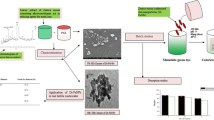
Abstract
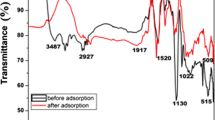
Waterborne hazardous organic pollutants, including malachite green (MG) dye, remain a major environmental concern due to their detrimental effects on ecosystems and humans. In the present paper, the MG dye removal from aqueous solutions by nickel oxide (NiO) is investigated; used NiO has been prepared using the precipitation method. Structural characterization and properties of the adsorbent were performed via various physicochemical methods such as X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, and N2 adsorption/desorption measurements. The results revealed that the maximum adsorption capacity and removal rate were 800 mg/g and about 99%, respectively. On the other hand, the adsorption kinetics follows the pseudo-second-order model. However, thermodynamics showed that: (i) ΔS° > 0 indicated an increase of disorder in the solid/liquid interface, (ii) ΔH° > 0 confirmed the endothermic nature of the adsorption process, and (iii) ΔG° < 0 demonstrated the spontaneous nature of MG adsorption. The calculated adsorption energies MCS/SAA EGas = − 409.638 kcal and EAqueous = − 416.856 kcal confirmed that the MG removal by adsorption on NiO is theoretically and experimentally favorable. In addition, MG molecules are adsorbed in a planar orientation on the NiO surface sites; the optimal interfacial contact is achieved. Finally, a mechanism was proposed to explain the adsorption of MG dye onto nickel oxide.
















Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdellaoui Y, Olguín MT, Abatal M, Ali B, Díaz Méndez SE, Santiago AA (2019) Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium- and sodium-forms. Superlattices Microstruct 127:165–175. https://doi.org/10.1016/j.spmi.2017.11.061
Abdellaoui Y, Abou Oualid H, Hsini A, El Ibrahimi B, Laabd M, El Ouardi M, Giácoman-Vallejos G, Gamero-Melo P (2020) Synthesis of zirconium-modified merlinoite from fly ash for enhanced removal of phosphate in aqueous medium: experimental studies supported by Monte Carlo/SA simulations. Chem Eng J 404:126600. https://doi.org/10.1016/j.cej.2020.126600
Abdellaoui Y, El Ibrahimi B, Abou Oualid H, Kassab Z, Quintal-Franco C, Giácoman-Vallejos G, Gemero-Melo P (2021) Iron-zirconium microwave-assisted modification of small-pore zeolite W and its alginate composites for enhanced aqueous removal of As(V) ions: experimental and theoretical studies. Chem Eng J 421:129909. https://doi.org/10.1016/j.cej.2021.129909
Abou Oualid H, Abdellaoui Y, Laabd M, Ouardi ME, Brahmi Y, Iazza M, Oualid JA (2020) Eco-efficient green seaweed codium decorticatum biosorbent for textile dyes: Characterization, mechanism, recyclability, and RSM optimization. ACS Omega. https://doi.org/10.1021/acsomega.0c02311
Abu Tarboush BJ, Husein MM (2012) Adsorption of asphaltenes from heavy oil onto in situ prepared NiO nanoparticles. J Colloid Interface Sci 378:64–69. https://doi.org/10.1016/j.jcis.2012.04.016
Afiqah N, Mohamad H, Biaw L, Lim L, Usman A (2019) Environmental technology & innovation enhancing adsorption of malachite green dye using base-modified Artocarpus odoratissimus leaves as adsorbents. Environ Technol Innov 13:211–223. https://doi.org/10.1016/j.eti.2018.12.002
Ahmad AA, Ahmad MA, Yahaya NKEM, Karim J (2021) Adsorption of malachite green by activated carbon derived from gasified Hevea brasiliensis root. Arab J Chem 14:103104. https://doi.org/10.1016/j.arabjc.2021.103104
