Abstract
Nanomaterials (NMs) are nanoscale materials of different shapes that are made of a variety of materials such as carbon, silicon, and transition metals. Wastewater treatment plants and nano-enabled consumer products have been identified as major sources of nanomaterials in the environment. Findings from many research studies revealed nanomaterials to be toxic to non-targeted species. Despite presenting an ecological risk to the environment and human health, little is known about the occurrence, formation, transport, and environmental impacts, owing to limited regulated quantitative and sensitive techniques for their assessment. More also, the realisation of nanomaterials' impact on the environment depends on emerging analytical methods. Therefore, it is paramount to continuously develop and optimise analytical methods that are promising to sensitively detect and quantify the nanomaterials in trace and ultra-trace levels in the environment. In this review, we discuss engineered metal/carbonaceous nanomaterials; production, pathways, fate, impact, toxicity, and their analytical methods of detection and quantification from the current literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials (NMs) are nanoscale materials of different sizes and morphologies. They exist in the environment naturally (Lespes et al. 2020) or as a result of the release of engineered nanomaterials of different morphology such as tubes, particles, wires, and sheets of nanometre order sizes (1–100 nm) into the environment, by humans or via industrial processes (Farré et al. 2009; Wang et al. 2020). One of the most recent promising and advanced environmental applications of nanotechnology has been in water treatment and remediation (Shon et al. 2007; Hotze and Lowry 2010). Different NMs are used in water purification through different mechanisms including the adsorption of heavy metals and other pollutants (Pan et al. 2009; Mahmoud et al. 2015), for the inactivation of pathogens and transformation of the toxic contaminants into less toxic compounds (Pan et al. 2009). Nanotechnology has transformed science, technology, engineering, and different other fields (Hotze and Lowry 2010; Ghasemzadeh et al. 2014).
Sources of nanomaterials in the environment are diverse and include natural occurrences such as forest fires, volcanic eruptions, dust storms, and soil erosion; and unintentional releases like vehicle exhaust, fossil fuel burning, mining, welding, and industrial processes. Engineered nanomaterials (ENMs) are manufactured for different applications in engineering (Saleem and Zaidi 2020), biomedical imaging, and drug delivery (Choi et al. 2017; Kabir et al. 2018). Of particular interest among many ENMs are nano-plastics, organic nanomaterials, and metal-engineered nanoparticles (Fig. 1) which are all considered contaminants of emerging concerns (CECs). The CECs have recently drawn the attention of many researchers globally (Donovan et al. 2016).
Metal ENPs based on titanium dioxides, silicon dioxide, cerium oxide, and iron oxides have been widely produced and incorporated into NMs. Due to the demand in the industry for their different uses and applications (Sengul and Asmatulu 2020; Sun et al. 2022). Hence, the understanding of the variation in metals in ENPs is necessary and their monitoring in the environment is of paramount importance. The behaviour of metal ENMs in different environmental matrixes is currently understudied. It is important to study these ENPs since they have significant negative effects on our ecosystem (Wang et al. 2022). Engineered NMs occur in the environment in different forms, and certain classes dominate our ecosystems. However, the occurrence and fate of these ENMs are poorly understood, and the methods currently used to analyse them have some limitations. This review aims to show the current research on the nature of ENMs in ecosystems and the analytical methods used thus far, for the analysis of these materials.
Materials and methods
Metal-engineered nanoparticles
Metal-engineered nanoparticles are anthropogenic microscopic particles whose dimensions are about 1–100 nm (Simonet and Valcárcel 2008; Navratilova et al. 2015; Rabajczyk 2016; Mahlalela et al. 2017). In the environment, metal ENPs coexist together with natural nanoparticles (NNPs). NNPs are produced from natural biochemical and geochemical processes, and they display similar physicochemical properties, sizes, and shapes as ENPs (Banfield and Zhang 2001; Hochella Jr, 2008; Hochella et al. 2008; Theng and Yuan 2008). This inadvertently poses a challenge of identifying and characterising metal ENPs in the environment. Consequently, detection and characterisation have been done only on few metal nanoparticles, which are only a small fraction of NNPs and ENPs compared with the quantity present in the environment. More also, exposure to these metal NNPs/ENPs in the environment is inevitable; hence, assessing the risks associated with them has become a major priority to researchers.
It is imperative to distinguish between the NNPs and ENPs in the environment for exposure risk assessment studies, in order to determine separately the impact of nanoparticles fabricated by humans. Gondikas et al. (Gondikas et al. 2018) suggested that the concentration could be used as a factor to distinguish between the NNPs and the ENPs in the environment, where the size and composition are the same. It has also been suggested by Kabir et al. (Kabir et al. 2018) that analytical method development to assess exposure to metal ENPs and effect on the environment must be a requirement for their production from industries, due to the bioaccumulation and toxicity concerns associated with the transportation and transformation of nanoparticles in the environment. Unfortunately, nanotechnology health is not prioritised when solving social issues pertaining to ENPs, as result of the huge economic impact it may have on the general public (Kabir et al. 2018). ENPs are fabricated in the industry in abundance for different purposes, without proper life cycle assessment (Som et al. 2011). Many contemporary products contain metal ENPs, and this has led to their entering the food chain. The production process depends on desired application, which determines the reaction mechanism and synthesis route of metal ENPs.
Synthesis of metal-engineered nanoparticles
Engineered nanoparticles have been synthesised using bottom-up and top-down approaches through chemical or mechanical means. The most commonly used method for synthesis is chemical reduction in metal salts (Hassellöv et al. 2008; Navratilova et al. 2015; Choi et al. 2017; Luo et al. 2018). (Khan et al. 2016), developed a method for the synthesis of copper nanoparticles using a reduction method. They reduced copper salts to copper nanoparticles using ascorbic acid. The synthesis reaction required heating at 80 °C and stirring for 30 min, and the reaction completion was indicated by the colour change. The resulting solution was left overnight to settle at room temperature, the supernatant was discarded, and the product was washed with ethanol to remove excess ascorbic acid. The copper nanoparticles had a diameter of 28.73 nm, and their successful synthesis was confirmed using scanning electron microscopy (SEM), energy-dispersive x-ray (EDX), and x-ray diffraction (XRD).
Milam (Milam 2010) synthesised silver (Ag) nanoparticles using different methods by varying reducing agents. In the first method, AgNO3 was reduced by NaBH4 in the presence of 0.35 polyvinyl alcohol as capping agent. The NaBH4 was kept on ice to prevent decomposition and was added dropwise. The second method was the citrate method: which was done at a high temperature (100 °C), where AgNO3 was reduced using sodium citrate on a hot plate. The solution turned faint yellow at 100 °C, indicating the formation of AgNPs. The third method used is called a 1:6 borohydride/silver ratio, where AgNO3 was added dropwise for 2–4 min with continuous stirring. The fourth method used was seed growth method, in which two different reducing agents, ascorbic acid and sodium citrate, were added to AgNO3 solution to increase the size of AgNPs. The progress of the reaction was monitored by colour change; yellow colour indicated the formation of AgNPs, clear solution indicated the reaction failure to form AgNPs, and a grey murky colour indicated that the occurrence of side reaction due to excessive reduction and aggregation of AgNPs. The size of nanoparticles fabricated using reducing agents, such as NaBH4 polyvinyl alcohol as capping agent (5–10 nm), citrate (20–30 nm), the 1borohydride:6silver (5–15 nm), ascorbic acid and NaBH4–AgNPs (60–80 nm), was obtained by transmission electron microscopy (TEM). The AgNPs were of spherical shapes, with absorbance at 380–460 nm using ultraviolet–visible spectrometer (UV–vis spectroscopy).
Gumbi et al. (Gumbi et al. 2014) also synthesised AuNPs using the citrate reduction method. Tri-sodium citrate was added to the boiling gold salt solution (100 °C) under constant stirring. The colour of the solution changed from yellow to colourless and to deep red indicating the formation of AuNPs. The synthesised nanoparticles were characterised using TEM and UV–vis. The AuNPs were spherical in shape with an average diameter of 27 nm, and the plasmonic peak of AuNPs was at 526 nm. Piella et al. (Piella et al. 2016) synthesised citrate-stabilised gold nanoparticles between the sizes 3.3 nm and 10 nm by stepwise seed growth method. The synthesis of the 3.5 nm was done under controlled conditions at 70 °C and pH of 8. Also, the synthesised AuNPs were characterised using TEM, HRTEM (spherical), UV–vis, and EDX.
Hendricks et al. (Hendricks et al. 2022) investigated AuNPs in a model to determine the number of metal nanoparticles using ICP-OES. AuNPs were synthesised by chemical reduction method, using different reducing agents. The AuNPs synthesised using NaBH4 as reducing agent had a diameter of less than 10 nm; ascorbic acid (ASCB) as reducing agent had diameter between 5 and 15 nm, while trisodium citrate (TSC) reducing agent had an average diameter of 20 nm. The seed growth method using NaBH4–ASCB–TSC had particle sizes greater that 50 nm. These AuNPs were used to develop a model; TSC-AuNPs were used to find the model that uses shape and size to find the number of nanoparticles in a sample.
In general, the size, shape, and morphology of ENPs depend on the synthesis method used, the reducing agent type used, synthesis temperature, and the pH of the reaction solution.
