and polyethylene fragments on soil properties in a five-week experiment (Souza Machado et al. 2018). The authors hypothesized about possible connection with the impact on soil aggregation. Fojt et al., (Fojt et al. 2022) demonstrated that poly-3-hydroxybutyrate particles changed the soil organic matter by altering its supramolecular structure. Such behavior of plastics in soil affects water holding capacity (WHC) and dynamics, which could impact plant-available water, plant growth and fitness. However, applying organic amendment, such as compost, to P3HB-enriched soil may aid in restoring WHC values as well as providing nitrogen supplementation to preserve degradation capability and activity (Rosa et al. 2023; Suzuki et al. 2007).
In this work, we continue our previous research (Brtnicky et al. 2022) that enabled understanding the issues related to the impact of P3HB biodegradation on soil under various content of soil organic matter and soil texture. The main issue appeared the competition between soil microorganisms and plants for nutrients, which cannot easily be solved by inoculation of soil by plant growth-promoting rhizobacteria and N2-fixing microorganisms, as shown recently (Brtnicky et al. 2024). Apparently, this issue can be solved by a direct supply of nutrients, the most feasible is the addition of either a fertilizer or a nutrient-rich amendment. However, NPK fertilizers, particularly those with higher nitrogen content in the form of ammonium or urea, tend to decrease soil pH over time (Hao et al. 2020). Although the P3HB-related acidification is not the primary factor inhibiting plant growth (released 3-hydroxybutyric acid has pKa of 4.41) (Bruss et al. 2008), addition of NPK fertilizers may worsen this issue. On the contrary, the application of stabilized amendments such as digestate or compost generally increases soil pH, and possibly counterbalance the effect of 3-HB and the nutrient shortage during biodegradation. In addition, use of such amendments is more sustainable and less demanding to soil heath compared to application of synthetic NPK fertilizers (Panuccio et al. 2019).
To the best of our knowledge, this approach has not been tested up to now. However, understanding the effects of organic amendments sources on the degradability of P3HB and the overall soil microbial and physico-chemical properties is vital for strategies for bioplastics waste disposal and also application of fast biodegradable plastics in agriculture, which is on rise (Santagata et al. 2017) in applications such as mulching, fertilizer coating and delivery of active compounds (Touchaleaume et al. 2016; Vroman and Tighzert 2009).
Therefore, this study aimed to evaluate the effect of solely applied poly-3-hydroxybutyrate (P3HB) and P3HB co-applied with stabilized organic matter (compost or digestate) on the biological properties of soil and the biomass of lettuce (Lactuca sativa).
It was hypothesized that:
-
1.
P3HB is utilized as a readily catabolized energy and carbon source in soil, resulting in enhanced degradation and respiration, but it reduces the intrinsic utilization and transformation of soil organic carbon.
-
2.
Plant aboveground and root biomass is decreased in P3HB-amended variants compared to unamended ones, due to competition from metabolic active and abundant soil microorganisms with plants for nutrients and due to the negative impact of P3HB on soil hydrophysical properties.
-
3.
Organic amendments provide more nutrients, enhance the transformation and mineralization activities of soil organisms, and increase respiration rate. They can improve plant biomass yield compared to both control and P3HB-affected plants.
-
4.
The organic amendment, by improving soil properties and nutrient status, will accelerate the degradation of biodegradable P3HB.
Materials and methods
Experimental design and treatment description
The study was carried od as a short-termed pot experiment under controlled conditions in growth chamber. The soil used for preparation of the experimental substrate was an arable Haplic Luvisol (WRB soil classification), silty clay loam (USDA Textural Triangle) (WRB Soil Classification 2021). It was a topsoil (0–15 cm), collected at field near Troubsko town, Czech Republic (49°10′28″ N 16°29′32″ E), with the following properties: pH (CaCl2) 7.3; total C 14.0, total N 1.60, S 0.145, P 0.097, K 0.231, Ca 3.26, Mg 0.236 g kg−1. Before use, a sieving (through a mesh) to size ≤ 2 mm was done (Hammerschmiedt et al. 2022). This sieved soil was mixed with a fine quartz sand (0.1–1.0 mm; ≥ 95% SiO2) in weight ratio 1:1 to gain an experimental substrate. One kg of experimental substrate was mixed with additives (Table 1) and put into 1-L plastic pots (height 13 cm, top diameter 11 cm, bottom diameter 9 cm) (Przygocka-Cyna et al. 2018). 3 replicates (pots) were prepared for each treatment. Commercial CMC compost (Fertia s.r.o., Czech Republic; in fresh matter C 127.9, N 11.8 g kg−1, C:N 10.8) and digestate (in fresh matter C 14,2, N 1.59 g kg−1, C:N 8.9) obtained from agricultural biogas plant were used as additives.
The pots were seeded with lettuce (Lactuca sativa L. var. capitata L.) cv. Brilliant, 3 sprouted lettuce seeds per each pot and cultivated in growth chamber under controlled conditions (as follow): photoperiod 12 h (Cervera-Mata et al. 2018), light intensity 20 000 lx (Bankole et al. 2018; Zhang et al. 2018), relative air humidity 70% (Chrysargyris et al. 2018), night/day temperature 18/22 °C; soil moisture was ~ 60% of water holding capacity. The one most robust plant was left in each pot after 10-day-growth of seedlings. A randomized placement of pots in the growth chamber was used and once per week, the pots were variably rotated (Iocoli et al. 2019). After 8 weeks from sowing, the plants were harvested by cutting the shoots at ground level (Trinchera et al. 1041). The roots were released from soil, cleaned gently and washed with water. The fresh lettuce aboveground and roots biomass was weighed on the analytical scales. The dry biomass was determined after drying the shoots and roots at 60 °C to a constant weight and weighting on the analytical scales again.
Poly-3-hydroxybutyrate (P3HB) used in this work was obtained from TianAn Biologic Materials Co., Ltd. (Ningbo City, China), grade ENMAT Y3000, powder with particle size < 63 μm. The particles were of spherical shapes with density of approximately 1.20 g·cm−3 (Höhnemann and Windschiegl 2023). The crystallinity of the polymer was 49% (Procházková et al. 2024). Further specifications of the used P3HB is reported in Fojt et al. 2022 and Y3000P 2023 (Fojt et al. 2022; Y3000P 2023).
Soil analysis
A mixed soil sample was taken from experimental substrate of each pot after harvesting the lettuce. Samples were sieved on mesh to size ≤ 2 mm and used for determination of soil properties, with 3–6 analytical replication (according to measured property) per each sample replication. The soil pH of air-dried samples was determined (ISO_10390 2005). The samples used for determination of dehydrogenase activity (DHA) (Ranamukhaarachchi 2009), soil basal respiration (BR) and substrate induced respirations (IR) were stored at 4 °C. Soil respiration analyses were detected using MicroResp® device (The James Hutton Institute, Scotland), which performance is regularly verified according to the instructions of the provider. The tests were carried out according to Campbell et al. (2003), with following induction substrates–D-glucose (Glc-IR), N-acetyl-β-D-glucosamine (NAG-IR), D-trehalose (Tre-IR), L-lysine (Lys-IR), L-alanine (Ala-IR), and L-arginine (Arg-IR). The values of induced respiration were used to calculate the microbial functional diversity (MFD) according to Iovieno et al. (2021) as a Shannon’s index according to equation:
pi = the activity on a particular substrate with respect to the sum of activities on all substrates.