Ali I, Alothman ZA, Alwarthan A (2017) Uptake of propranolol on ionic liquid iron nanocomposite adsorbent: kinetic, thermodynamics and mechanism of adsorption. J Mol Liq 236:205–213. https://doi.org/10.1016/j.molliq.2017.04.028
Al-Sehemi AG, Al-Shihri AS, Kalam A, Dud G, Ahmad T (2014) Microwave synthesis, optical properties and surface area studies of NiO nanoparticles. J Mol Struct 1058:56–61. https://doi.org/10.1016/j.molstruc.2013.10.065
Amuguni H, Mmari J, Mwanza P (2019) Brewers ’ spent grain in adsorption of aqueous congo red and malachite green dyes: Batch and continuous flow systems. J Hazard Mater 380:120897. https://doi.org/10.1016/j.jhazmat.2019.120897
Ba Mohammed B, Yamni K, Tijani N, Lee HS, Dehmani Y, El Hamdani H, Alrashdi AA, Ramola S, Belwal T, Lgaz H (2021) Enhanced removal efficiency of NaY zeolite toward phenol from aqueous solution by modification with nickel (Ni–NaY). J Saudi Chem Soc 25:101224. https://doi.org/10.1016/j.jscs.2021.101224
Behnajady MA, Bimeghdar S (2014) Synthesis of mesoporous NiO nanoparticles and their application in the adsorption of Cr(VI). Chem Eng J 239:105–113. https://doi.org/10.1016/j.cej.2013.10.102
Ben SK, Gupta S, Raj KK, Chandra V (2023) Adsorption of malachite green from polyaniline facilitated cobalt phosphate nanocomposite from aqueous solution. Chem Phys Lett 820:140469. https://doi.org/10.1016/j.cplett.2023.140469
Bhardwaj S, Sarkar T (2020) Core–shell type magnetic Ni/NiO nanoparticles as recyclable adsorbent for Pb (II) and Cd (II) ions: one-pot synthesis, adsorption performance, and mechanism. J Taiwan Inst Chem Eng 113:223–230. https://doi.org/10.1016/j.jtice.2020.08.011
Bourzi H, Oukhrib R, Ibrahimi B. El, Oualid HA, Abdellaoui Y, Balkard B, Hilali M, Issami S. El (2020) Understanding of anti-corrosive behavior of some tetrazole derivatives in acidic medium: adsorption on Cu (111) surface using quantum chemical calculations and Monte Carlo simulations. Surf Sci 702:121692. https://doi.org/10.1016/j.susc.2020.121692
Chadi A, Yani N, Bukhari F, Suah M (2020) International journal of biological macromolecules adsorption and desorption of malachite green by using chitosan-deep eutectic solvents beads. Int J Biol Macromol 164:3965–3973. https://doi.org/10.1016/j.ijbiomac.2020.09.029
Chen H, Liu T, Meng Y, Cheng Y, Lu J, Wang H (2020) Novel graphene oxide/aminated lignin aerogels for enhanced adsorption of malachite green in wastewater. Colloids Surf A 603:125281. https://doi.org/10.1016/j.colsurfa.2020.125281
Chen L, Mi B, He J, Li Y, Zhou Z, Wu F (2023) Functionalized biochars with highly-efficient malachite green adsorption property produced from banana peels via microwave-assisted pyrolysis. Bioresour Technol 376:128840. https://doi.org/10.1016/j.biortech.2023.128840
Choudhary M, Kumar R, Neogi S (2020) Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J Hazard Mater 392:122441. https://doi.org/10.1016/j.jhazmat.2020.122441
Claude V, Mahy JG, Lohay T, Tilkin RG, Micheli F, Lambert SD (2020) Sol–gel synthesis of Ni/Al2O3 catalysts for toluene reforming: Support modification with alkali, alkaline earth or rare-earth dopant (Ca, K, Mg or Ce). Surf Interfaces 20:100511. https://doi.org/10.1016/j.surfin.2020.100511
Dehbi A, Dehmani Y, Omari H, Lammini A, Elazhari K, Abdallaoui A (2019) Hematite iron oxide nanoparticles (α-Fe2O3): synthesis and modelling adsorption of malachite green. J Environ Chem Eng 8:103394. https://doi.org/10.1016/j.jece.2019.103394