Application of metal-engineered nanoparticles
Nanoparticles are acknowledged for their useful applications in society due to their unique properties such as high specific surface area. Nanoparticles are heavily used in daily products such as cosmetics, personal care products, food, and for drug delivery (Navratilova et al. 2015; Zhang et al. 2015; Choi et al. 2017). For instance, titanium dioxide nanoparticles (TiO2NPs) are commonly used in a variety of food, personal care, and other consumer products as additives. It was reported that TiO2NPs enters the environment after application as organic manure onto agricultural land. Also, titanium is constituent of those nanoparticles that result from the incineration of solids waste in landfills. Furthermore, food candies, sweets, and chewing gums were found to contain a high content of TiO2NPs. Brar et al. (Brar et al. 2010) reported that nanotechnology was applied widely in environmental, industrial, and agricultural sectors. These applications range from fabrication of molecular assemblies to microbial array chips. Variety of nanoparticles that are present in commercial products and industrial raw materials enter wastewater treatment plants (WWTP) facilities through sewage, and end up in wastewater effluent and sludge. Some of these nanoparticles were introduced in the WWTP, via incorporation into activated carbon for absorption and removal of pathogens.
The addition of nanoparticles to cosmetics has been reported to improve the quality of ingredients and for better maintenance of the skin (Katz et al. 2015). Nanoparticles are also added into formulations to stabilise components that can decay due to oxidation. TiO2 and ZnO nanoparticles are frequently employed in personal care products such as sunscreens and in most non-prescription drug products (Katz et al. 2015). These nanoparticles are desired due to being excellent UV radiation blockers. Moreover, silver nanoparticles are utilised in consumable products because of their anti-bacterial or preservative properties (Katz et al. 2015).
Ovissipour et al. (Ovissipour et al. 2013) reported that the growing utilisation of metal ENPs can result in the contamination of the aquatic system, which might influence the risk to living organisms in the aquatic environment. Their fastest growing utilisation has been in medicine whereby they are used in drug formulations, body visualisation, ageing prevention in cosmetic lotions, and pharmaceuticals in general. In energy industries, they are used for generation of power and for data storage (Silva et al. 2011). Copper nanoparticles are utilised in manufacturing industries for extension of conductivity, as fillers, expanding the wear resistance, decreasing friction, and inflexible in the material (Pham et al. 2012; Ovissipour et al. 2013; Navratilova et al. 2015; Khan et al. 2016). They also behave as stimulants on activated carbons in the removal of nitrate from waste stream to prevent their escape into the aquatic environment (Asl et al. 2016). Copper nanoparticles have the binary magnitude to behave as an imperative co-factor and bio-stimulants with a critical balance for proper intracellular metal homeostasis and metabolism, though it has been implicated in triggering certain diseases (Usman et al. 2013; Zazo et al. 2016).
Application of metal-engineered nanoparticles in water treatment
In particular, the application of metal ENPs such as silver nanoparticles (AgNPs), iron nanoparticles (FeNPs), titanium oxide nanoparticles (TiO2NPs), zinc oxide nanoparticles (ZnO2NPs), and iron oxide nanoparticles (FeO2NPs) in water and wastewater treatment has gain wide attention (Roy et al. 2021; Rajput et al. 2023). The antimicrobial properties of AgNPs have made them popular disinfectant agents in water treatment. Silver nanoparticles are mainly incorporated in nanomembrane for effective removal of E. coli and prevention of fouling due to it toxicity towards viruses, fungi, and bacteria (Mahjoubian et al. 2023; Xu et al. 2023). However, the mechanism of the antimicrobial effects of AgNPs is not well understood, but several theories describing disinfection process have been put forward (Husain et al. 2023; Khalil et al. 2023; Wen et al. 2023). AgNPs attach to the cell wall and eventually enter to cause the cell membrane structural damage. Inside the cell, it is thought to generate free radical and interact with thiol groups of many vital enzymes, which leads to disruption of normal function eventual cell death. Application of AgNPs has drawbacks due to their tendency to aggregate in water. Hence, in many cases AgNPs are incorporated in membrane or ceramic membranes for filtration (Latwal et al. 2023). Therefore, for water contaminated with bacteria filtration through membrane decorated with AgNPs exhibits strong antimicrobial properties and this might be the future of water treatment (Behboudi et al. 2023).
Iron nanoparticles are the most studied zero-valent metal ENPs among Zn, Al, and Ni in water treatment due to their moderate standard potential to act as reducing agents relative to many redox-labile contaminants (Okpara et al. 2023). Aluminium and nickel are unstable in water, likely to aggregates and form oxides. Although Ni has suitable standard potential for water treatment, FeNPs have more advantages over NiNPs because of excellent adsorption properties, precipitation, and oxidation (Ramadevi et al. 2023). The mechanisms in which FeNPs work are based on the oxidation–reduction redox reaction between zero-valent and contaminants, depend Fe2+, and can be oxidised to Fe3+. If the pH is increased, Fe(OH)3 can be formed, which can facilitate removal of contaminants. In the presence of dissolved oxygen, zero-valent can transfer two electrons to O2 to produce H2O2 which reacts with Fe2+ (fenton reaction); this can generate hydroxyl radicals (HO.) which have strong oxidising ability towards a wide range of organic compounds. Zero-valent metal ENPs have already applied in removal of heavy metal organic dyes, phenols, inorganic anions, and nitrates (Li et al. 2022; Mantovani et al. 2023). Application of zero-valent metal ENPs industrial scale has limitations due to aggregation, oxidation, and separation difficulties in a aging system (Barka et al. 2023). Doping with other metal has been solution to aggregation challenges in water treatment application (Haribhau Waghchaure et al. 2022).
Photocatalytic degradation is a best method at present for the removal of pollutant from wastewater process. Among studied, metal oxide ENPs such as zinc oxide (ZnO2), iron oxide (FeO2), and titanium oxide (TiO2) are extensively investigated because of their photocatalytic activity, cost-effectiveness, and stability (Haribhau Waghchaure et al. 2022; Pandey and Saha 2023). These catalysts when come in contact with contaminants gradually oxidise them into low molecular weight products like CO2, H2O, and NO. Many metal oxides ENPs photocatalyst requires ultraviolet excitation to induce charge separation within particles. This process generates reactive oxygen species and hydroxyl radicals which give photocatalysts the ability to damage the function and structure of various cells. However, the energy band gap is a disadvantage as this makes many metal oxide ENPs to need light to work. The activity under visible light is reduced; hence, many metal oxide photocatalysts need metal doping to improve adsorption of light (Rajput et al. 2023). Also, production of metal oxide complicated and their removal in wastewater process is cumbersome, especially when applied in suspension. Incorporation of metal oxide ENPs in membrane provided a temporary solution to these outlined problems (Behboudi et al. 2023).
Emission pathways of metal-engineered nanoparticles to the environment
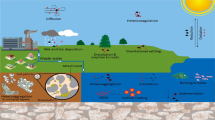
ENPs are produced as a result of human needs and utilised in operation such WWTP processes, from where they are introduced in different environmental matrices such as dumpsites, soil, and water. Peters et al. (Peters et al. 2018) reported the sources and pathways of NPs, namely manufacturing, industrial application, and wastewater treatment plants, from where they may enter food chain. Metal nano-abled products include personal care products (PCPs) such as cosmetics, sunscreens and gels, construction materials; paints; and cement (Keller et al. 2013). These products are used on the daily bases, and their residues are released into sewerage and waterways; hence, there are high chances for it to negatively affect animals and plants in different ecosystems, due to accumulation in different environmental matrices, and even the humans. The emission of ENPs into the environment and its distribution pathways are depicted in Fig. 2.
Engineered nanoparticles are classified as emerging pollutants (Hassellöv et al. 2008; Silva et al. 2011; Navratilova et al. 2015; Luo et al. 2018). While their production is intended for specific applications, some portion may unintendedly end up in the environment through many different ways, with potential to cause harmful effects (Hassellöv et al. 2008; Simonet and Valcárcel 2008; Silva et al. 2011; Polesel et al. 2018). The release of engineered metal nanoparticles such as TiO2 in the environment directly affects microorganisms in the aquatic environment. They can be transported to WWTPs from surface waters, sewage, or lakes where they were initially introduced. The aggregation of this nanomaterial occurs due to their increased stability in the presence of dissolved organic matter (Gondikas et al. 2018).
Disposal in landfill and illegal dumping may introduce metal ENPs in the environment as shown in Fig. 2. Also, traces of metal ENPs such as catalysts used in the production of other chemicals may leach into and remain in the products and enter the environment upon product’s disposal (Simonet and Valcárcel 2008; Silva et al. 2011). In WWTPs, nanoparticles that are used for the treatment of water are not entirely removed during the treatment processes (Simonet and Valcárcel 2008). This may lead to nanomaterials reaching drinking water reservoirs such as rivers and dams (Silva et al. 2011; Donovan et al. 2016). Furthermore, reservoirs near landfills or dumpsites may be affected due to runoffs containing NMs from these sites (Simonet and Valcárcel 2008; Silva et al. 2011). Compost and sludge containing engineered nanoparticles used in agriculture may also be carried with surface runoffs into adjoining water bodies.
Domercq et al. (Domercq et al. 2018) reported that, to assess the impact of nanoparticles in urban areas, all possible ENPs emission sources must be identified and documented. These possible sources include households, industries, hospitals, and land cover. It was noted that household products including cleaning products, cosmetics, clothing, and food additives are used on daily bases, from which the wastes discarded end up in wastewaters, with a potential to release ENPs to the environment (Keller and Lazareva 2014). However, industries are considered as the most likely source of ENPs in the urban area environment. This is because manufacturing industries discharge effluent directly into nearby ponds/rivers, and runoffs from the industries further contaminate surface waters. ENPs which are most likely to be present in discharges from the manufacturing industries include catalysts from fuel additives and cleaning products (Domercq et al. 2018).
