Abstract
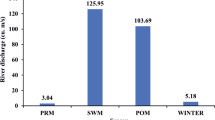
The present study investigated the prevalence of pathogenic organisms (Salmonella spp, Vibrio cholerae, and Shigella spp) and their correlation to the abundance of faecal indicator organisms in water and riverbed sediments in the Apies River, South Africa. In all, 558 water and sediment samples were collected from 10 sites in the river (May 2013–February 2014) and analysed through culture and molecular (real-time PCR) techniques. Concentrations of faecal indicator organisms in sediments reached 1.39 × 105 (±standard deviation) CFU/100 mL. All three pathogens were detected in water and sediments. Pathogens were mostly detected in sediments at sites influenced either by wastewater treatment works or by informal settlements. During the wet and dry seasons (water column), a strong positive correlation was observed between E. coli and all pathogens; C. perfringens only correlated with V. cholerae. Within sediments, strong positive correlations were only observed between E. coli and Salmonella spp, E. coli and V. cholerae (dry season); E. coli and V. cholerae and E. coli and Shigella spp (wet season). No correlation was observed between sediments C. perfringens counts and all the pathogens. Thus, sediments of the Apies River harbour pathogenic organisms. Correlation between E. coli and pathogenic organisms in the sediments suggests that E. coli could also be an indicator of pathogens’ presence. However, the lack of a correlation between E. coli and some pathogens in sediments and between C. perfringens and all the pathogens highlights the need to investigate for more indicators of pathogens’ presence in this complex matrix.


Similar content being viewed by others
References
Abdallah SA, Elmanama AA, Fahd MI, Afifi S (2005) Microbiological beach sand quality in the Gaza Strip in comparison to seawater. Polish J Environ Stud 14:841–850
Abia ALK, Ubomba-Jaswa E, du Preez M, Momba MNB (2015a) Riverbed sediments in the Apies River, South Africa: recommending the use of both Clostridium perfringens and Escherichia coli as indicators of faecal pollution. J Soils Sediments 15:2412–2424. doi:10.1007/s11368-015-1209-0
Abia ALK, Ubomba-Jaswa E, Momba MNB (2015b) Impact of seasonal variation on Escherichia coli concentrations in the riverbed sediments in the Apies River, South Africa. Sci Total Environ 537:462–469. doi:10.1016/j.scitotenv.2015.07.132
Abia LKA, Ubomba-Jaswa E, Ssemakalu CC, Momba MNB (2015c) Development of a rapid approach for the enumeration of Escherichia coli in riverbed sediment: case study, the Apies River, South Africa. J Soils Sediments 15:2425–2432. doi:10.1007/s11368-015-1081-y
Abraham W-R (2011) Megacities as sources for pathogenic bacteria in rivers and their fate downstream. Int J Microbiol. doi:10.1155/2011/798292
Akoachere J-FT, Masalla T, Njom H (2013) Multi-drug resistant toxigenic Vibrio cholerae O1 is persistent in water sources in New Bell-Douala, Cameroon. BMC Infect Dis 13:366. doi:10.1186/1471-2334-13-366
Al-bashan MM (2012) Influences of some physico-chimical stress conditions on the survivality and resistibility of Shigella flexneri and Shigella sonnei. Glob Vet 9:706–716. doi:10.5829/idosi.gv.2012.9.6.52224
APHA/AWWA/WEF (2001) American Public Health Association, American Water Works Association, Water Environment Federation (APHA-AWWA-WEF) .2001. Standard methods for the examination of water and wastewater, 22nd edn. Washington
Ashbolt N, Grabow W, Snozzi M (2001) Indicators of microbial water quality. In: Fewtrell L, Barthram J (eds) water qual. IWA Publishing, London, pp 289–316
Ashida H, Ogawa M, Mimuro H et al (2011) Shigella are versatile mucosal pathogens that circumvent the host innate immune system. Curr Opin Immunol 23:448–455. doi:10.1016/j.coi.2011.06.001