Dehbi A, Dehmani Y, Omari H, Lammini A, Elazhari K, Abouarnadasse S, Abdallaoui A (2020) Comparative study of malachite green and phenol adsorption on synthetic hematite iron oxide nanoparticles (α-Fe2O3). Surf Interfaces 21:100637. https://doi.org/10.1016/j.surfin.2020.100637
Dehmani Y, Abouarnadasse S (2020) Study of the adsorbent properties of nickel oxide for phenol depollution. Arab J Chem 13:5312–5325. https://doi.org/10.1016/j.arabjc.2020.03.010
Dehmani Y, Sellaoui L, Alghamdi Y, Lainé J, Badawi M, Amhoud A, Abouarnadasse S (2020) Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay Younes Dehmani. J Mol Liquids 312:113383
Dehmani Y, Dridi D, Lamhasni T, Abouarnadasse S, Chtourou R, Lima EC (2022) Review of phenol adsorption on transition metal oxides and other adsorbents. J Water Process Eng 49:102965. https://doi.org/10.1016/j.jwpe.2022.102965
Eda Y (2020) Enhancement of adsorption capacity of reduced graphene oxide by sulfonic acid functionalization: malachite green and Zn (II) uptake. Karatas 256:123662. https://doi.org/10.1016/j.matchemphys.2020.123662
El Ibrahimi B (2020) Atomic-scale investigation onto the inhibition process of three 1,5-benzodiazepin-2-one derivatives against iron corrosion in acidic environment. Colloids Interface Sci Commun 37:100279. https://doi.org/10.1016/j.colcom.2020.100279
El Ibrahimi B, El Mouaden K, Jmiai A, Baddouh A, El Issami S, Bazzi L, Hilali M (2019) Understanding the influence of solution’s pH on the corrosion of tin in saline solution containing functional amino acids using electrochemical techniques and molecular modeling. Surf Interfaces 17:100343. https://doi.org/10.1016/j.surfin.2019.100343
El Ibrahimi B, Bazzi L, El Issami S (2020) The role of pH in corrosion inhibition of tin using the proline amino acid: theoretical and experimental investigations. RSC Adv 10:29696–29704. https://doi.org/10.1039/d0ra04333h
Eltaweil AS, Mohamed HA, El-monaem EMA (2020) Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv Powder Technol 31:1253–1263. https://doi.org/10.1016/j.apt.2020.01.005
Fan X, Deng L, Li K, Lu H, Wang R, Li W (2021) Adsorption of malachite green in aqueous solution using sugarcane bagasse-barium carbonate composite. Colloid Interface Sci Commun 44:100485. https://doi.org/10.1016/j.colcom.2021.100485
Feitoza U, Dos S, Thue PS, Lima EC, dos Reis GS, Rabiee N, de Alencar WS, Mello BL, Dehmani Y, Rinklebe J, Dias SLP (2022) Use of biochar prepared from the açaí seed as adsorbent for the uptake of catechol from synthetic effluents. Molecules 27:21758. https://doi.org/10.3390/molecules27217570
Fernandes DM, Hechenleitner AAW, Silva MF, Lima MK, Bittencourt PRS, Silva R, Melo MAC, Pineda EAG (2009) Preparation and characterization of NiO, Fe2O3, Ni0.04Zn0.96O and Fe0.03Zn0.97O nanoparticles. Mater Chem Phys 118:447–452. https://doi.org/10.1016/j.matchemphys.2009.08.016
Galvani GM, Zito CA, Perfecto TM, Malafatti JOD, Paris EC, Volanti DP (2022) Two-dimensional NiO nanosheets for efficient Congo red adsorption removal. Mater Chem Phys 290:126591. https://doi.org/10.1016/j.matchemphys.2022.126591
Ghafari M, Cui Y, Alali A, Atkinson JD (2019) Phenol adsorption and desorption with physically and chemically tailored porous polymers: mechanistic variability associated with hyper-cross-linking and amination. J Hazard Mater 361:162–168. https://doi.org/10.1016/j.jhazmat.2018.08.068