Also, the emission of nanomaterials from industries could occur during production, application, and recycling processes (Levin et al. 2015). The amount assimilated into a product determines the amount of ENMs that will be released into the environment. For instance, cosmetics which contain high amount of TiO2, silica, and aluminium oxide are anticipated to be released into the environment due to the high rate of the product usage (Levin et al. 2015).
The physical state of materials and the resulting energy from certain processes are the factors influencing mechanisms of ENMs emission pathways, which determine the type of ENMs released in the process. The physical state includes powder, suspension, and solid. For powders, the concentration of water vapour present in the air and the liquid substances in the environment control their release levels.
Occurrence and behaviour of metal-engineered nanoparticles in the environment
Little has been published on the occurrence, fate, and behaviour of engineered nanoparticles in the environment. This could partly be attributed to expensive analytical methods required to effectively detect, quantify, and characterise them. There is therefore a need to develop less expensive analytical methods that are sensitive and robust to detect ENMs in the environment. Engineered nanoparticles in the environment are present in low concentrations ranging from few ng L−1 to µg L−1, which make their detection difficult due to matrix complexity (Hassellöv et al. 2008). ENPs are continuously produced based on needful exigencies, and this has led to increased discharge into the environment. The distribution pathways and behaviour of these ENPs are illustrated in Fig. 3. They reach the aquatic environment and soil where they change their form either by agglomerating or degrading, followed by transport to the living organisms (exposure) (Giese et al. 2018).
In a study conducted by Silva et al. (Silva et al. 2011), it was observed that aggregated engineered nanoparticles are the dominant form of NP contaminants in surface waters. These contaminants do not accumulate in river sediments due to their size dimension, thus making them hydrodynamic and thereby remaining in suspension in water flow. The availability of soluble substances which can interact with natural organic materials (NOM) in aquatic systems can cause agglomeration and contribute to the occurrence of high ENPs levels in the environment. Engineered nanoparticles behave differently in water due to their properties, synthesis route taken, and components of the environmental matrices. Properties that affect the fate of ENPs the most in the environment include their size, dispensation, catalytic activities, and the pollutants that coexist together with engineered nanoparticles in the aquatic system (Silva et al. 2011).
Kaegi et al. (Kaegi et al. 2013) concluded that engineered nanomaterials in the environment occur in different species and are controlled by environmental conditions. They noted as an example that silver exists in the environment as Ag (0) and can be transformed into a variety of species such as Ag, Ag2O, and Ag2S nanoparticles. It was found that AgNPs released into sewage that contain sulphur resulted in a formation of Ag2S during the wastewater treatment process (Kaegi et al. 2013). Bolyard et al. (Bolyard et al. 2013) also reported high release and disposal of nanomaterials containing products in the landfill. Landfill leachates have many elements that can influence the behaviour of the nanoparticles in landfill sites. For instance, landfill materials change with time due to degradation, but organic matter as a component of leachates takes a longer time to biodegrade (Bolyard et al. 2013). Titanium is one of the ten abundant elements that are reportedly found incorporated in other NMs in the environment (Gondikas et al. 2018).
The production, use, and disposal of nanomaterials with nanomaterial-containing products result in their presence in the environment. Although they may subsist in their original forms or as a modified version of the original materials, their environmental behaviour and fate are not well known. Lead and Wilkinson (Lead and Wilkinson 2006) were of the opinion that the behaviour and fate of nanomaterials in the environment can be drawn from the knowledge of the science of colloids. Accordingly, the two major primary factors that may predispose the fate and behaviour of nanomaterials in the environment are their physical and chemical characteristics and that of the receiving environment (Chen and Burda 2008; Saleh et al. 2008; Tiraferri et al. 2008). A review report presented by Klaine et al. (Klaine et al. 2008) provided an informative insight into the fate and behaviour of nanomaterials in the environment. A general scheme of ultra-small-sized particles is aggregation/agglomeration with one and other with dissolved/colloidal particles within the environmental matrices in which they occur. This may be followed by nanomaterial speciation where they interact with ionic or molecular dissolved chemical substances, disaggregation/deagglomeration, biological/chemical transformation, silting/settling, or complete mineralisation.
Environmental impact, toxicity, and transformation of metal-engineered nanoparticles
ENPs tend to persist in the environment due to the properties they possess; this causes negative environmental impacts (Mueller and Nowack 2008; Silva et al. 2011). There has been some reported negative health impact due to critical exposure to ENPs. Indirect effect of ENPs in the environment results from the interaction of engineered nanoparticles with natural organic materials. This interaction varies depending on where they occur, i.e., in biological systems or in the environment, and the occurrence of nutrients in the biosystem or environment (Simonet and Valcárcel 2008). The increase in engineered nanoparticles reaching the aquatic environment negatively impacts aquatic organisms/ecology due to the alteration of nutrients supply needed for their existence/living. Exposure to ENPs can lead to different types of toxicity. It may result into immediate fatality (acute exposure) or chronic exposure (Giese et al. 2018) as a result of persistent exposure that may fatally affect organisms in a long run. More also, pollutants in the environment make nanoparticles readily bioavailable. Exposure of some organisms to ENPs can result in changes in metabolic processes of the exposed living organisms, causing unreactive systems and adverse biological effects (Simonet and Valcárcel 2008; Ovissipour et al. 2013). Bioavailable nanoparticles can also affect inhibitor resistance mechanism leading to processes that will cause cell death on the living tissue (an events called apoptosis) and necrosis (Garner et al. 2017).
ENPs negative effect on aquatic environment organisms involves critical subjection, persistence subjection, fish cell line culture, food chain, and dietary exposure (Ovissipour et al. 2013). ENPs bind to certain hormones/proteins or other physiological components of an organism resulting in certain malfunctions, such as morphological malformation that causes wounds, spinal abnormalities, and heart malformations. Erosion of the intestinal epithelia in fishes has been observed in the food chain and dietary exposure studies. Variation in the surface charge, cluster formation of ENPs, and the ability to adhere onto the surface of the tissues are affected by the pH, ionic strength, concentration of divalent cations, and composition (Ovissipour et al. 2013).
Garner et al. (Garner et al. 2017) reported that ENMs can accumulate as nanoparticles in the environment to enough concentrations that exceed the minimum toxic threshold in freshwater and some soils, and this may arise as a result of high manufacturing of ENMs such as TiO2 and ZnO. This study showed that the negative impact of ENPs on the environment and whether they will cause acute or chronic effect in the organism depend on the concentration levels of these ENPs in the environment.
In a study conducted to assess the ecotoxicological impact of chronic exposure to ENPs in the marine wildlife (Matranga and Corsi 2012), it was observed that the exposure of ENPs to invertebrates and fishes in the marine environment could have adverse effects on these organisms. However, there is limited knowledge on their ecotoxicological impacts in river and the marine ecosystems; hence, ENPs are considered as emerging class of pollutants. There is also a need to model their environmental behaviour and concentration.
Metal ENPs can be transformed in different matrices as a result of variety of factors. In soil matrices, parameters such as the temperature, pH, ionic strength, water content, and organic matter influence the transformation of ENPs (Santiago-Martín et al. 2016; Kumar et al. 2022). These factors change the surface and structure of ENPs, including the net charge. Factors such as pH and water content influence the enhancement of the decoupling and breaking of the ENPs composition, thus freeing the ions (Kabata-Pendias 2004; Navarro et al. 2008). In aquatic environment, factors such as light, temperature, ionic strength, and pH affect the transformation of ENPs (Chen et al. 2012). Factors such as pH in particular affect the surface charge of the nanoparticles and ionic strength that changes the electrostatic double layer in the surface of the particles, and this plays an important role in transforming ENPs (Peng et al. 2017).
Analytical methods
Different analytical techniques have been developed for the detection and quantification of engineered nanoparticles in the environment. These include techniques such as ICP, IC, UV–vis and SP-ICP, with different detectors. Qualitative analysis is mostly done using microscopic, spectroscopic and chromatographic techniques, such as transmission electron microscopy (TEM), scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM), energy dispersive spectroscopy (EDS), hydrodynamic chromatography (HDC), ultraviolet–visible (UV–Vis), and field emission scanning electron microscopy (FESEM). The SP-ICP-MS is widely used as a detection method in recent times; however, it is limited to the detection of spherical lower (between 20 and 60 nm)-sized nanomaterials. Different sample preparation techniques such as liquid–liquid extraction, centrifugation, solid-phase extraction, and microwave-assisted extraction have been developed for the extraction of ENPs. Centrifuge extraction is the frequently used technique because it is fast and minimises sample losses.
El Hadri et al. (El Hadri et al. 2018) reported different techniques to evaluate soil and sediments interactions with metal ENPs. The extraction was done using centrifugation which extracted less than 10 nm AuNPs from the sample. Other analytical methods such as asymmetric flow, field flow fractionation, and single-particle inductively coupled plasma mass spectrometry (SP-ICP-MS) were employed to detect metal ENPs in the extracts. Their results showed that the adsorption of AuNPs to the soil particles is not significantly dependent on their composition or surface coating. They conducted a kinetic experiment over 48 h to determine if the attachment of ENPs to the soil is rapid and if hetero-aggregation is abundant. Their results showed that the attachment is rapid and dominant.