Enzymatic activities were measured in the freeze-dried samples: β-glucosidase (GLU), N-acetyl-β-D-glucosaminidase (NAG), phosphatase (Phos), arylsulfatase (ARS), urease (Ure) (ISO 2013 0:2018). The p-nitrophenole (PNP)-derivatives of the specific soil substrates were used for Vis spectrophotometric measurement (Infinite M Nano, Tecan Trading AG, Switzerland) at λ = 405 nm (β-glucosidase, N-acetyl-β-D-glucosaminidase, phosphatase, arylsulfatase). Urease activity was determined as an amount of ammonium produced from the substrate urea, detected Vis spectrophotometrically by the reagent cyanurate (λ = 650 nm). Using enzyme activities, nutrient acquisition ratios were calculated, based on the formulae presented in Cui et al. (2022):
When demand of soil microbiome for carbon increases and more carbon utilizing enzymes are secreted to obtain C, this results to higher C acquisition ratio. Similarly, when soil microorganisms are deficient for nitrogen, they produce additional enzymes catalyzing N decomposition, resulting in higher N acquisition ratio.
The theory of enzymatic stoichiometry (Moorhead et al. 2016) was used to compute the vector length and angle in order to estimate the microbial resource limitation. Microbial C limitation aggravates with the increase in the vector length. While the vector angle of < 45° indicates microbial N limitation, the vector angle > 45° indicates microbial P limitation. The following formulae based on (Moorhead et al. 2016) were used, ARCTG2 refers to arcus tangens:
The quality control (QC) and quality assurance (QA) of the used devices and obtained data was performed as follows: QC: Analytical equipment used for enzymatic assays, respirometry, balances and thermogravimetry are regularly calibrated and verified, to ensure accuracy in measurements. In some cases (enzymes, respirometry), we also incorporate positive and negative controls in experiments, i.e. positive controls ensure the assay is capable of producing a positive result when expected, and negative controls confirm the absence of contamination and non-specific signals. All measurements are done at least in triplicate or more to assess the reproducibility of the results. In addition, standard curves for quantification in enzymatic assays and respiration are used to ensure linearity of the detection range and to validate the assay's sensitivity and specificity. QA: We keep all detailed documentation of all protocols of standardization and procedures used in the experiments to unsure that the procedures can be repeated under the same conditions and produce similar results. All personnel involved in the experiments are properly trained and competent in the specific techniques and equipment used. We implement a process for checking the raw data for errors or inconsistencies before analysis, i.e. cross-checking data entries, verifying calculation methods, or using software for data integrity checks.
Thermogravimetric analysis (TG)
The thermogravimetric analysis was conducted in order to analyze residual plastic in soils after termination of the experiment. The instrument was calibrated for temperature using Curie point of alumel, nickel and iron; mass loss was controlled by calcium oxalate degradation. These values were verified regularly each month to assure the accurate performance of the device. The analysis was conducted using thermogravimeter Q550 TA Instruments (Delaware, USA). The homogenized soils were placed to the pre-weighed alumina pans and heated from laboratory temperature up to 650 °C, with heating rate 5 °C min−1 under the dynamic air atmosphere enriched by water vapors to 43% relative humidity, flow rate 90 ml min−1. The TG records were elaborated using TRIOS from TA Instruments. Measurements of each variant were done in triplicate, the obtained mass losses were averaged. To obtain the residual P3HB, the TG data were treated according to the procedure reported recently by Palucha et al. 2024 (Palucha et al. 2024). Briefly, as the pure P3HB thermally degrades in the interval from 200 to 300 °C, mass loss obtained in this temperature area of the control sample was subtracted from the respective sample containing P3HB and adjusted for dry mass by considering the moisture contents (100% minus mass loss in the interval 25–200 °C). Results of repeated measurements were averaged and standard deviation was calculated.
Statistical analyses
The Software R, version 3.6.1. (R_Core_Team. R 2020) was used for the following data processing and statistical analyses to detect statistically significant difference among factor level means through methods of one-way analysis of variance (ANOVA) type I (sequential), using sum of squares at 5% significance level (Zar 1984), Tukey’s HSD (honestly significant difference) test and “treatment contrast” to calculate factor level means for each treatment. The linear dependence between all soil properties was determined by Pearson’s correlation analysis to reveal the mutual relationships between individual enzymes, IR and yield of plants. The interpretation of Pearson’s correlation coefficient (r) was as follows: 0.0 < r < 0.3 (negligible correlation), 0.3 < r < 0.5 (low correlation), 0.5 < r < 0.7 (moderate correlation) and 0.7 < r < 0.9 (high correlation), 0.9 < r < 1.0 (very high correlation) (Hinkle et al. 2003). After all statistical analyses the assumptions of selected models was also checked at significance level of 0.05. For testing the normality, it was used Kolmogorov–Smirnov test and for testing the homoscedasticity, it was used Bartlett’s test of homogeneity of variances. Besides, the model checking was also performed using different diagnostic plots.
Results and discussion
Effect of P3HB on plant aboveground, root biomass, and enzyme activity
The application of P3HB significantly reduced the abovegound fresh and dry biomass (AGB fresh, AGB dry) of lettuce when applied alone or in combination with compost (Co) (Fig. 1a, b). Compost alone did not enhance AGB fresh and AGB dry compared to the control, while digestate (Di) notably improved lettuce biomass, both alone and when combined with P3HB. Moreover, digestate co-applied with P3HB mitigated the decline in AGB fresh and AGB dry caused by P3HB alone, even surpassing the biomass in the Co-only variant (Fig. 1a). However, both P3HB and P3HB + compost treatments negativelly impacted root biomass (fresh and dry) compared to the control. In contrast, Di co-applied with P3HB improved root dry biomass, though it remained lower then in the digestate, compost, and control treatments (Fig. 1d). Fresh root biomass varied significantly across treatments (Fig. 1c), with the highets fresh biomass observed in the digstate-amended soils.
Soil degradation activities, as indicated by dehydrogenase (DHA), increased across all amended variants compared to the control, particularly in the Di + P3HB variant, highlighting enhanced carbon mineralization (Fig. 2a). Conversely, β-glucosidase (GLU) activity, another carbon mineralizing enzyme, was suppressed by P3HB with significant enhancement only observed with digestate addition (Fig. 2f). P3HB presence in the soil reduced carbon transformation efficiency, as indicated by lower C acquisition ratios (Table 2). Higher vector length values (Table 2) in P3HB-unamended variants indicated greater C limitation.
Enzyme activities of the soil amended with P3HB, compost, digestate, and combination Dehydrogenase activity (a), N-acetyl-β-D-glucosaminidase (b), arylsulfatase (c), urease (d), phosphatase (e) and β-glucosidase (f) activities. Lowercase letters indicate the differences between variants on the statistical significance level p ≤ 0.05
Arylsulfatase (ARS) activity increased across all treatments, particularly in the Co + P3HB variant (Fig. 2c). Enzymes involved in nitrogen mineralization, such as N-acetyl-β-D-glucosaminidase (NAG) and urease (Ure), were mainly elevated by P3HB, whether applied alone or with digestate or compost (Fig. 2b and d). The co-application of P3HB with digestate further boosted NAG activity, with the highest increase seen with digestate alone, while the combination P3HB and compost led to the highest Ure activity (Fig. 2d). Phosphatase (Phos) activity was enhanced under combined treatments, most notably in the Co + P3HB variant (Fig. 2e).