Guo F, Jiang X, Li X, Jia X, Liang S (2020) Synthesis of MgO/Fe3O4 nanoparticles embedded activated carbon from biomass for high-efficient adsorption of malachite green. Mater Chem Phys 240:122240. https://doi.org/10.1016/j.matchemphys.2019.122240
Hashemian S (2013) Fenton-like oxidation of malachite green solutions: kinetic and thermodynamic study. J Chem. https://doi.org/10.1155/2013/809318
Himanshu L, Chamoli S, Singh A, Kapoor RK, Singh S, Singh RK, Saini JK (2023) Purification and characterization of laccase from Ganoderma lucidum and its application in decolorization of malachite green dye. Bioresour Technol Rep 21:101368. https://doi.org/10.1016/j.biteb.2023.101368
Hosseini A, Karimi H, Foroughi J, Sabzehmeidani MM, Ghaedi M (2021) Heterogeneous photoelectro-Fenton using ZnO and TiO2 thin film as photocatalyst for photocatalytic degradation malachite green. Appl Surf Sci Adv 6:100126. https://doi.org/10.1016/j.apsadv.2021.100126
Javaid R, Qazi UY (2019) Catalytic oxidation process for the degradation of synthetic dyes: an overview. Int J Environ Res Public Health 16:1–27. https://doi.org/10.3390/ijerph16112066
Jawad AH, Abdulhameed AS (2020) Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surf Interfaces 18:100422. https://doi.org/10.1016/j.surfin.2019.100422
Kumar JP, Giri SD, Sarkar A (2018) ScienceDirect Mesoporous NiO with different morphology: synthesis, characterization and their evaluation for oxygen evolution reaction. Int J Hydrogen Energy 43:15639–15649. https://doi.org/10.1016/j.ijhydene.2018.06.097
Kundu A, Mondal A (2019) Kinetics, isotherm, and thermodynamic studies of methylene blue selective adsorption and photocatalysis of malachite green from aqueous solution using layered Na-intercalated Cu-doped Titania. Appl. Clay Sci. 183:105323. https://doi.org/10.1016/j.clay.2019.105323
Laabd M, Brahmi Y, El Ibrahimi B, Hsini A, Toufik E, Abdellaoui Y, Abou Oualid H, El Ouardi M, Albourine A (2021) A novel mesoporous Hydroxyapatite@Montmorillonite hybrid composite for high-performance removal of emerging Ciprofloxacin antibiotic from water: Integrated experimental and Monte Carlo computational assessment. J Mol Liq 58:116705. https://doi.org/10.1016/j.molliq.2021.116705
Li Y, Hu X, Liu X, Zhang Y, Zhao Q, Ning P, Tian S (2017) Adsorption behavior of phenol by reversible surfactant-modified montmorillonite: mechanism, thermodynamics, and regeneration. Chem Eng J. https://doi.org/10.1016/j.cej.2017.09.140
Lima EC, Hosseini-Bandegharaei A, Anastopoulos I (2019) Response to Some remarks on a critical review of the estimation of the thermodynamic parameters on adsorption equilibria Wrong use of equilibrium constant in the van’t Hoff equation for calculation of thermodynamic parameters of adsorption. J Mol Liquids 273, 425–434 (2019) DOI: https://doi.org/10.1016/j.molliq.2019.01.160
Lin L, Tang S, Wang X, Sun X, Yu A (2020) Adsorption of malachite green from aqueous solution by nylon microplastics: reaction mechanism and the optimum conditions by response surface methodology. Process Saf Environ Prot 140:339–347. https://doi.org/10.1016/j.psep.2020.05.019
Makhado E, Motshabi BR, Allouss D, Ramohlola KE, Modibane KD, Hato MJ, Jugade RM, Shaik F, Pandey S (2022) Development of a ghatti gum/poly (acrylic acid)/TiO2 hydrogel nanocomposite for malachite green adsorption from aqueous media: Statistical optimization using response surface methodology. Chemosphere 306:135524. https://doi.org/10.1016/j.chemosphere.2022.135524