Bag PKP, Bhowmik P, Hajra TK et al (2008) Putative virulence traits and pathogenicity of Vibrio cholerae non-o1, non-o139 isolates from surface waters in Kolkata, India. Appl Environ Microbiol 74:5635–5644. doi:10.1128/AEM.00029-08
Basson MS (2011) Water development in South Africa. UN-Water Int. Conf. Water Green Econ. Pract. Towar. Rio + 20. pp 1–12
Budillon F, Vicinanza D, Ferrante V, Iorio M (2006) Sediment transport and deposition during extreme sea storm events at the Salerno Bay (Tyrrhenian Sea): comparison of field data with numerical model results. Nat Hazards Earth Syst Sci 6:839–852. doi:10.5194/nhess-6-839-2006
Burton GAJ, Gunnison D, Lanza GR (1987) Survial of pathogenic bacteria in various freshwater sediments. Appl Environ Microbiol 53:633–638
Carey CM, Lee H, Trevors JT (2004) Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocyst. Water Res 38:818–862. doi:10.1016/j.watres.2003.10.012
CDC (2013) Turtles and other reptiles are risky pets. http://www.cdc.gov/Features/SalmonellaFrogTurtle/. Accessed 31 Mar 2015
Characklis GW, Dilts MJ, Simmons OD et al (2005) Microbial partitioning to settleable particles in stormwater. Water Res 39:1773–1782. doi:10.1016/j.watres.2005.03.004
Chase E, Hunting J, Staley C, Harwood VJ (2012) Microbial source tracking to identify human and ruminant sources of faecal pollution in an ephemeral Florida river. J Appl Microbiol 113:1396–1406. doi:10.1111/jam.12007
Chigor VN, Okoh AI (2012) Quantitative detection and characterization of human adenoviruses in the buffalo river in the Eastern Cape province of South Africa. Food Environ Virol 4:198–208. doi:10.1007/s12560-012-9090-0
Chitanand MP, Gyananath G, Lade HS (2008) Bacterial assessment of ground water: a case study of Nanded city. J Environ Biol 29:315–318
Craig DL, Fallowfield HJ, Cromar NJ (2004) Use of microcosms to determine persistence of Escherichia coli in recreational coastal water and sediment and validation with in situ measurements. J Appl Microbiol 96:922–930. doi:10.1111/j.1365-2672.2004.02243.x
Crowther J, Kay D, Wyer MD (2002) Faecal-indicator concentrations in waters draining lowland pastoral catchments in the UK: relationships with land use and farming practices. Water Res 36:1725–1734. doi:10.1016/S0043-1354(01)00394-3
de Magny GC, Mozumder PK, Grim CJ et al (2011) Role of zooplankton diversity in Vibrio cholerae population dynamics and in the incidence of cholera in the Bangladesh Sundarbans. Appl Environ Microbiol 77:6125–6132. doi:10.1128/AEM.01472-10
Decamp O, Warren A (2000) Investigation of Escherichia coli removal in various designs of subsurface flow wetlands used for wastewater treatment. Ecol Eng 14:293–299
Department of Environmental Affairs Republic of South Africa (2012) South African water quality guidelines for coastal marine waters. Volume 2: Guielines for recreational use
Devane ML, Moriarty EM, Wood D et al (2014) The impact of major earthquakes and subsequent sewage discharges on the microbial quality of water and sediments in an urban river. Sci Total Environ 485–486:666–680. doi:10.1016/j.scitotenv.2014.03.027
Djaouda M, Gaké B, Ebang Menye D et al (2013) Survival and Growth of Vibrio cholerae, Escherichia coli, and Salmonella spp. in well water used for drinking purposes in Garoua (North Cameroon). Int J Bacteriol. doi:10.1155/2013/127179
Agency Environment (2002) The Microbiology of Drinking Water (2002)—Part 10—Methods for the isolation of Yersinia, Vibrio and Campylobacter by selective enrichment. Methods exam Waters Assoc Mater 10:14–17
Faruque SM, Chowdhury N, Kamruzzaman M et al (2004) Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci 101:2123–2128. doi:10.1073/pnas.0308485100
Finkelstein RA (1996) Cholera, Vibrio cholerae O1 and O139, and other pathogenic vibrios. In: Baron S (ed) Medical microbiolology, 4th edn. University of Texas, Galveston