Statistical analysis (i.e. the correlation matrix in Figure S1) supported above-statements and revealed P3HB treatment strongly correlates with reduced aboveground biomass (AGB) and root biomass, as indicated by significant negative correlations with glucose (r = − 0.48 for AGB fresh) and glucosamine (r = − 0.62 for root dry). Conversely, dehydrogenase activity (DHA) showed a positive correlation with glucose and trehalose respiration (r = 0.64 and 0.67, respectively), highlighting P3HB’s impact on carbon cycling enzymes. Next, microbial activity, particularly β-glucosidase (GLU) and arylsulfatase (ARS), appeared to be inversely related to plant biomass under P3HB treatment, with significant negative correlations with root dry weight (r = − 0.53 and − 0.22, respectively).
Effect of P3HB on soil respiration
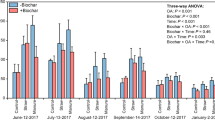
The introduction of stabilized organic matter (compost or digestate) did not significantly enhance basal respiration (BR) or substrate induced respiration (IR) compared to the control. In fact, digestate reduced all respiration indicators, and compost specfically decreased Glc-, Tre-, NAG, and Arg-IR relative to the control (Fig. 3). On the contrary, P3HB alone stimulated all forms of respiraton except Tre-IR and Arg-IR (Fig. 3c, g). The co-application of P3HB with compost generally reduced (BR, Tre-IR, Lys-IR) or mainained (Glc-, NAG-, Ala-IR) respiration levels compared to P3HB alone, though Glc- and Ala-IR in the P3HB + Co were higher than in the control. When P3HB was combined with digestate, it significantly enhanced all types of IR except for Arg-IR, which was higher in the P3HB + Di variant than in P3HB alone, but still lower then in the control (Fig. 3e). Interestingly, BR was more elevated in the P3HB treatment than in the P3HB + Di combination (Fig. 3a).
Respiration of the soil amended with P3HB, compost, digestate and combination Basal respiration (a) and substrate-induced respirations—Glc-IR (b), Tre-IR (c), NAG-IR (d), Ala-IR (e), Lys-IR (f), Arg-IR (g). Lowercase letters indicate the differences between variants on the statistical significance level p ≤ 0.05
The interactions among various respiration types were evident, as demonstrated by strong positive correlation between Glc-IR and other types including Tre-IR, NAG-IR, Ala-IR, and Lys-IR, with correlaton coeffiients of 0.8, 0.88, 0.92, 0.83, respectively (Figure S1). Additionally, Glc-IR correlated with NAG (r = 0.82), indicating interderdence among these respiration activities. Depite these correlations, the independence of these respiration types were reflected in the microbial functional diversity (MFD parameter), which showed any amendment reduced the functional diversity of the soil microbiome. Suprisingly, the sole addition of digestate had the most pronounced negative effect on diversity, while all three PHB-based treatments displayed comparable reductions in MFD (Table 3).
Thermogravimetry results
Figure 4 presents exemplary thermogravimetric (TG) results, showing mass loss percentages as the temperature increases in an oxidative atmosphere, ranging from laboratory temperature to 650 °C. According to Palucha et al. 2027 (see Sect. "Thermogravimetric analysis (TG)"), mass loss, particularly between 200 and 300 °C, corresponds to the degradation of P3HB together and the labile components of SOM, as indicated by dotted lines in the Fig. 4 (Palucha et al. 2024). Among all soils tested, the control exhibited the least mass lost, with progressively greater losses observed in soil amended with compost and digestate, and the most significant loss in soils treated with P3HB.
Residual P3HB content was quantified post-heating, revealing 0.37 ± 0.05% (from the original 1%) residual P3HB in the control soil, reduced to 0.22 ± 0.05 and 0.23 ± 0.04% in soils amended with digestate and compost, respectively.
Discussion
Effect of P3HB on plant aboveground and root biomass, and enzyme activity.
Effect on plant aboveground and root biomass
The effect of P3HB (applied solely or with compost) on both abovegound and root fresh and dry biomass (AGB fresh, AGB dry, Root fresh, Root dry) of lettuce was strongly negative. Brtnicky et al. 2022 (Brtnicky et al. 2022) reported a similar deleterious impact of P3HB on plant growth in both soil and soil-sand substrates (Brtnicky et al. 2022). In general, the adverse impact of P3HB on plant growth can be explained as the enhanced turnover of SOM and competition of highly active microbial biomass in degradation hotspots (Zhou et al. 2021b) and plants for nutrients (Zhou et al. 2021a). This competition subsequently enhances nutrient transformation and reduces their sequestration for plant nutrition, particularly phosphorus and nitrogen. It has been reported that a similar increase in the input of easily available carbon (C) into the rhizosphere (Kuzyakov and Xu 2013; Nguyen 2003) may escalate microbiome-plant competition due to higher microbial abundance, activity, and growth in the rhizosphere (Blagodatskaya et al. 2010). Consequently, this drains off the remaining nutrients available to plants due to higher microbial uptake and immobilization (Zak et al. 2000). This explanation is supported by the negative correlation between AGB fresh, AGB dry, Root fresh, Root dry and indicators of nitrogen mineralization such as Ure (r values were − 0.61, − 0.73, − 0.94, − 0.94) and Ala-IR (r values were − 0.5, − 0.56, − 0.43, − 0.71). The P3HB variant showed a higher nitrogen value acquisition ratio in comparison to the control (Table 2), which corroborates our hypothesis #2.
Morevover, the amendment of compost did not effectively nourish the lettuce, as its application increased signficantly only the root fresh biomass compared to the control. On the contrary, solely applied digestate had a positive impact on both dry and fresh AGB (Fig. 1a, b), albeit it showed a slightly negative effect on root fresh biomass (Fig. 1d). When applied with P3HB, digestate alleviated the deleterious impact of bioplastics on all determinants of lettuce biomass yield and even incerased the AGB fresh biomass more than the compost amendment. The properties of digestate, such as higher water content and readily available ammonium nitrogen, were presumably responsible for mitigation the P3HB-mediated repression of plant growth, despite potentially lower total nitrogen input (Table 1). These findings partially (in the case of digestate) confirmed our hypothesis #3.
As mentioned above, the increased water evaporation/drain-off, leading to decreased water content should be considered in P3HB-amended soil. The P3HB is a water insoluble material with a reported contact angle between 70° (Bonartsev et al. 2013) and approximately 81° (Pompe et al. 2007), thus, it slightly increases water repellency. Niu et al. (Niu et al. 2016) noted that the evaporation coefficient increased with higher doses of residual plastic film in soil, suggesting a decreased water holding capacity. Wan et al. (Wan et al. 2019) reported an increased rate of soil water evaporation with a higher amount of plastics in soil, and Fojt et al. (Fojt et al. 2022) observed a decrease in evaporation enthalpy after the adition of P3HB particles to soil organic matter. On the contrary, digestate amendment to soil could have a positive, evaporation-mitigating effect on soil and enhance the availability of water for plants, as referred by some authors (Beck-Broichsitter et al. 2020; Nabel et al. 2017). This feature, along with the reported beneficial effect of digestate on plant growth and the abundance of plant-promoting rhizobacteria (PGPR) in soil (Siebielec et al. 2018), might explain why the Di + P3HB variant did not exert any significant negative effects on plant biomass.