The SP-ICP-MS quantification method is based on the particle size distribution (PSD) of NPs in the aquatic matrix. Granting that this technique has been the method of choice for many researchers, it is limited to the analysis of spherical nanoparticles between 20 and 60 nm and not available in many laboratories as yet; hence, it is not a standard routine method. Aznar et al. (Aznar et al. 2017) carried out the analysis of AgNPs in consumer products and aqueous samples using SP- ICP-MS. In their study, they made use of number-based PSD in determining concentrations of AgNPs in drinking and river water samples, near manufacturing industries. This method detected nanoparticles with sizes starting from 30 nm, and an excellent linearity, repeatability, and justifiable biasness were established. They compared their results to those obtained from asymmetric flow-field flow fractionation and supernatant fractions after centrifugal filtration. The outcomes of the comparison suggested that the later method was able to determine the presence of AgNPs with support from the TEM and EDX characterisation. These methods could be exploited for quantitative and qualitative positive screening tests of metal ENPs in the environment (Aznar et al. 2017).
Differentiation between NNPs and ENPs before analysis is required. This is done by considering the resemblance in essential properties such as density, surface chemistry, size, and composition. Many studies mainly used particle size and composition for quantifying the ENPs in the environment with a noticeable background concentration of the targeted metal. This is achieved by employing SP-ICP-MS and the multi-element detection (time of flight) SP-ICP-MS, with TEM, autoSEM, and bulk elemental analysis instruments. Gondikas et al. (Gondikas et al. 2018) reported the analytical attempts for the analysis of TiO2NPs in surface waters. They compared nanoparticles consisting of titanium during the bathing season and outside the bathing season in order to differentiate from natural and engineered nanoparticles. This was done using the multi-element analyser SP-ICP-TOFMS. It was also found that Al, Fe, Mn, and Pb nanoparticles are the mostly found natural NPs attached to Ti. Their study showed that the exposure of TiO2 in the environment can be linked to bathing activities, and this may eventually lead to the formation of TiO2NPs clusters in sediments. The combination of SP-ICP-TOFMS and SP-ICP-MS enabled the differentiation and variation between NNPs and ENPs in environment (Gondikas et al. 2018).
Analytical methods such as single particles ICP-MS, ICP-MS, ICP-OES, TEM, and UV–Vis have been used to quantify total concentration of ENPs in aquatic samples. Table 1 shows that SP-ICP-MS is the method of choice used by most researchers in recent years. Microscopic techniques are mostly used in the method development, along with centrifuge extraction method, though sonication is most preferred (Gondikas et al. 2018; Jiang et al. 2022). ENPs such as Cu, Ag, Ni, and TiO2 are the most studied in recent years because of their high usage, hence, their abundance in the environment.
Although a reliable, inexpensive analytical method to quantify and qualitatively analyse in-depth engineered nanoparticles in the environmental samples has not been determined, developed analytical methods should be able to provide size, composition of nanomaterials, and differentiate between the ENPs and NNPs. Current methods are developed to find the total amount of nanoparticles present in aquatic samples.
Organic nanomaterials
Globally, organic nanomaterials have attracted great interest in the past decades as a result of their increased scientific and industrial applications (Kaegi et al. 2008; Sanchis et al. 2018). Their size, hydrophobicity, 3D, and electronic configuration have further led to the generation interest in them. Organic nanomaterials comprise a large group of nanomaterials which include carbon nanotubes, fullerenes, dendrimers, liposomes, and nano-celluloses (Fig. 4) (Nikalje 2015). Due to the high production and usage of carbon nanotubes and fullerenes among the organic nanomaterials, much data on fate and behaviour of these nanomaterials in the environment are required. Finding from some studies has also shown that they have some negative ecological and health effects that need to be understood.
Carbon nanotubes may be classified into different classes: single-walled (SWCNT) or multi-walled (MWCNT) carbon nanotubes, which may be filled or surface-modified. SWCNTs have wide range of applications in many popular market products such as microelectronics and composite materials (Schierz et al. 2012). This is as a result of their excellent physical, mechanical, electronic, and optical properties. The back effect of this is the increase in the volume of these carbonaceous nanomaterials entering the environment. SWCNTs are now considered as one of the new classes of emerging contaminants (Schierz et al. 2012). To assess the potential impacts of the released SWCNTs into the environment, an informative study should focus on the transport, fate, and ecotoxicity of SWCNTs upon environmental and living organism’s exposure.
Other examples are fullerenes, known as C60 consisting of 20 hexagons and 12 pentagons. They contain a total of 60 carbon atoms, which form a perfectly symmetrical cage type structure. Fullerenes are polyhedrons that are hollow allotropes of carbon, and have dimensions below 1.0 nm (Sanchís et al. 2018). Sources of fullerenes into the environment can either be from natural or anthropogenic sources. Natural sources of fullerenes are from volcanic eruptions, lightning strikes, and wildfires (Sanchís et al. 2018). Fullerenes can also be formed from anthropogenic sources such as combustion processes (Gottschalk et al. 2009, 2010) and as a by-product from different hydrocarbon flame types (Gottschalk et al. 2009; Tiwari et al. 2016). There has been an increase in commercial interest in C60 fullerenes because of its unique physical and chemical properties (Jurkowska et al. 2006; Zakaria et al. 2018). The aqueous solubility of fullerenes in water is lower than 8 ng L−1 due to their ability to form stable aggregates in water (van Wezel et al. 2011a, b). In medicine, fullerenes aqueous dispersions are utilised to adsorb free radicals and prevent various pathological diseases, and help immobilise the transport of medicines on a cellular level (Mikheev et al. 2016a, b).
Application of engineered organic nanomaterials in water treatment
The utilisation of organic nanoparticles depends on type. They have found use in computer chips, and for displays, nano-tools, and in energy applications. They are also used in the tips of atomic force microscopes (AFM) and as potential materials to store hydrogen in hydrogen fuel cells (Schierz et al. 2012). Also, fullerenes nanoparticles are used in photovoltaics as polymer additives, in organic field-effect transistors, catalysts, antioxidants and bio-pharmaceuticals (Emke et al. 2015). Additionally, they are applied in many industrial processes such as constituents of sensor membranes or perm-selective membranes (Chen et al. 2008a, b; Emke et al. 2015; Roy et al. 2021). Fullerenes have many applications; hence, they are listed as the most-used organic nanoparticles (Sanchís et al. 2018).
Lyon et al. (Lyon et al. 2008) found that one of the key properties of fullerenes is their capacity to pass through cell membranes. This enables fullerenes to be used in drug delivery. The most commonly used fullerenes in photovoltaics are C60 and C70, but C70 has been shown to have a 25% higher power conversion efficiency than C60. Other derivatives such as Phenyl-C61-butyric acid hexyl ester (C61-PCBH) have been shown to increase conversion efficiency by over 40% when compared to [6, 6]-phenyl-C61-butyric acid methyl ester (C61-PCBM) in similar systems. Fullerenes also react readily with free radicals and thus act as powerful antioxidants. These properties are desired in health and personal care applications, especially in the prevention of brain cells damages. This has led pharmaceutical industries to explore the use of fullerenes to managing neurological diseases such as Alzheimer and Gehrig’s disease (Lyon et al. 2008).
Throughout history and into the present, water filtration and purification components are primarily made of carbon. Activated carbon has been utilised before BC, but it has low adsorption affinity for lower molecular weight polar organic compounds. However, organic-engineered nanomaterials have interesting adsorption agents because of their structural electronic properties and are explored as substitute for activated carbon. Organic nanomaterials such as CNTs, graphene, dendrimers nanocomposites, and carbon nanorod-based photocatalysts have been explored in water and wastewater for removal of wide variety of contaminants due to their tuneable physicochemical properties (Singh et al. 2022; Jafarian et al. 2023). The capacity to adsorb contaminants is accompanied by fast kinetics due to large surface area, and the selectivity towards aromatics is advantageous. There are still engineering challenges for the application of organic-engineered nanomaterials for large-scale water treatment. But some studies have proposed that organic-engineered nanomaterials can be used infinitely for certain wastewater systems (Saravanan et al. 2022).
Carbon nanotubes have gained much focus from researchers because of their ability to adsorb range of pollutants including polar compounds compared to activated carbons, because of their diverse contaminant-CNT interactions in the form of hydrogen bonding, covalent bonding, and interactions. CNTs are found to be efficient in water and wastewater treatment than other organic-engineered nanomaterials due to availability of longer pores in bundles and more accessible sorption sites (Mazrouaa et al. 2023). Most of the CNT water treatment application was reported for heavy materials elimination. Drawbacks of CNTs are their hydrophobic surface which makes them form loose bundles/aggregates in aqueous medium, which reduces the active sorption surface area. Dendrimers can also be used for removal of heavy metals in water or wastewater, as their solubility property makes them efficient adsorbents. Another organic-engineered nanomaterial with a promising water and wastewater application is graphene nanosphere. Graphene exhibits high efficient for removal of antibiotic because of its single-stratum carbon moiety characteristic property (Masri et al. 2019). In addition, the permeable assembly and large surface size of graphene materials combination make this material more promising for removal of antibiotic (Masri et al. 2019). Despite promising removal capacity, the price of a commercial-scale generation of graphene is much higher than conventional treatment materials.
To ensure complete removal of diverse group of contaminants, organic-engineered nanomaterials are normally prepared in composites. In theory, composites for water and wastewater application should be continuous, bulk immobile material, where nanoreactivity is obtained by impregnating parent material with organic nanomaterials (Jafarian et al. 2023). Researchers globally are in quest of desired nanomaterials for water treatment. The focus is on adsorption mechanism of adsorbates and organic nanomaterials that can treat different water systems. Since pi–pi electron donor–acceptor interactions have been proved to be a main adsorption mechanism, materials with an increased surface area are normal desired (Kumar et al. 2022; Yu et al. 2022). Apart from adsorption properties, organic nanomaterials that exhibited great photocatalytic activities have been proposed degradation of contaminants as well. Carbon nanorods with high surface area have attracted a lot of attention in photodegradation of contaminants. Water and wastewater treatment demands for nontoxic, long-term stable, and low-cost materials for water treatment (Luo et al. 2021).