Mandal A, Das SK (2019) Journal of Environmental Chemical Engineering Phenol adsorption from wastewater using clari fi ed sludge from basic oxygen furnace. J Environ Chem Eng 7, 103259 DOI: https://doi.org/10.1016/j.jece.2019.103259
Mehraban Z, Farzaneh F, Shafiekhani A (2007) Synthesis and characterization of a new organic – inorganic hybrid NiO – chlorophyll- a as optical material. Optical Mater 29:927–931. https://doi.org/10.1016/j.optmat.2006.02.007
Michel CR, Martínez-Preciado AH (2023) Adsorption and photocatalytic degradation of Congo red and malachite green by nanostructured Y2O3 synthesized by the coprecipitation method. Open Ceram 13:100336. https://doi.org/10.1016/j.oceram.2023.100336
Modwi A, Abbo MA, Hassan EA, Al-duaij OK, Houas A (2017) Journal of environmental chemical engineering adsorption kinetics and photocatalytic degradation of malachite green (MG) via Cu/ZnO nanocomposites. J Environ Chem Eng 5:5954–5960. https://doi.org/10.1016/j.jece.2017.11.024
Moosavi A, Amooey AA, Alinejad A, Marzbali MH (2020) Chinese journal of chemical engineering extraordinary adsorption of acidic fuchsine and malachite green onto cheap nano-adsorbent derived from eggshell. Chin J Chem Eng 28:1591–1602. https://doi.org/10.1016/j.cjche.2020.02.031
Oukhrib R, Abdellaoui Y, Berisha A, Abou Oualid H, Halili J, Jusufi K, Ait El Had M, Bourzi H, El Issami S, Asmary FA, Parmar VS, Len C (2021) DFT, Monte Carlo and molecular dynamics simulations for the prediction of corrosion inhibition efficiency of novel pyrazolylnucleosides on Cu(111) surface in acidic media. Sci Rep 11:82927. https://doi.org/10.1038/s41598-021-82927-5
Patil PS, Kadam LD (2002) Preparation and characterization of spray pyrolyzed nickel oxide (NiO) thin films. Appl Surf Sci 199:211–221. https://doi.org/10.1016/S0169-4332(02)00839-5
Raval NP, Shah PU, Shah NK (2017) Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl Water Sci 7:3407–3445. https://doi.org/10.1007/s13201-016-0512-2
Ren X, Zhai Y, Zhou Q, Yan J, Liu S (2020) Fabrication of nanoporous Ni and NiO via a dealloying strategy for water oxidation catalysis. J Energy Chem 50:125–134. https://doi.org/10.1016/j.jechem.2020.03.020
Rogozea EA, Petcu AR, Olteanu NL, Lazar CA, Cadar D, Mihaly M (2017) Tandem adsorption-photodegradation activity induced by light on NiO-ZnO p–n couple modified silica nanomaterials. Mater Sci Semicond Process 57:1–11. https://doi.org/10.1016/j.mssp.2016.10.006
Rubio-clemente A, Gutiérrez J, Henao H, Melo AM, Pérez JF, Chica E (2021) Journal of King Saud University – engineering sciences adsorption capacity of the biochar obtained from Pinus patula wood micro-gasification for the treatment of polluted water containing malachite green dye. J King Saud Univ Eng Sci. https://doi.org/10.1016/j.jksues.2021.07.006
Saad AM, Abukhadra MR, Abdel-Kader Ahmed S, Elzanaty AM, Mady AH, Betiha MA, Shim JJ, Rabie AM (2020) Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J Environ Manage 258:110043. https://doi.org/10.1016/j.jenvman.2019.110043
Saleh AM, Hassan YM, Selim S, AbdElgawad H (2019) NiO-nanoparticles induce reduced phytotoxic hazards in wheat (Triticum aestivum L.) grown under future climate CO2. Chemosphere 220:1047–1057. https://doi.org/10.1016/j.chemosphere.2019.01.023