Effect on soil enzymes
DHA was enhanced by all tested soil amendments, but more significantly with P3HB and digestate, and to a lesser extent with compost. The addition of P3HB induced soil degradation as this compound was primarily utilized as a carbon and energy source by microbes (Bonartseva et al. 2003; Rizzarelli et al. 2004), and the results correspond to previous findings (Brtnicky et al. 2022). An even higher induction of P3HB-derived carbon mineralization was expected under conditions of higher nitrogen access (and also other nutrients) in the variants co-amended with P3HB and stabilized organic matter. However, only the P3HB + Di amendment lead to higher DHA compared to the sole P3HB addition, as shown in Fig. 2a. There was a markable tendency towards higher carbon limitation in P3HB-unamended variants compared to those supplied with bioplastics (Table 2). This difference in nutrient availability and the interplay between bioplastics, P3HB-degrading microbes, and the plant rhizobiome presumably mediated the enhanced DHA activity in a similar manner as observed recently (Bai et al. 2020) when di(2-ethylhexyl)phthalate (DEHP) promoted increased biodegradation under soil bioaugmentation with PGPR and changes in dominant genera (Allorhizobium, Neorhizobium, Pararhizobium, Rhizobium, Caulobacteraceae) in the consortium. Similar changes in the microbiome composition can be inferred from the differences in the microbial functional diversity (MFD), which significantly varied mainly between the control and all P3HB-based variants (Table 3). This presumption was supported by the observations that several types of respiration showed positive correlations with DHA: Glc-IR (r = 0.64), Ala-IR (r = 0.67), Lys-IR (r = 0.67, Figure S1).
In contrast to DHA, other enzymes (especially GLU) were more likely involved in the transformation of intrinsic SOM (or external organic matter, EOM, of compost and digestate amendments). GLU was lower in the presence of P3HB than under amendment of digestate or compost, or without any amendment (Fig. 2f), suggesting that P3HB may mitigate the mineralization of cellulose and its derivatives in soil. This observation aligns with findings from Brtnicky et al. 2022 (Brtnicky et al. 2022) and with reports of P3HB being used as a single carbon and energy source (Bonartseva et al. 2003; Rizzarelli et al. 2004), which is preferred over organic C from either SOM and extraneous organic matter (EOM) of stabilized organic amendments (compost and digestate). This assumption could be related to suprisingly unchanged values of of the C acquisition ratio (Table 2) in P3HB-supplied variants. In these variants, values of the ratio were relatively decreased due to the mitigated carbon uptake from intrinsic SOM, as most of the utilized carbon was derived from P3HB. This finding verified our hypothesis #1. Significant GLU enhancement was achieved only with the addition of digestate, which possibly also provided higher inoculation with cellulolytic microbes.
Although ARS activity was induced by all tested amendments (most significantly by P3HB + Co), it only showed a weak correlation with other enzymes, except for Ure (where r = 0.59, Figure S1). Poly-3-hydoxybutyre did not significantly stimulate ARS compared to the solely applied digestate or compost. Therefore, no P3HB specific impact was ascribed to the results, aligning with the findings of Brtnicky et al. 2022 (Brtnicky et al. 2022). The highest ARS value (Fig. 2c) was presumably due to the highest available sulphur content in the compost, as shown by Prasad et al. 2022 (Prasad et al. 2012), and the increased microbial biomass due to the supply of P3HB.
NAG, an indicator or fungal biomass degradation and turnover, was found to be significantly increased in all P3HB variant (compared to the control), similarly as referred in Brtnicky et al. 2022 (Brtnicky et al. 2022). However, the combinations of P3HB + Co and P3HB + Di were even more beneficial for NAG activity (Fig. 2b). It was observed P3HB in the soil contributed to the multiplication of saprophytic fungi, similarly as reported e.g. by the study (Janczak et al. 2020), and these fungi exhibit the ability to catabolize P3HB (Altaee et al. 2016; Sang et al. 2002). The significant increase in NAG, whether due to P3HB-, digestate- (alone or with P3HB) or compost, also indicated an enhancement of nutrient (including nitrogen) mineralization due to increased depolymerization of P3HB. The mutual relationship between nutrient uptake due to partial organic matter degradation and related fungal biomass turnover was documented by a positive correlation between DHA and NAG (r = 0.67, Figure S1).
Furthermore, another nitrogen mineralizing enzyme, urease (Ure), was induced by the addition of P3HB to soil, whether applied alone or in combination with digestate or compost (Fig. 2d). The highest Ure value was found in soil amended with P3HB and compost, presumably due to the highest nitrogen dose provided by compost (Table 1). These results, similar those Brtnicky et al. 2022 (Brtnicky et al. 2022), aligned with reports of higher early losses of available nitrogen in soil amended with either compost or digestate (Nicholson et al. 2017). However, the enhancement of Ure activity was significantly related to the simultaneous application of P3HB, as neither the Ure values nor the nitrogen acquisition ratios of the digestate, compost, and control differed markedly. The related enhanced N acquisition ratios in P3HB-amended variants were found to be significantly higher than in the unamended variants (Table 2). The relationship between Ure and catabolism in SOM (and carbon mineralization) was documented by a positive correlation of Ure with either GLU (r = 0.63) or Al-IR (r = 0.54) (Figure S1). The results of NAG and Ure (and partially also ARS) determination corroborated Hypothesis #3: i.e. organic amendments provided more nutrients, enhanced their transformation mineralization activities.
Phosphatase activity, similar to the results in Brtnicky et al. 2022 (Brtnicky et al. 2022) and to ARS, was mostly insignificantly affected either by P3HB or stabilized organic matter, with the only significant effect detected in the P3HB + Co variant (Fig. 2e). This was due to the presumed highest phosphorus availability in this blend, an assumption that aligns with reported general levels of phosphorus in various stabilized organic matters (Prasad et al. 2012; Manasa et al. 2020). Values in Table 2 showed that biodegradable plastic played role in higher nitrogen acquisition, whereas P3HB-unamendment variant appeared much less limited by nitrogen than by phosphorus. The Angle values (Table 2) indicated that increased degradation of organic matter in all amended variants was likely the reason for phosphorus limitation (compared to the weakest limitation in the control), but this limitation was least severe (lowest value) in the P3HB + Co soil. Again, presumably the highest phosphorus content led to significantly higher Phos activity.
Effect of P3HB on soil respiration
Among all three amended materials, only the P3HB amendment lead to an increase in respiration potential compared to the control (as previously was reported in Brtnicky et al. 2022 (Brtnicky et al. 2022)), indicated by stimulated respiration of all types except for Tre-IR and Arg-IR (Fig. 3c, g). These results might also be presumably due to a P3HB-derived amplification of aerobic degraders, which corroborates our hypothesis #1. On the contrary, the digestate amendment decreased all respiration indicators, presumably due to the second lowest input of organic C in external organic matter (Table 1), which likely also had higher recalcitrance than the more labile P3HB-associated organic carbon. Moreover, the minimal respiration activity of the digestate and compost variants, coupled with the lowest carbon mineralization, was caused by carbon limitation (with the lowest values being 1.6, 1.55, respectively, as shown in Table 2). Compost added to the soil decreased Glc-, Tre-, NAG, and Arg-IR compared to the control (Fig. 3).