Emission pathways of organic nanoparticles in the environment
The pathways for the release of carbonaceous ENMs into the environment from different sources are shown in Fig. 5. Tiwari et al. (Tiwari et al. 2016) noted that, as man-made organic nanomaterials are increasingly manufactured, releases of these nanomaterials into the environment through utilisation, disposal, and unintentional disposal cannot be avoided.
Occurrence and environmental impact of carbonaceous nanomaterials
Organic nanomaterials are carbonaceous nanoparticles that occur in the environment in trace levels. Carbon nanotubes and fullerenes in the environment behave differently in the environment due to their characteristic wide surface areas compared with usual organic contaminants such as pesticides and pharmaceuticals (Chen et al. 2008a, b). This uniqueness is attributed to the van der Waals and hydrophobic interaction of carbonaceous nanoparticles which could lead to aggregation and sedimentation in an aqueous environment (Pérez et al. 2009; Ross and Knightes 2022). Different types and degree of agglomeration affect the toxicity of carbonaceous nanoparticles (Wick et al. 2007). Also, the changing of the functional groups and coating of the surfaces of carbonaceous nanoparticles improve their properties and stabilise them in the aqueous environment, thus influencing their toxicity and fate in the environment (Pérez et al. 2009). Direct disposal of products containing fullerenes in the aquatic system may result in their occurrence in the environment (Chen et al. 2008a, b; Avant et al. 2019).
Nanoparticles have been reported to cause stress in the metabolism of aquatic organisms even at environmental levels after chronic exposure (Mikheev et al. 2016a, b). In aquatic environments, nanoparticles might accumulate within the body of fish and be complexed via organic phenomenon during its life cycle (Zhang et al. 2009; Avant et al. 2019). The size dimension of nanoparticles presents a high possibility of conveying virulent substances that could have an adverse impact on human health (Tran et al. 2000; Wick et al. 2007; Pérez et al. 2009; Zhang et al. 2009). Organs in fishes such as brain, gut, liver, etc. are mostly affected by the carbonaceous nanomaterials in the aquatic ecosystem. Some organisms in the aquatic environment such as algae and unicellular protozoa are affected by the direct contact with this carbon nanoparticle (Zhang et al. 2009; Schwab et al. 2011). The risks associated with the carbonaceous nanoparticles have made them to be listed as one of the prominent contaminants whose steadiness needs to be examined (Zhang et al. 2009).
Aside from DNA damage and stunting of growth, some other harmful health impacts on living organisms have been reported. These include oxidative stress and even mortality at acute levels, while many low concentration or chronic toxicities have also been identified (Isaacson et al. 2007; Becker et al. 2011; Astefanei et al. 2014; Emke et al. 2015; Zakaria et al. 2018).
Analysis of organic-engineered nanomaterials in environmental samples
One of the major challenges associated with assessing the risks of fullerene nanoparticles in the environment is the lack of standard analytical methods to quantify them. However, in recent years’ various sensitive methods for the determination and extraction of fullerenes from environmental matrices have been developed. The common analytical methods for characterising C60 in aqueous environments include liquid–liquid extraction (LLE), high-performance liquid chromatography (HPLC), solid-phase extraction (SPE), UV detection, and mass spectrometry (MS) as shown in Table 2 (Isaacson et al. 2007; Chen et al. 2008a, b; Hyung and Kim 2009; van Wezel et al. 2011a, b; PÉREZ et al. 2013; Carboni et al. 2014; Sanchís et al. 2015, 2018). HPLC is a method of choice to separate fullerenes from samples matrices using toluene or benzene as a mobile phase (Chen et al. 2008a, b). LLE and SPE consist of two steps, through which fullerene may be extracted using LLE from an aqueous phase into toluene and then followed by SPE clean-up step. The water sample may be directly analysed by LC–MS, after the removal of interferences by the clean-up step (Astefanei et al. 2014).
Results and discussion
Environmental fate of nanomaterials
Understanding the fate of nanomaterials is complex, partly because they are different types with distinct physical and chemical properties, as well as the lack of information on their occurrence levels in various environmental compartments, including air, water, and soils. This inevitably affects the ability to evaluate the potential negative effects and toxicities of nanomaterials in the environment. While the exact quantity (concentration) of nanomaterials present in the environment is not exactly known, their existence and levels in different compartments of the environment have been predicted based on the pattern of use (Boxall et al. 2007a, b; Boxall et al. 2007a, b). In air, their occurrence is vapour pressure dependent wherewith nanomaterials may exist as aerodynamic fine particles. They may readily associate with colloidal suspension of humic and fulvic substances in water, while they are mainly sequestered in bentonitic materials and sediments in aquatic and marine beds, and soils (Baalousha 2009).
It was reported that higher proportion of metal ENPs are partitioned into sediments compared to water, due to the aggregation of ENPs (Zhou et al. 2012). Mostly, metal oxides ENPs including zinc oxide, titanium oxide, and cerium oxide have high aggregation and sedimentation rate when introduced to the marine environment (Keller et al. 2010). Hanna et al. (Hanna et al. 2013) investigated the accumulation and toxicity of metal oxides NPs in soft sediments estuarine amphipod. This study suggested that there are some ENPs that are dissolved rapidly when entering the aquatic systems, and that those ENPs are more toxic compared to those that settle and bind to the sediments. Nevertheless, the metal ENPs that are dissolved completely are less toxic compared to the slowly dissolving metal ENPs. It is evidenced that the amphipods in the sediments will be slowly affected by the ENPs; this affects their life cycle, food chain, and can cause lethal effect in some organisms. Carbon fullerenes (C60) are also engineered nanoparticles (ENPs) but are non-toxic compared to metal ENPs. However, they do not degrade easily in the environment; instead, they aggregate and settle to sediments. Understanding the behaviour of nanoparticles and their environmental concentration is important to develop policies that prevent their release from nano-enabled commercial product formulations into water ways (Tiwari et al. 2016).
Future recommendations
There is a need to develop new methods that can be used to predict the risk associated with ENMs into our ecosystems. The occurrence of ENMs has been studied in various matrixes, although there is a gap in finding routine, easy, and cheap methods to assess the occurrence of these nanomaterials. The fate and behaviour of ENMs in the environments have been poorly understood and studied due to lack of standard methods, for which there is a need for more studies. In filling the gap, development of sensitive methods that can detect these contaminants in complex environments in their trace level and different forms is imperative. Furthermore, development of the methods to extract nanomaterials from different matrixes is of important, to accurately quantify their occurrence levels. The standardisation of methods to be used to analyse and assess the risk of ENMs in the environment is also imperative.
Conclusion
Engineered nanomaterials have been produced and used in abundance in recent decades for various applications. They end-up in the environment, soils, rivers and the marines through several routes. ENMs can accumulate in the environment, and exposure beyond certain thresholds could cause adverse effects to aquatic organisms. Various analytical methods have been developed to monitor the levels of their occurrence in environmental systems; however, more work is required in that direction due to the increasing complexity of their formulations and occurrence in environmental matrices. Mostly, the SP-ICP-MS have been used to analyse the metal ENPs. However, this technique is not frequently used due to its limitations. ICP-OES when combined with appropriate extraction techniques is currently regarded as the possible method to analyse the ENPs.
References
Asl MK, Hasani AH, Naserkhaki E (2016) Evaluation of nitrate removal from water using activated carbon and clinoptilolite by adsorption method. Biosci Biotechnol Res Asia 13(2):1045–1054
Astefanei A, Núñez O, Galceran MT (2014) Analysis of c60-fullerene derivatives and pristine fullerenes in environmental samples by ultrahigh performance liquid chromatography–atmospheric pressure photoionization-mass spectrometry. J Chromatogr A 1365:61–71. https://doi.org/10.1016/j.chroma.2014.08.089
Avant B, Bouchard D, Chang X, Hsieh H-S, Acrey B, Han Y, Spear J, Zepp R, Knightes CD (2019) Environmental fate of multiwalled carbon nanotubes and graphene oxide across different aquatic ecosystems. NanoImpact 13:1–12. https://doi.org/10.1016/j.impact.2018.11.001
Aznar R, Barahona F, Geiss O, Ponti J, José Luis T, Barrero-Moreno J (2017) Quantification and size characterisation of silver nanoparticles in environmental aqueous samples and consumer products by single particle-icpms. Talanta 175:200–208. https://doi.org/10.1016/j.talanta.2017.07.048
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, ph and natural organic matter. Sci Total Environ 407(6):2093–2101
Banfield JF, Zhang H (2001) Nanoparticles in the environment. Rev Mineral Geochem 44(1):1–58
Barka E, Noutsopoulos C, Galani A, Panagou I, Kalli M, Koumaki E, Malamis S, Mamais D (2023) Removal of contaminants of emerging concern from wastewater using an integrated column system containing zero valent iron nanoparticles. Water 15(3):598
Becker H, Herzberg F, Schulte A, Kolossa-Gehring M (2011) The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int J Hygiene Environ Health 214(3):231–238. https://doi.org/10.1016/j.ijheh.2010.11.004
Behboudi A, Mohammadi T, Ulbricht M (2023) High performance antibiofouling hollow fiber polyethersulfone nanocomposite membranes incorporated with novel surface-modified silver nanoparticles suitable for membrane bioreactor application. J Ind Eng Chem 119:298–314. https://doi.org/10.1016/j.jiec.2022.11.049
Bolyard SC, Reinhart DR, Santra S (2013) Behavior of engineered nanoparticles in landfill leachate. Environ Sci Technol 47(15):8114–8122. https://doi.org/10.1021/es305175e
Bouchard D, Zhang W, Chang XJ (2013) A rapid screening technique for estimating nanoparticle transport in porous media. Water Res 47(12):4086–4094. https://doi.org/10.1016/j.watres.2012.10.026
Boxall, A., K. Tiede, Q. Chaudhry, R. Aitken, A. Jones, B. Jefferson and J. Lewis, 2007a. Current and future predicted exposure to engineered nanoparticles. Safety of Nanomaterials Interdisciplinary Research Centre Report: 1–13.