Sharma P, Laddha H, Agarwal M, Gupta R (2022) Selective and effective adsorption of malachite green and methylene blue on a non-toxic, biodegradable, and reusable fenugreek galactomannan gum coupled MnO2 mesoporous hydrogel. Microporous Mesoporous Mater 338:111982. https://doi.org/10.1016/j.micromeso.2022.111982
Sun L, Hu S, Sun H, Guo H, Zhu H, Liu M, Sun H (2015) Malachite green adsorption onto Fe3O4@SiO2-NH2: isotherms, kinetic and process optimization. RSC Adv 5:11837–11844. https://doi.org/10.1039/c4ra13402h
Villanueva ME, Salinas A, Copello GJ, Díaz LE (2014) Point of zero charge as a factor to control biofilm formation of Pseudomonas aeruginosa in sol-gel derivatized aluminum alloy plates. Surf Coatings Technol 254:145–150. https://doi.org/10.1016/j.surfcoat.2014.05.074
Wu J, Yang J, Feng P, Wen L, Huang G, Xu C, Lin B (2022) Highly efficient and ultra-rapid adsorption of malachite green by recyclable crab shell biochar. J Ind Eng Chem 113:206–214. https://doi.org/10.1016/j.jiec.2022.05.047
Yasin Y, Hussein M, Ahmad Amat F (2007) Adsorption of methylene blue onto treated activated carbon. Malays J Anal Sci 11:400–406
Zhang K, Monteiro MJ, Jia Z (2016) Stable organic radical polymers: synthesis and applications. Polym Chem 7:5589–5614. https://doi.org/10.1039/c6py00996d
Zhang K, Li H, Xu X, Yu H (2017) Synthesis of reduced graphene oxide/NiO nanonanocomposites for the removal of Cr(VI) from aqueous water by adsorption. Microporous Mesoporous Mater 255:7–14. https://doi.org/10.1016/j.micromeso.2017.07.037
Zhang K, Li H, Xu X, Yu H (2018) Synthesis of reduced graphene oxide/NiO nanocomposites for the removal of Cr(VI) from aqueous water by adsorption. Microporous Mesoporous Mater 255:7–14. https://doi.org/10.1016/j.micromeso.2017.07.037
Zhao S, Xie H, Tang X, Lu G, Zhang Y (2023) Oxidized dextran-crosslinked ferrocene-chitosan-PEI composite porous material integrating adsorption and degradation to malachite green. Carbohydr Polym 312:120770. https://doi.org/10.1016/j.carbpol.2023.120770
Acknowledgements
The authors would like to thank Dr. Youness Abdellaoui the Universidad Autónoma de Yucatán, Mérida, Mexico. The work is supported by National Natural Science Foundation of China (52305103), Changsha Natural Science Foundation Project (kq2208025), Distinguished Young Scholars Fund of National Natural Science Foundation of China (52025082) and Key Support Project of National Natural Science Foundation of China - "Ye Qisun" Science Foundation (U2141242).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The preparation of the material, data collection, and analysis were carried out by Y.D., Y.B., R.O., A.D., M.A.E.H., and I.A. The first version of the manuscript was written by Y.D., Y.B., R.O., A.D., M.A.E.H., I.A., B.E.I., R.C., Y.B., T.L., A.A., G.G-V., A.S., and E.C.L. All authors have commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consentfor publication
Not applicable.
Ethics approval and consentto participate
Not applicable.
Additional information
Editorial responsibility: Palanivel Sathishkumar.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dehmani, Y., Bengamra, Y., Aadnan, I. et al. Efficient removal of malachite green dye onto nickel oxide-based adsorbent: experimental and theoretical approaches. Int. J. Environ. Sci. Technol. 21, 3037–3052 (2024). https://doi.org/10.1007/s13762-023-05153-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05153-8