Co-amendment of P3HB with stabilized organic matter (compost or digestate) further altered the respiration potencial (and likely the abundance of aerobes) in comparison to the single P3HB amendment (Fig. 3). When P3HB was co-applied with compost, it decreased BR, Tre-IR, Lys-IR, despite the presumed availability of nutrients (nitrogen, sulphur, phosphorus) inferred from the values of enzyme activities (NAG, Ure, ARS, Phos). Some adversely acting factors could be assumed, such as low plant root biomass and assumed lower water content (compared to the digestate-treated variant), which slightly negatively effected microbial respiration in the soil compared to the impact of sole P3HB. The important role of soil mositure in the degradation of plastic residues in soil has already been described (King et al. 2015). The study by Almethyeab et al. (Almethyeb et al. 2013) showed how amplified PGPR and root symbionts significantly affected plant growth and nutrient uptake, and in return, increased soil respiration. In contrats, the P3HB co-applied with digestate was presumed to cause the most significant amplification of aerobic soil degraders, as indicated by the enhancement of all IR types except for Arg-IR. The most significant decline in Arg-IR (in comparison to the control) across all IRs in P3HB, P3HB + Co, and P3HB + Di variants (Fig. 3g) possibly indicated that the functional fraction of the soil microbiome, capable of utilizing L-arginine (Arg) utilization, was also affected by plant–microbe competition for amino acids, as described in study (Owen and Jones 2001). This competition was expected to be highest particularly in P3HB and P3HB + Co variants. Therefore, the results of respiration determination led to the partial rejection of Hypothesis #3, that posited that organic amendment(s) should enhance both nutrient transformation (mineralization) activities and respiration rate, as the respiration types in sole compost- or digestate-amended variants were mitigated. The variability in the response of each respiration type to respective amendments was best documented by the parameter MFD (microbial functional diversity, Table 3), which as it showed that each amendment reduced functional diversity of the soil in comparison to the control, leading to a less diverse microbiome in P3HB-treated soil and the lowest diversity in the digestate variant.
Effect of organic amendments on P3HB degradation
As demonstarted by this and previous works (e.g. (Zhou et al. 2021a; Brtnicky et al. 2022)) the biodegradation of polyalkanoates is connected with a demand for nutrients, in particular nitrogen and phosphorus. In addition, the presence of plastic residue accelerates moisture evaporation (Fojt et al. 2022) thereby stressing plants due to a lack of moisture. The TG results obtained here suggest that biodegradation was incomplete when the experiment ended, with both P3HB degrading organisms and residues of P3HB still present in the soil. In addition, it was obseved that the rate of P3HB degradation was higher in soils amended with compost and digestate, while it was lower in control soil. This supports the Hypothesis 4 that the amendment accelerates the degradation of biodegradable P3HB. However, the degree of degradation in compost and digestate correlate neither with the enzyme content nor with the AGB results. In particular, compost apeared to be a good substrate for P3HB biodegradation, but, at the same time, did not support the plant growth. On the contrary, digestate supports both biodegradation and plant growth. Considering that Ure levels were higher in compost and Phos were comparable in both variants, we can only speculate that this may be related to the mechanisms of nutrient fixation in both substrates and their differing impacts on soil physical properteis (porosity, density).
To the best of our knowledge, no study has compared those amendments in terms of their influence on the rate of biodegradation. On the contrary, the effectiveness of digestate comparing to compost in promoting plant growth and enhancing soil biochemical processes has been studied and the authors obtained various results (Aguilar-Benítez et al. 2020; Tambone and Adani 2017). We conclude that the specific effect would depend on feedstock for compost and digestate production, technoogy used for their production, soil type and many other factors, which were not included in this study. Thus, understanding this problem requires futher research involving different composts and digestates obtained from various feedstocks, various soil types mainly in terms of soil texture and soil organic matter contest, various temperatures and moisture levels. Nevertheless, in light of the results obtained in this study, the application of an amendment providing nutrinets and water and suporting the soil aeration seem to be necessary to mitigate the negative effects of bioplastic biodegradation on plant growth.
Conclusion
In this study, we addressed the issues connected with nutrient demand of biodegradable plastics biodegradation in arable soils and their negative impact on plant growth. Applying compost alone boosted the root dry biomass of lettuce, but has limited effect on aboveground biomass. Application of digestate showed, that alone, it enhanced both aboveground biomass (dry and fresh) and specific enzyme activity (β-glucosidase), indicating a beneficial impact on lettuce growth and certain soil biochemical functions. The application of P3HB alone strongly reduced both root and aboveground biomass, indicating a detrimental effect on plant growth. However, it increased activity of dehydrogenase and urease enzymes and various types of respiration, which implied enhanced microbial activity in the soil. The co-application of compost and P3HB enhanced all tested enzyme activities except for β-glucosidase, which suggested that compost can mitigate some of the negative effects of P3HB on biochemical processes in the soil. The most effective combination was digestate and P3HB, their co-application significantly countered the negative impact of P3HB on lettuce biomass and further enhanced certain enzyme activities and types of respiration. This combination appeared to be the most effective in supporting both plant growth and soil health.
In fact, both digestate and compost are valuable organic amendments that can improve soil health and plant growth, the compost seemed less advantageous for the purpose of mitigating possible adverse environmental impact of microbioplastics. Apart from lower impact of anaerobic digestion on the environment, digestate may offer additional advantages, likely due to its degree and mechanisms of organic matter decomposition, nutrient composition, moisture content, and the nature of its microbial activity. These factors make digestate particularly effective in certain agricultural contexts, though it is important to monitor conditions like salt content and potential over-fertilization. Further research is needed to optimize the use of digestate for support of bioplastic degradation in specific soil types, climates, and cropping systems to harness its full potential effectively. Nevertheless, the implications of our findings are significant for agricultural practices, particularly in managing soil health and plant growth in environments impacted by biodegradable plastics.
Furthermore, this study also confirms recent observations that integrating biodegradable plastics into agricultural practices requires a comprehensive understanding of how these materials interact with soil amendments, particularly in terms of nutrient availability, microbial activity, and water retention. These findings advocate for further research and development of best practices for the use of biodegradable plastics in different agricultural systems, ensuring that their environmental benefits are maximized while minimizing potential negative impacts on soil health and plant growth.
In summary, while biodegradable plastics offer a sustainable alternative to traditional plastics, their integration into agriculture must be managed carefully. The strategic use of organic amendments like digestate can play a vital role in this process, supporting both the degradation of bioplastics and the maintenance of healthy, productive soils. This approach is essential for the effective management of bioplastic residues, particularly in the context of global efforts to enhance sustainability in agriculture.
Data availability
The data presented in this study are available on request from the corresponding author.