Boxall, A.B., K. Tiede and Q. Chaudhry, 2007b. Engineered nanomaterials in soils and water: How do they behave and could they pose a risk to human health?
Brar SK, Verma M, Tyagi RD, Surampalli RY (2010) Engineered nanoparticles in wastewater and wastewater sludge–evidence and impacts. Waste Manag 30(3):504–520. https://doi.org/10.1016/j.wasman.2009.10.012
Carboni A, Emke E, Parsons JR, Kalbitz K, de Voogt P (2014) An analytical method for determination of fullerenes and functionalized fullerenes in soils with high performance liquid chromatography and uv detection. Anal Chim Acta 807:159–165
Chang Y-J, Shih Y-H, Su C-H, Ho H-C (2017) Comparison of three analytical methods to measure the size of silver nanoparticles in real environmental water and wastewater samples. J Hazard Mater 322:95–104. https://doi.org/10.1016/j.jhazmat.2016.03.030
Chen X, Burda C (2008) The electronic origin of the visible-light absorption properties of c-, n-and s-doped tio2 nanomaterials. J Am Chem Soc 130(15):5018–5019
Chen Z, Westerhoff P, Herckes P (2008b) Quantification of c60 fullerene concentrations in water. Environ Toxicol Chem Int J 27(9):1852–1859
Chen G, Liu X, Su C (2012) Distinct effects of humic acid on transport and retention of tio2 rutile nanoparticles in saturated sand columns. Environ Sci Technol 46(13):7142–7150
Chen, Z., P. Westerhoff and P. Herckes, 2008a. Quantification of c60 fullerene concentration in water.
Choi S, Johnston MV, Wang GS, Huang CP (2017) Looking for engineered nanoparticles (enps) in wastewater treatment systems: qualification and quantification aspects. Sci Total Environ 590–591:809–817. https://doi.org/10.1016/j.scitotenv.2017.03.061
Domercq P, Praetorius A, Boxall ABA (2018) Emission and fate modelling framework for engineered nanoparticles in urban aquatic systems at high spatial and temporal resolution. Environ Sci-Nano 5(2):533–543. https://doi.org/10.1039/c7en00846e
Donovan AR, Adams CD, Ma Y, Stephan C, Eichholz T, Shi H (2016) Single particle icp-ms characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere 144:148–153. https://doi.org/10.1016/j.chemosphere.2015.07.081
El Hadri H, Louie SM, Hackley VA (2018) Assessing the interactions of metal nanoparticles in soil and sediment matrices–A quantitative analytical multi-technique approach. Environ Sci Nano 5(1):203–214. https://doi.org/10.1039/C7EN00868F
Emke E, Sanchis J, Farre M, Bäuerlein P, De Voogt P (2015) Determination of several fullerenes in sewage water by lc hr-ms using atmospheric pressure photoionisation. Environ Sci Nano 2(2):167–176
Farré M, Gajda-Schrantz K, Kantiani L, Barceló D (2009) Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal Bioanal Chem 393(1):81–95. https://doi.org/10.1007/s00216-008-2458-1
Garner KL, Suh S, Keller AA (2017) Assessing the risk of engineered nanomaterials in the environment: development and application of the nanofate model. Environ Sci Technol 51(10):5541–5551. https://doi.org/10.1021/acs.est.6b05279
Ghasemzadeh G, Momenpour M, Omidi F, Hosseini MR, Ahani M, Barzegari A (2014) Applications of nanomaterials in water treatment and environmental remediation. Front Environ Sci Eng 8(4):471–482. https://doi.org/10.1007/s11783-014-0654-0
Giese B, Klaessig F, Park B, Kaegi R, Steinfeldt M, Wigger H, von Gleich A, Gottschalk F (2018) Risks, release and concentrations of engineered nanomaterial in the environment. Sci Rep 8(1):1–18
Gondikas A, von der Kammer F, Kaegi R, Borovinskaya O, Neubauer E, Navratilova J, Praetorius A, Cornelis G, Hofmann T (2018) Where is the nano? Analytical approaches for the detection and quantification of tio2 engineered nanoparticles in surface waters. Environ Sci Nano 5(2):313–326. https://doi.org/10.1039/C7EN00952F
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (tio2, zno, ag, cnt, fullerenes) for different regions. Environ Sci Technol 43(24):9216–9222
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2010) Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ Toxicol Chem 29(5):1036–1048
Gumbi B, Ngila JC, Ndungu PG (2014) Gold nanoparticles for the quantification of very low levels of poly-diallyldimethylammonium chloride in river water. Anal Methods 6(17):6963–6972
Hanna SK, Miller RJ, Zhou D, Keller AA, Lenihan HS (2013) Accumulation and toxicity of metal oxide nanoparticles in a soft-sediment estuarine amphipod. Aquat Toxicol 142–143:441–446. https://doi.org/10.1016/j.aquatox.2013.09.019
Haribhau Waghchaure R, Ashok Adole V, Sonu Jagdale B, Bhimrao Koli P (2022) Fe3+ modified zinc oxide nanomaterial as an efficient, multifaceted material for photocatalytic degradation of mb dye and ethanol gas sensor as part of environmental rectification. Inorg Chem Commun 140:109450. https://doi.org/10.1016/j.inoche.2022.109450
Hassellöv M, Readman JW, Ranville JF, Tiede K (2008) Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 17(5):344–361. https://doi.org/10.1007/s10646-008-0225-x
Helfrich A, Bettmer J (2011) Analysis of gold nanoparticles using icp-ms-based hyphenated and complementary esi-ms techniques. Int J Mass Spectr 307(1):92–98. https://doi.org/10.1016/j.ijms.2011.01.010
Hendricks N, Olatunji O, Gumbi B (2022) A simple model to estimate the number of metal engineered nanoparticles in samples using inductively coupled plasma optical emission spectrometry. Molecules 27(18):5810
Hochella MF Jr (2008) Nanogeoscience: from origins to cutting-edge applications. Elements 4(6):373–379
Hochella MF, Lower SK, Maurice PA, Penn RL, Sahai N, Sparks DL, Twining BS (2008) Nanominerals, mineral nanoparticles, and earth systems. Science 319(5870):1631–1635
Hotze M, Lowry G (2010) Nanotechnology for sustainable water treatment. Sustain Water 31:138–164
Husain S, Nandi A, Simnani FZ, Saha U, Ghosh A, Sinha A, Sahay A, Samal SK, Panda PK, Verma SK (2023) Emerging trends in advanced translational applications of silver nanoparticles: a progressing dawn of nanotechnology. J Funct Biomater 14(1):47
Hyung H, Kim J-H (2009) Dispersion of c60 in natural water and removal by conventional drinking water treatment processes. Water Res 43(9):2463–2470
Isaacson CW, Usenko CY, Tanguay RL, Field JA (2007) Quantification of fullerenes by lc/esi-ms and its application to in vivo toxicity assays. Anal Chem 79(23):9091–9097
Jafarian H, Dadashi Firouzjaei M, Aghapour Aktij S, Aghaei A, Pilevar Khomami M, Elliott M, Wujcik EK, Sadrzadeh M, Rahimpour A (2023) Synthesis of heterogeneous metal organic framework-graphene oxide nanocomposite membranes for water treatment. Chem Eng J 455:140851. https://doi.org/10.1016/j.cej.2022.140851
Jiang, C., S. Liu, T. Zhang, Q. Liu, P.J. Alvarez and W. Chen, 2022. Current methods and prospects for analysis and characterization of nanomaterials in the environment. Environmental Science & Technology.