Abbreviations
- 3-HB:
-
3-Hydroxybutyric acid
- AGB dry:
-
Dry aboveground lettuce biomass
- AGB fresh:
-
Fresh aboveground lettuce biomass
- ANOVA:
-
Analysis of variance
- ARS:
-
Arylsulfatase
- BR:
-
Basal respiration
- Cinput :
-
Amount of carbon added to soil with amendments
- Co:
-
Compost
- DHA:
-
Dehydrogenase activity
- DEHP:
-
Di(2-ethylhexyl)phthalate
- Di:
-
Digestate
- GLU:
-
β-Glucosidase
- EOM:
-
External organic matter
- IR:
-
Substrate induced respirations D-glucose (Glc-IR), D-trehalose (Tre-IR), N-acetyl-β-D-glucosamine (NAG-IR), L-alanine (Ala-IR), L-lysine (Lys-IR) and L-arginine (Arg-IR)
- MFD:
-
Microbial functional diversity
- NAG:
-
N-Acetyl-β-D-glucosaminidase
- Ninput :
-
Amount of nitrogen added to soil with amendments
- p:
-
p-Value
- PHA:
-
Polyhydroxyalkanoates
- P3HB:
-
Poly-3-hydroxybutyrate
- Phos:
-
Phosphatase
- r:
-
Pearson’s correlation coefficient
- SOM:
-
Soil organic matter
- Ure:
-
Urease
- WHC:
-
Water holding capacity
References
Abou-Zeid DM, Muller RJ, Deckwer WD (2004) Biodegradation of aliphatic homopolyesters and aliphatic-aromatic copolyesters by anaerobic microorganisms. Biomacromol 5(5):1687–1697. https://doi.org/10.1021/bm0499334
Aguilar-Benítez G, Solís-Oba MM, Castro-Rivera R, López-Gayou V, Lara-Ávila JP, Esteves-Luna MA (2020) Effect of PGPB bacteria, compost and digestate on the dry matter yield of cocksfood. Rev Mex Cienc Agríc 11:117–127. https://doi.org/10.29312/remexca.v0i24.2363
Alcântara JM, Distante F, Storti G, Moscatelli D, Morbidelli M, Sponchioni M (2020) Current trends in the production of biodegradable bioplastics: the case of polyhydroxyalkanoates. Biotechnol Adv 42:107582. https://doi.org/10.1016/j.biotechadv.2020.107582
Almethyeb M, Ruppel S, Paulsen HM, Vassilev N, Eichler-Lobermann B (2013) Single and combined applications of arbuscular mycorrhizal fungi and Enterobacter radicincitans affect nutrient uptake of faba bean and soil biological characteristics. Landbauforschung 63(3):229–234. https://doi.org/10.3220/lbf_2013_229-234
Altaee N, El-Hiti GA, Fahdil A, Sudesh K, Yousif E (2016) Biodegradation of different formulations of polyhydroxybutyrate films in soil. Springerplus 5(1):762. https://doi.org/10.1186/s40064-016-2480-2
Baei MS, Najafpour GD, Younesi H, Tabandeh F, Eisazadeh H (2009) Poly (3-hydroxybutyrate) synthesis by Cupriavidus necator DSMZ 545 utilizing various carbon sources. World Appl Sci J 7(2):157–161
Bai N, Li S, Zhang J, Zhang H, Zhang H, Zheng X et al (2020) Efficient biodegradation of DEHP by CM9 consortium and shifts in the bacterial community structure during bioremediation of contaminated soil. Environ Pollut 266(Pt 2):115112. https://doi.org/10.1016/j.envpol.2020.115112
Bankole A, Umebese CE, Feyisola RT, Bamise O (2018) Influence of salicylic acid on the growth of lettuce (Lactuca sativa var longifolia) during reduced leaf water potential. J Appl Sci Environ Manag 22:543. https://doi.org/10.4314/jasem.v22i4.18
Beck-Broichsitter S, Ruth S, Schröder R, Fleige H, Gerke HH, Horn R (2020) Simultaneous determination of wettability and shrinkage in an organic residue amended loamy topsoil. J Hydrol Hydromech 68(2):111–118. https://doi.org/10.2478/johh-2020-0007
Blagodatskaya E, Littschwager J, Lauerer M, Kuzyakov Y (2010) Growth rates of rhizosphere microorganisms depend on competitive abilities of plants and N supply. Plant Biosyst–int J Dealing All Asp Plant Biol 144(2):408–413. https://doi.org/10.1080/11263501003718596
Bonartsev AP, Yakovlev SG, Zharkova II, Boskhomdzhiev AP, Bagrov DV, Myshkina VL et al (2013) Cell attachment on poly(3-hydroxybutyrate)-poly(ethylene glycol) copolymer produced by Azotobacter chroococcum 7B. BMC Biochem 14(1):12. https://doi.org/10.1186/1471-2091-14-12
Bonartsev AP, Voinova VV, Bonartseva GA (2018) Poly(3-hydroxybutyrate) and human microbiota (review). Appl Biochem Microbiol 54(6):547–568. https://doi.org/10.1134/s0003683818060066
Bonartseva GA, Myshkina VL, Nikolaeva DA, Kevbrina MV, Kallistova AY, Gerasin VA et al (2003) Aerobic and anaerobic microbial degradation of poly-beta-hydroxybutyrate produced by Azotobacter chroococcum. Appl Biochem Biotechnol 109(1–3):285–301. https://doi.org/10.1385/abab:109:1-3:285
Brtnicky M, Pecina V, Holatko J, Hammerschmiedt T, Mustafa A, Kintl A et al (2022) Effect of biodegradable poly-3-hydroxybutyrate amendment on the soil biochemical properties and fertility under varying sand loads. Chem Biol Technol Agric 9(1):75. https://doi.org/10.1186/s40538-022-00345-9
Brtnicky M, Pecina V, Kucerik J, Hammerschmiedt T, Mustafa A, Kintl A et al (2024) Biodegradation of poly-3-hydroxybutyrate after soil inoculation with microbial consortium: Soil microbiome and plant responses to the changed environment. Sci Total Environ 946:174328. https://doi.org/10.1016/j.scitotenv.2024.174328
Bruss ML (2008) Chapter 4–Lipids and ketones. In: Kaneko JJ, Harvey JW, Bruss ML (eds) Clinical Biochemistry of Domestic Animals, 6th edn. Academic Press, San Diego, pp 81–115
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69(6):3593–3599. https://doi.org/10.1128/AEM.69.6.3593-3599.2003
Cervera-Mata A, Pastoriza S, Rufián-Henares JÁ, Párraga J, Martín-García JM, Delgado G (2018) Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch Agron Soil Sci 64:790–804. https://doi.org/10.1080/03650340.2017.1387651
Chrysargyris A, Xylia P, Anastasiou M, Pantelides I, Tzortzakis N (2018) Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J Sci Food Agric 98:5861–5872. https://doi.org/10.1002/jsfa.9139
Corrêa MCS, Rezende ML, Rosa DS, Agnelli JAM, Nascente PAP (2008) Surface composition and morphology of poly(3-hydroxybutyrate) exposed to biodegradation. Polym Test 27(4):447–452. https://doi.org/10.1016/j.polymertesting.2008.01.007
Cui J, Zhang S, Wang X, Xu X, Ai C, Liang G et al (2022) Enzymatic stoichiometry reveals phosphorus limitation-induced changes in the soil bacterial communities and element cycling: evidence from a long-term field experiment. Geoderma 426:116124. https://doi.org/10.1016/j.geoderma.2022.116124
Dahal S, Yilma W, Sui Y, Atreya M, Bryan S, Davis V, Whiting GL, Khosla R (2020) Degradability of biodegradable soil moisture sensor components and their effect on maize (Zea mays L.) growth. Sensors 20(21):6154. https://doi.org/10.3390/s20216154
de Souza Machado AA, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC (2018) Impacts of microplastics on the soil biophysical environment. Environ Sci Technol 52(17):9656–9665. https://doi.org/10.1021/acs.est.8b02212
Deroiné M, César G, Le Duigou A, Davies P, Bruzaud S (2015) Natural degradation and biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in liquid and solid marine environments. J Polym Environ 23(4):493–505. https://doi.org/10.1007/s10924-015-0736-5
Eya H, Otuji Y, Nagai T, Hattori K (1997) Biodegradability in soil and composting of microbial polyester. Kobunshi Ronbunshu 54(8):463–470. https://doi.org/10.1295/koron.54.463
Fojt J, Denkova P, Brtnicky M, Holatko J, Rezacova V, Pecina V et al (2022) Influence of poly-3-hydroxybutyrate micro-bioplastics and polyethylene terephthalate microplastics on the soil organic matter structure and soil water properties. Environ Sci Technol 56(15):10732–10742. https://doi.org/10.1021/acs.est.2c01970
González-Pleiter M, Tamayo-Belda M, Pulido-Reyes G, Amariei G, Leganés F, Rosal R et al (2019) Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environ Sci Nano 6(5):1382–1392. https://doi.org/10.1039/c8en01427b
Grousseau E, Blanchet E, Deleris S, Albuquerque MG, Paul E, Uribelarrea JL (2013) Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour Technol 148:30–38. https://doi.org/10.1016/j.biortech.2013.08.120
Guo W, Tao J, Yang C, Zhao Q, Song C, Wang S (2010) The rapid evaluation of material biodegradability using an improved ISO 14852 method with a microbial community. Polym Test 29(7):832–839. https://doi.org/10.1016/j.polymertesting.2010.07.004
Hammerschmiedt T, Holatko J, Kucerik J, Mustafa A, Radziemska M, Kintl A et al (2022) Manure maturation with biochar: effects on plant biomass, manure quality and soil microbiological characteristics. Agriculture 12(3):314. https://doi.org/10.3390/agriculture12030314
Hao T, Zhu Q, Zeng M, Shen J, Shi X, Liu X et al (2020) Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J Environ Manag 270:110888. https://doi.org/10.1016/j.jenvman.2020.110888
Hinkle DE, Wiersma W, Jurs SG (2003) Applied statistics for the behavioral sciences. 5th ed. Boston, Mass.: Houghton Mifflin
Höhnemann T, Windschiegl I (2023) Influence of rheological and morphological characteristics of polyhydroxybutyrate on its meltblown process behavior. Materials 16(19):6525. https://doi.org/10.3390/ma16196525
Iocoli GA, Zabaloy MC, Pasdevicelli G, Gómez MA (2019) Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: Characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci Total Environ 647:11–19. https://doi.org/10.1016/j.scitotenv.2018.07.444
Iovieno P, Scotti R, Zaccardelli M (2021) Functional diversity of soil microbial community after conversion of a chestnut forest to an agricultural system. Agriculture 11(1):43
ISO_10390 (2005) Soil quality -Determination of pH. International Organization for Standardization, Geneva, Switzerland
Janczak K, Dąbrowska GB, Raszkowska-Kaczor A, Kaczor D, Hrynkiewicz K, Richert A (2020) Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int Biodeterior Biodegrad 155:105087. https://doi.org/10.1016/j.ibiod.2020.105087
Kadouri D, Jurkevitch E, Okon Y (2003) Poly beta-hydroxybutyrate depolymerase (PhaZ) in Azospirillum brasilense and characterization of a phaZ mutant. Arch Microbiol 180(5):309–318. https://doi.org/10.1007/s00203-003-0590-z
King MA, Piper KL, MacDonald G, Sherman SE, Francis H, Hopkins CJ, et al. (2015) Role of moisture in determining compostable bag degradation. BioCycle. 14
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198(3):656–669. https://doi.org/10.1111/nph.12235
Lee SY (1996) Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol 14(11):431–438. https://doi.org/10.1016/0167-7799(96)10061-5
Luckachan GE, Pillai CKS (2011) Biodegradable polymers–a review on recent trends and emerging perspectives. J Polym Environ 19(3):637–676. https://doi.org/10.1007/s10924-011-0317-1
Malik MR, Yang W, Patterson N, Tang J, Wellinghoff RL, Preuss ML et al (2015) Production of high levels of poly-3-hydroxybutyrate in plastids of Camelina sativa seeds. Plant Biotechnol J 13(5):675–688. https://doi.org/10.1111/pbi.12290
Manasa MRK, Katukuri NR, Darveekaran Nair SS, Haojie Y, Yang Z, Guo RB (2020) Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J Environ Manag 269:110737. https://doi.org/10.1016/j.jenvman.2020.110737
Mierziak J, Wojtasik W, Kulma A, Dziadas M, Kostyn K, Dymińska L, Hanuza J, Żuk M, Szopa J (2020) 3-Hydroxybutyrate is active compound in flax that upregulates genes involved in dna methylation. Int J Mol Sci 21(8):2887. https://doi.org/10.3390/ijms21082887
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7. https://doi.org/10.1016/j.soilbio.2015.10.019
Muneer F, Rasul I, Azeem F, Siddique MH, Zubair M, Nadeem H (2020) Microbial polyhydroxyalkanoates (PHAs): efficient replacement of synthetic polymers. J Polym Environ 28(9):2301–2323. https://doi.org/10.1007/s10924-020-01772-1
Nabel M, Schrey SD, Poorter H, Koller R, Jablonowski ND (2017) Effects of digestate fertilization on Sida hermaphrodita : boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 107:207–213. https://doi.org/10.1016/j.biombioe.2017.10.009
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23(5–6):375–396. https://doi.org/10.1051/agro:2003011
Nicholson F, Bhogal A, Cardenas L, Chadwick D, Misselbrook T, Rollett A et al (2017) Nitrogen losses to the environment following food-based digestate and compost applications to agricultural land. Environ Pollut 228:504–516. https://doi.org/10.1016/j.envpol.2017.05.023
Nishida H, Tokiwa Y (1993) Distribution of poly(b-hydroxybutyrate) and poly(e-caprolactone)aerobic degrading microorganisms in different environments. J Environ Polym Degrad 1(3):227–233. https://doi.org/10.1007/bf01458031
Nishide H, Toyota K, Kimura M (1999) Effects of soil temperature and anaerobiosis on degradation of biodegradable plastics in soil and their degrading microorganisms. Soil Sci Plant Nutr 45(4):963–972. https://doi.org/10.1080/00380768.1999.10414346
Niu W, Zou X, Liu J, Zhang M, Lü W, Gu J (2016) Effects of residual plastic film mixed in soil on water infiltration, evaporation and its uncertainty analysis. Trans Chin Soc Agric Eng 32(14):110–119
Owen AG, Jones DL (2001) Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol Biochem 33(4–5):651–657. https://doi.org/10.1016/s0038-0717(00)00209-1
Palucha N, Fojt J, Holátko J, Hammerschmiedt T, Kintl A, Brtnický M, Řezáčová V, De Winterb K, Uitterhaegen E, Kučerík J (2024) Does poly-3-hydroxybutyrate biodegradation affect the quality of soil organic matter? Chemosphere 352:141300. https://doi.org/10.1016/j.chemosphere.2024.141300
Panayotidou E, Baklavaridis A, Zuburtikudis I, Achilias DS (2014) Nanocomposites of poly(3-hydroxybutyrate)/organomodified montmorillonite: effect of the nanofiller on the polymer’s biodegradation. J Appl Polym Sci. https://doi.org/10.1002/app.41656
Panuccio MR, Papalia T, Attinà E, Giuffrè A, Muscolo A (2019) Use of digestate as an alternative to mineral fertilizer: effects on growth and crop quality. Arch Agron Soil Sci 65(5):700–711. https://doi.org/10.1080/03650340.2018.1520980
Pompe T, Keller K, Mothes G, Nitschke M, Teese M, Zimmermann R et al (2007) Surface modification of poly(hydroxybutyrate) films to control cell-matrix adhesion. Biomaterials 28(1):28–37. https://doi.org/10.1016/j.biomaterials.2006.08.028
Prasad M, Lee A, Gaffney MT (2012) A detailed chemical and nutrient characterisation of compost and digestate fibre including a comparative release of Nitrogen and Phosphorus. Dublin. West Pier Business Campus, Dún Laoghaire, Co, Ireland
Procházková P, Kalčíková G, Maršálková E, Zlámalová Gargošová H, Kučerík J (2024) Innovative approach for quantitative determination of ingested microplastics by Daphnia magna: use of differential scanning calorimetry and thermogravimetry. J Therm Anal Calorim. https://doi.org/10.1007/s10973-024-12985-0