Jurkowska B, Jurkowski B, Kamrowski P, Pesetskii S, Koval V, Pinchuk L, Olkhov YA (2006) Properties of fullerene-containing natural rubber. J Appl Polym Sci 100(1):390–398
Kabata-Pendias A (2004) Soil–plant transfer of trace elements—An environmental issue. Geoderma 122(2–4):143–149
Kabir E, Kumar V, Kim K-H, Yip ACK, Sohn JR (2018) Environmental impacts of nanomaterials. J Environ Manag 225:261–271. https://doi.org/10.1016/j.jenvman.2018.07.087
Kaegi R, Ulrich A, Sinnet B, Vonbank R, Wichser A, Zuleeg S, Simmler H, Brunner S, Vonmont H, Burkhardt M, Boller M (2008) Synthetic tio2 nanoparticle emission from exterior facades into the aquatic environment. Environ Pollut 156(2):233–239. https://doi.org/10.1016/j.envpol.2008.08.004
Kaegi R, Voegelin A, Ort C, Sinnet B, Thalmann B, Krismer J, Hagendorfer H, Elumelu M, Mueller E (2013) Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res 47(12):3866–3877. https://doi.org/10.1016/j.watres.2012.11.060
Katz LM, Dewan K, Bronaugh RL (2015) Nanotechnology in cosmetics. Food Chem Toxicol 85:127–137. https://doi.org/10.1016/j.fct.2015.06.020
Keller AA, Lazareva A (2014) Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett 1(1):65–70. https://doi.org/10.1021/ez400106t
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15(6):1–17
Khalil S, Mehmood A, Khan MAR, Ahmad KS, Abasi F, Raffi M, Ali K, Khan MEH, Jones DA, Abdelkarim M (2023) Antibacterial, antioxidant and photocatalytic activity of novel rubus ellipticus leaf mediated silver nanoparticles. J Saudi Chem Soc 27(1):101576. https://doi.org/10.1016/j.jscs.2022.101576
Khan A, Rashid A, Younas R, Chong R (2016) A chemical reduction approach to the synthesis of copper nanoparticles. Int Nano Lett 6(1):21–26. https://doi.org/10.1007/s40089-015-0163-6
Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem Int J 27(9):1825–1851
Kolkman A, Emke E, Bäuerlein PS, Carboni A, Tran DT, ter Laak TL, van Wezel AP, de Voogt P (2013) Analysis of (functionalized) fullerenes in water samples by liquid chromatography coupled to high-resolution mass spectrometry. Anal Chem 85(12):5867–5874. https://doi.org/10.1021/ac400619g
Kumar CV, Karthick V, Kumar VG, Inbakandan D, Rene ER, Suganya KU, Embrandiri A, Dhas TS, Ravi M, Sowmiya P (2022) The impact of engineered nanomaterials on the environment: release mechanism, toxicity, transformation, and remediation. Environ Res 212:113202
Latwal, M., S. Arora, A. Joshi, M. Irfan and G. Pandey, 2023. Sustainable ceramic membrane for decontamination of water: A cost-effective approach. Heliyon.
Lead JR, Wilkinson KJ (2006) Aquatic colloids and nanoparticles: current knowledge and future trends. Environ Chem 3(3):159–171
Lespes G, Faucher S, Slaveykova VI (2020) Natural nanoparticles, anthropogenic nanoparticles, where is the frontier? Front Environ Sci 8:71
Levin M, Rojas E, Vanhala E, Vippola M, Liguori B, Kling KI, Koponen IK, Molhave K, Tuomi T, Gregurec D, Moya S, Jensen KA (2015) Influence of relative humidity and physical load during storage on dustiness of inorganic nanomaterials: Implications for testing and risk assessment. J Nanoparticle Res 17(8):1–13. https://doi.org/10.1007/s11051-015-3139-6
Li M, Kuang S, Kang Y, Ma H, Dong J, Guo Z (2022) Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment. Sci Total Environ 819:153157. https://doi.org/10.1016/j.scitotenv.2022.153157
Luo P, Roca A, Tiede K, Privett K, Jiang J, Pinkstone J, Ma G, Veinot J, Boxall A (2018) Application of nanoparticle tracking analysis for characterising the fate of engineered nanoparticles in sediment-water systems. J Environ Sci 64:62–71. https://doi.org/10.1016/j.jes.2016.07.019
Luo JM, Fu KX, Yu DY, Hristovski KD, Westerhoff P, Crittenden JC (2021) Review of advances in engineering nanomaterial adsorbents for metal removal and recovery from water: synthesis and microstructure impacts. Acs Es&T Eng 1(4):623–661. https://doi.org/10.1021/acsestengg.0c00174
Lyon D, Brown D, Alvarez P (2008) Implications and potential applications of bactericidal fullerene water suspensions: effect of nc60 concentration, exposure conditions and shelf life. Water Sci Technol 57(10):1533–1538
Mahjoubian M, Naeemi AS, Moradi-Shoeili Z, Tyler CR, Mansouri B (2023) Toxicity of silver nanoparticles in the presence of zinc oxide nanoparticles differs for acute and chronic exposures in zebrafish. Arch Environ Contam Toxicol 84(1):1–17. https://doi.org/10.1007/s00244-022-00965-0
Mahlalela LC, Ngila JC, Dlamini LN (2017) Monitoring the fate and behavior of tio2 nanoparticles: Simulated in a wwtp with industrial dye-stuff effluent according to oecd 303a. J Environ Sci Health Part A Toxic/Hazard Subst Environ Eng 52(8):794–803. https://doi.org/10.1080/10934529.2017.1305176
Mahmoud AM, Ibrahim FA, Shaban SA, Youssef NA (2015) Adsorption of heavy metal ion from aqueous solution by nickel oxide nano catalyst prepared by different methods. Egypt J Petrol 24(1):27–35. https://doi.org/10.1016/j.ejpe.2015.02.003
Mantovani M, Collina E, Lasagni M, Marazzi F, Mezzanotte V (2023) Production of microalgal-based carbon encapsulated iron nanoparticles (me-nfe) to remove heavy metals in wastewater. Environ Sci Pollut Res 30(3):6730–6745. https://doi.org/10.1007/s11356-022-22506-x
Masri, A.K., T.W. Yen, M.A. Ahmad and S. Karim, 2019. Graphene-based nanomaterial for the removal of sulfamethoxazole in water. In: 4th International Conference on Green Chemical Engineering and Technology (GCET) - Materials Science. Meleka, MALAYSIA: pp: 198–201.
Matranga V, Corsi I (2012) Toxic effects of engineered nanoparticles in the marine environment: model organisms and molecular approaches. Marine Environ Res 76:32–40. https://doi.org/10.1016/j.marenvres.2012.01.006
Mazrouaa, A.M., A.A. Mousa and M.G. Mohamed, 2023. Chemically modified carbon nanotubes for water purification system. Chemically Modified Carbon Nanotubes for Commercial Applications: 197–214.
Mikheev, I., T. Bolotnik, D. Volkov, M. Korobov and M. Proskurnin, 2016a. Approaches to the determination of c60 and c70 fullerene and their mixtures in aqueous and organic solutions. Haнocиcтeмы: физикa, xимия, мaтeмaтикa, 7(1).
Mikheev, I.V., T.A. Bolotnik, D.S. Volkov, M. Korobov and M. Proskurnin, 2016b. Approaches to the determination of c60 and c70 fullerene and their mixtures in aqueous and organic solutions.
Milam, S., 2010. Effects of silver nanoparticles on photochemical processes focusing on luminol chemiluminescence. 303: 4-9
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42(12):4447–4453. https://doi.org/10.1021/es7029637
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008) Toxicity of silver nanoparticles to chlamydomonas reinhardtii. Environ Sci Technol 42(23):8959–8964
Navratilova J, Praetorius A, Gondikas A, Fabienke W, Von der Kammer F, Hofmann T (2015) Detection of engineered copper nanoparticles in soil using single particle icp-ms. Int J Environ Res Public Health 12(12):15020
Nikalje AP (2015) Nanotechnology and its applications in medicine. Med Chem 5(2):081–089
Okpara EC, Olatunde OC, Wojuola OB, Onwudiwe DC (2023) Applications of transition metal oxides and chalcogenides and their composites in water treatment: a review. Environ Adv 11:100341. https://doi.org/10.1016/j.envadv.2023.100341
Ovissipour M, Rasco B, Sablani S (2013) Impact of engineered nanoparticles on aquatic organisms. J Fisheries Livest Prod 1:e106
Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S (2009) Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J 151(1):19–29. https://doi.org/10.1016/j.cej.2009.02.036
Pandey K, Saha S (2023) Encapsulation of zero valent iron nanoparticles in biodegradable amphiphilic janus particles for groundwater remediation. J Hazard Mater 445:130501. https://doi.org/10.1016/j.jhazmat.2022.130501
Peng Y-H, Tsai Y-C, Hsiung C-E, Lin Y-H, Shih Y-H (2017) Influence of water chemistry on the environmental behaviors of commercial zno nanoparticles in various water and wastewater samples. J Hazard Mater 322:348–356
Pérez S, la Farré M, Barceló D (2009) Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment. TrAC, Trends Anal Chem 28(6):820–832
Pérez RA, Albero B, Miguel E, Tadeo JL, Sánchez-Brunete C (2013) A rapid procedure for the determination of c60 and c70 fullerenes in soil and sediments by ultrasound-assisted extraction and hplc-uv. Anal Sci 29(5):533–538
Peters RJB, van Bemmel G, Milani NBL, den Hertog GCT, Undas AK, van der Lee M, Bouwmeester H (2018) Detection of nanoparticles in dutch surface waters. Sci Total Environ 621:210–218. https://doi.org/10.1016/j.scitotenv.2017.11.238
Pham LQ, Sohn JH, Kim CW, Park JH, Kang HS, Lee BC, Kang YS (2012) Copper nanoparticles incorporated with conducting polymer: effects of copper concentration and surfactants on the stability and conductivity. J Colloid Interface Sci 365(1):103–109. https://doi.org/10.1016/j.jcis.2011.09.041
Piella J, Bastús NG, Puntes V (2016) Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem Mater 28(4):1066–1075. https://doi.org/10.1021/acs.chemmater.5b04406
Polesel F, Farkas J, Kjos M, Almeida Carvalho P, Flores-Alsina X, Gernaey KV, Hansen SF, Plósz BG, Booth AM (2018) Occurrence, characterisation and fate of (nano)particulate ti and ag in two norwegian wastewater treatment plants. Water Res 141:19–31. https://doi.org/10.1016/j.watres.2018.04.065
Rabajczyk A (2016) Possibilities for analysis of selected nanometals in solid environmental samples. Desalin Water Treat 57(3):1598–1610. https://doi.org/10.1080/19443994.2015.1030109
Rajput A, Rahman MA, Rahman MH, Kuila A (2023) Visible light photocatalytic degradation of organic pollutants in industrial wastewater by engineered tio2 nanoparticles. Biomass Conver Bioref 58:89. https://doi.org/10.1007/s13399-023-03783-z
Ramadevi P, Shanmugavadivu R, Venkatesan R, Mayandi J, Sagadevan S (2023) Photocatalytic dye degradation efficiency and reusability of aluminium substituted nickel ferrite nanostructures for wastewater remediation. Inorg Chem Commun 150:110532. https://doi.org/10.1016/j.inoche.2023.110532
Ross BN, Knightes CD (2022) Simulation of the environmental fate and transformation of nano copper oxide in a freshwater environment. ACS Es&t Water 2(9):1532–1543
Roy, A., A. Sharma, S. Yadav, L.T. Jule and R. Krishnaraj, 2021. Nanomaterials for remediation of environmental pollutants. Bioinorganic Chemistry and Applications, 2021.