Przygocka-Cyna K, Grzebisz W, Biber M (2018) Evaluation of the potential of bio-fertilizers as a source of nutrients and heavy metals by means of the exhaustion lettuce test. J Elementol. https://doi.org/10.5601/jelem.2017.22.4.1494
Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25(2):262–284. https://doi.org/10.1016/j.cmet.2016.12.022
R_Core_Team (2020) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing
Ranamukhaarachchi SL (2009) Soil dehydrogenase in a land degradation-rehabilitation gradient: observations from a savanna site with a wet/dry seasonal cycle. Rev Biol Trop 57(1–2):223–234
Rizzarelli P, Puglisi C, Montaudo G (2004) Soil burial and enzymatic degradation in solution of aliphatic co-polyesters. Polym Degrad Stab 85(2):855–863. https://doi.org/10.1016/j.polymdegradstab.2004.03.022
Roohi ZMR, Kuddus M (2018) PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polymers Adv Technol 29(1):30–40
Rosa DD, Rodrigues TC, Gracas Fassina Guedes CD, Calil MR (2003) Effect of thermal aging on the biodegradation of PCL, PHB-V, and their blends with starch in soil compost. J Appl Pol Sci 89(13):3539–3546
Rychter P, Biczak R, Herman B, Smylla A, Kurcok P, Adamus G, Kowalczuk M (2006) Environmental degradation of polyester blends containing atactic poly (3-hydroxybutyrate). Biodegradation in soil and ecotoxicological impact. Biomacromol 7(11):3125–3131
Sanchez-Hernandez JC, Capowiez Y, Ro KS (2020) Potential use of earthworms to enhance decaying of biodegradable plastics. ACS Sustain Chem Eng 8(11):4292–4316. https://doi.org/10.1021/acssuschemeng.9b05450
Sang BI, Hori K, Tanji Y, Unno H (2002) Fungal contribution to in situ biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) film in soil. Appl Microbiol Biotechnol 58(2):241–247. https://doi.org/10.1007/s00253-001-0884-5
Sang BI, Hori K, Unno H (2004) Comparison of the degradation characteristics of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in water and soil by isolated soil microorganisms. European Symposium on Environmental Biotechnology. Oostende, Belgium. p 327–30
Santagata G, Schettini E, Vox G, Immirzi B, Scarascia Mugnozza G, Malinconico M (2017) Biodegradable spray mulching and nursery pots: new frontiers for research. In: Malinconico M, editor Soil Degradable Bioplastics for a Sustainable Modern Agriculture. Springer Berlin Heidelberg, Berlin, Heidelberg. p 105–37
Savenkova L, Gercberga Z, Nikolaeva VJ, Dzene A, Bibers I, Kalnin M (2000) Mechanical properties and biodegradation characteristics of PHB-based films. Process Biochem 35(6):573–579
Shah AA, Hasan F, Hameed A, Ahmed S (2007) Isolation and characterisation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) degrading Actinomycetes and purification of PHBV depolymerase from newly isolated Streptoverticillium kashmirense AF1. Ann Microbiol 57(4):583–588. https://doi.org/10.1007/bf03175359
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24(13):2452. https://doi.org/10.3390/molecules24132452
Siebielec G, Siebielec S, Lipski D (2018) Long-term impact of sewage sludge, digestate and mineral fertilizers on plant yield and soil biological activity. J Clean Prod 187:372–379. https://doi.org/10.1016/j.jclepro.2018.03.245
Sudesh K, Abe H, Doi Y (2005) Synthesis structure and properties of polyhydroxyalkanoates: biological polyesters. Progress Polymer Sci 25(10):1503–1555
Suzuki S, Noble AD, Ruaysoongnern S, Chinabut N (2007) Improvement in water-holding capacity and structural stability of a sandy soil in Northeast Thailand. Arid Land Res Manag 21(1):37–49. https://doi.org/10.1080/15324980601087430
Tambone F, Adani F (2017) Nitrogen mineralization from digestate in comparison to sewage sludge, compost and urea in a laboratory incubated soil experiment. J Plant Nutr Soil Sci 180:355–365. https://doi.org/10.1002/jpln.201600241
Touchaleaume F, Martin-Closas L, Angellier-Coussy H, Chevillard A, Cesar G, Gontard N et al (2016) Performance and environmental impact of biodegradable polymers as agricultural mulching films. Chemosphere 144:433–439. https://doi.org/10.1016/j.chemosphere.2015.09.006
Trinchera A, Baratella V, Rinaldi S, Renzaglia M, Marcucci A, Rea E (2013) Greenhouse lettuce: assessing nutrient use efficiency of digested livestock manure as organic N-fertilizer. In: Proceedings of the II International Symposium on Organic Greenhouse Horticulture 1041, p 63–69, ISHS: Leuven, Belgium
Vogel FA, Schlundt C, Stote RE, Ratto JA, Amaral-Zettler LA (2021) Comparative genomics of marine bacteria from a historically defined plastic biodegradation consortium with the capacity to biodegrade polyhydroxyalkanoates. Microorganisms 9(1):27. https://doi.org/10.3390/microorganisms9010186
Vroman I, Tighzert L (2009) Biodegradable polymers. Materials 2(2):307–344. https://doi.org/10.3390/ma2020307
Wan Y, Wu C, Xue Q, Hui X (2019) Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci Total Environ 654:576–582. https://doi.org/10.1016/j.scitotenv.2018.11.123
WRB Soil Classification, ISBN 978-92-5-108369-7 (print), E-ISBN 978-92-5-108370-3 (PDF). Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 22 April 2021)
Y3000P (2023) Technical Data Sheet & Processing Guide for ENMAT™ Thermoplastics Resin Y3000P, 2023. Access date 2024/05/06, http://en.tianan-enmat.com/pdf/TDS_Y3000P.pdf
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147(1):201–222. https://doi.org/10.1046/j.1469-8137.2000.00687.x
Zar JH (1984) Biostatistical Analysis, 2nd edn. Prentice-Hall, Inc., New Jersey
Zhang T, Shi Y, Piao F, Sun Z (2018) Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult (PCTOC) 134:231–240. https://doi.org/10.1007/s11240-018-1415-8
Zhou J, Wen Y, Marshall MR, Zhao J, Gui H, Yang Y et al (2021a) Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci Total Environ 787:147444. https://doi.org/10.1016/j.scitotenv.2021.147444
Zhou J, Gui H, Banfield CC, Wen Y, Zang H, Dippold MA et al (2021b) The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol Biochem 156:108211. https://doi.org/10.1016/j.soilbio.2021.108211
Acknowledgements
This research was supported by the project FCH-S-24-8591 of Ministry of Education, Youth and Sports of the Czech Republic, by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO1224, MZE-RO1724.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.H., J.K., and M.B.; methodology, M.B., J.H., A.K., O.M., and A.M.; resources A.K., T.B., O.M., M.R., E.K. and A.M.; writing—original draft preparation, M.B., J.H.; visualization, T.H. and T.B.; supervision, J.K., and J.H.; project administration, M.B., A.K., and J.H. All authors contributed to the data interpretation, writing—review and editing and final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relation-ships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brtnicky, M., Holatko, J., Hammerschmiedt, T. et al. Effect of stabilized organic amendments on biodegradability of poly-3-hydroxybutyrate, soil biological properties, and plant biomass. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-06061-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-06061-1