Saleem H, Zaidi SJ (2020) Developments in the application of nanomaterials for water treatment and their impact on the environment. Nanomaterials 10(9):1764
Saleh N, Kim H-J, Phenrat T, Matyjaszewski K, Tilton RD, Lowry GV (2008) Ionic strength and composition affect the mobility of surface-modified fe0 nanoparticles in water-saturated sand columns. Environ Sci Technol 42(9):3349–3355. https://doi.org/10.1021/es071936b
Sanchis J, Bozovic D, Al-Harbi NA, Silva LF, Farre M, Barcelo D (2013) Quantitative trace analysis of fullerenes in river sediment from spain and soils from saudi arabia. Anal Bioanal Chem 405(18):5915–5923. https://doi.org/10.1007/s00216-013-6924-z
Sanchis J, Milacic R, Zuliani T, Vidmar J, Abad E, Farre M, Barcelo D (2018) Occurrence of c60 and related fullerenes in the sava river under different hydrologic conditions. Sci Total Environ 643:1108–1116. https://doi.org/10.1016/j.scitotenv.2018.06.285
Sanchís J, Bosch-Orea C, Farré M, Barceló D (2015) Nanoparticle tracking analysis characterisation and parts-per-quadrillion determination of fullerenes in river samples from barcelona catchment area. Anal Bioanal Chem 407(15):4261–4275
Sanchís J, Milačič R, Zuliani T, Vidmar J, Abad E, Farré M, Barceló D (2018) Occurrence of c60 and related fullerenes in the sava river under different hydrologic conditions. Sci Total Environ 643:1108–1116. https://doi.org/10.1016/j.scitotenv.2018.06.285
Santiago-Martín AD, Constantin B, Guesdon G, Kagambega N, Raymond S, Cloutier RG (2016) Bioavailability of engineered nanoparticles in soil systems. J Hazard Toxic Radioact Waste 20(1):B4015001
Saravanan A, Kumar PS, Hemavathy RV, Jeevanantham S, Jawahar MJ, Neshaanthini JP, Saravanan R (2022) A review on synthesis methods and recent applications of nanomaterial in wastewater treatment: challenges and future perspectives. Chemosphere 307:135713. https://doi.org/10.1016/j.chemosphere.2022.135713
Schierz A, Parks AN, Washburn KM, Chandler GT, Ferguson PL (2012) Characterization and quantitative analysis of single-walled carbon nanotubes in the aquatic environment using near-infrared fluorescence spectroscopy. Environ Sci Technol 46(22):12262–12271
Schwab F, Bucheli TD, Lukhele LP, Magrez A, Nowack B, Sigg L, Knauer K (2011) Are carbon nanotube effects on green algae caused by shading and agglomeration? Environ Sci Technol 45(14):6136–6144. https://doi.org/10.1021/es200506b
Sengul AB, Asmatulu E (2020) Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett 18(5):1659–1683
Shon H, Vigneswaran S, Kandasamy J, Cho J (2007) Characteristics of effluent organic matter in wastewater. Eolss Publishers, Oxford, UK
Silva BFD, Pérez S, Gardinalli P, Singhal RK, Mozeto AA, Barceló D (2011) Analytical chemistry of metallic nanoparticles in natural environments. TrAC Trends Anal Chem 30(3):528–540. https://doi.org/10.1016/j.trac.2011.01.008
Simonet BM, Valcárcel M (2008) Monitoring nanoparticles in the environment. Anal Bioanal Chem 393(1):17. https://doi.org/10.1007/s00216-008-2484-z
Singh KK, Singh A, Rai S (2022) A study on nanomaterials for water purification. Mater Today: Proc 51:1157–1163. https://doi.org/10.1016/j.matpr.2021.07.116
Som C, Wick P, Krug H, Nowack B (2011) Environmental and health effects of nanomaterials in nanotextiles and facade coatings. Environ Int 37(6):1131–1142
Sun C, Hu K, Mu D, Wang Z, Yu X (2022) The widespread use of nanomaterials: the effects on the function and diversity of environmental microbial communities. Microorganisms 10(10):2080
Theng BK, Yuan G (2008) Nanoparticles in the soil environment. Elements 4(6):395–399
Tiraferri A, Chen KL, Sethi R, Elimelech M (2008) Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J Colloid Interface Sci 324(1–2):71–79
Tiwari AJ, Ashraf-Khorassani M, Marr LC (2016) C60 fullerenes from combustion of common fuels. Sci Total Environ 547:254–260. https://doi.org/10.1016/j.scitotenv.2015.12.142
Tran C, Buchanan D, Cullen R, Searl A, Jones A, Donaldson K (2000) Inhalation of poorly soluble particles. Ii. Influence of particle surface area on inflammation and clearance. Inhalation Toxicol 12(12):1113–1126
Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA (2013) Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed 8:4467–4479. https://doi.org/10.2147/IJN.S50837
van Wezel AP, Morinière V, Emke E, ter Laak T, Hogenboom AC (2011) Quantifying summed fullerene nc60 and related transformation products in water using lc ltq orbitrap ms and application to environmental samples. Envir Internl 37(6):1063–1067. https://doi.org/10.1016/j.envint.2011.03.020
van Wezel AP, Morinière V, Emke E, ter Laak T, Hogenboom AC (2011b) Quantifying summed fullerene nc60 and related transformation products in water using lc ltq orbitrap ms and application to environmental samples. Environ Int 37(6):1063–1067
Wang J, Nabi MM, Mohanty SK, Afrooz ARMN, Cantando E, Aich N, Baalousha M (2020) Detection and quantification of engineered particles in urban runoff. Chemosphere 248:126070. https://doi.org/10.1016/j.chemosphere.2020.126070
Wang, Y., Z. Yang, M. Yu, R. Lin, L. Zhu and F. Bai, 2022. Integrating ecosystem health and services for assessing ecological risk and its response to typical land-use patterns in the eco-fragile region, north china. Environmental Management: 1–18.
Wen H, Shi H, Jiang N, Qiu J, Lin F, Kou Y (2023) Antifungal mechanisms of silver nanoparticles on mycotoxin producing rice false smut fungus. iScience 26(1):105763. https://doi.org/10.1016/j.isci.2022.105763
Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A (2007) The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett 168(2):121–131
Xu M, Luo H, Rong H, Wu S, Zheng Z, Chen B (2023) Calcium alginate gels-functionalized polyurethane foam decorated with silver nanoparticles as an antibacterial agent for point-of-use water disinfection. Int J Biol Macromol 231:123289. https://doi.org/10.1016/j.ijbiomac.2023.123289
Yang Y, Long C-L, Li H-P, Wang Q, Yang Z-G (2016) Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci Total Environ 563–564:996–1007. https://doi.org/10.1016/j.scitotenv.2015.12.150
Yu S, Tang H, Zhang D, Wang S, Qiu M, Song G, Fu D, Hu B, Wang X (2022) Mxenes as emerging nanomaterials in water purification and environmental remediation. Sci Total Environ 811:152280. https://doi.org/10.1016/j.scitotenv.2021.152280
Zakaria S, Fröhlich E, Fauler G, Gries A, Weiß S, Scharf S (2018) First determination of fullerenes in the austrian market and environment: quantitative analysis and assessment. Environ Sci Pollut Res 25(1):562–571
Zazo H, Colino CI, Lanao JM (2016) Current applications of nanoparticles in infectious diseases. J Controll Release 224:86–102. https://doi.org/10.1016/j.jconrel.2016.01.008
Zhang Y, Chen Y, Westerhoff P, Crittenden J (2009) Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res 43(17):4249–4257
Zhang Y, Leu Y-R, Aitken RJ, Riediker M (2015) Inventory of engineered nanoparticle-containing consumer products available in the singapore retail market and likelihood of release into the aquatic environment. Int J Environ Res Public Health 12(8):8717–8743. https://doi.org/10.3390/ijerph120808717
Zhou D, Bennett SW, Keller AA (2012) Increased mobility of metal oxide nanoparticles due to photo and thermal induced disagglomeration. PLoS ONE 7(5):e37363
Funding
Open access funding provided by University of KwaZulu-Natal. This work was supported by [Water Research Commission] (Grant numbers [K5/280] and National Research Foundation of South Africa, 120221]). Gumbi BP. has received research support from Water Research Commission and National Research Foundation.
Author information
Authors and Affiliations
Contributions
[NH, OSO, IO and BPG] authors contributed to the study conception and design. The first draft of the manuscript was written by [NH], and [NH, OSO, IO and BPG] authors commented on previous versions of the manuscript. [NH, OSO, IO and BPG] authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hendricks, N., Olatunji, O., Ofori, I. et al. Occurrence, fate, and impact of engineered metal/carbonaceous nanomaterials in the environment, detection, and quantitation methods. Int. J. Environ. Sci. Technol. 20, 12937–12954 (2023). https://doi.org/10.1007/s13762-023-04977-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04977-8