Fries JS, Characklis GW, Noble RT (2008) Sediment—water exchange of Vibrio sp. and fecal indicator bacteria: implications for persistence and transport in the Neuse River Estuary, North Carolina. USA Water Res 42:941–950. doi:10.1016/j.watres.2007.09.006
Gao G, Falconer R, Lin B (2011) Numerical modelling sediment-bacteria interaction processes in the Severn Estuary. J Water Resour Prot 3:22–31. doi:10.4236/jwarp.2011.31003
Garzio-hadzick A, Shelton DR, Hill RL et al (2010) Survival of manure-borne E. coli in streambed sediment: effects of temperature and sediment properties. Water Res 44:2753–2762. doi:10.1016/j.watres.2010.02.011
Gemmell ME, Schmidt S (2013) Is the microbiological quality of the Msunduzi River (KwaZulu-Natal, South Africa) suitable for domestic, recreational, and agricultural purposes? Environ Sci Pollut Res 20:6551–6562. doi:10.1007/s11356-013-1710-1
Goel AK, Tamrakar AK, Nema V et al (2005) Detection of viable toxigenic Vibrio cholerae from environmental water sources by direct cell duplex PCR assay. World J Microbiol Biotechnol 21:973–976. doi:10.1007/s11274-004-7317-4
Gonzalez RA, Conn KE, Crosswell JR, Noble RT (2012) Application of empirical predictive modeling using conventional and alternative fecal indicator bacteria in eastern North Carolina waters. Water Res 46:5871–5882. doi:10.1016/j.watres.2012.07.050
Hendriksen SWM, Orsel K, Wagenaar JA, Miko A (2004) Animal-to-human transmission of Salmonella Typhimurium DT104A variant. Emerg Infect Dis 10:2225–2227
Hsu B-M, Wu S-F, Huang S-W et al (2010) Differentiation and identification of Shigella spp. and enteroinvasive Escherichia coli in environmental waters by a molecular method and biochemical test. Water Res 44:949–955. doi:10.1016/j.watres.2009.10.004
Ibekwe A, Papiernik S (2010) Quantification of persistence of Escherichia coli O157: H7 in contrasting soils. Int J Microbiol. doi:10.1155/2011/421379
Islam MS, Hossain MA, Khan SI et al (2001) Survival of Shigella dysenteriae type 1 on fomites. J Heal Popul Nutr 19:177–182
Jamieson R, Gordon R, Joy D, Lee H (2004) Assessing microbial pollution of rural surface waters a review of current watershed scale modeling approaches. Agric Water Manag 70:1–17. doi:10.1016/j.agwat.2004.05.006
Jamieson RC, Joy DM, Lee H et al (2005a) Resuspension of sediment-associated Escherichia coli in a natural stream. J Environ Qual 34:581. doi:10.2134/jeq2005.0581
Jamieson RC, Joy DM, Lee H et al (2005b) Transport and deposition of sediment-associated Escherichia coli in natural streams. Water Res 39:2665–2675. doi:10.1016/j.watres.2005.04.040
Jeong K, Zhang Q, Nunnari J, Tzipori S (2010) A piglet model of acute gastroenteritis induced by Shigella dysenteriae Type 1. J Infect Dis 201:903–911. doi:10.1086/650995
Jiang J, Wang P, Pan G, Kang L (2005) Isolation and identification of rabbits Shigella dysenteriae in a large scale warren. J Anhui Agric Sci 33:1666–1667
Kaur J, Jain SK (2012) Role of antigens and virulence factors of Salmonella enterica serovar Typhi in its pathogenesis. Microbiol Res 167:199–210. doi:10.1016/j.micres.2011.08.001
Kay D, Crowther J, Fewtrell L et al (2008) Quantification and control of microbial pollution from agriculture: a new policy challenge? Environ Sci Policy 11:171–184. doi:10.1016/j.envsci.2007.10.009
Khan S, Khan W (2012) Isolation and identification of bacterial pollutants from the Berg and Plankenburg Rivers in the Western Cape, South Africa. Water SA 38:819–824
Kinge CW, Mbewe M (2010) Characterisation of Shigella spesies isolated from river catchments in the North West Province of South Africa. S Afr J Sci 106:211. doi:10.4102/sajs.v106i11/12.211
Kinzelman JL, McLellan SL (2009) Success of science-based best management practices in reducing swimming bans—a case study from Racine, Wisconsin, USA. Aquat Ecosyst Health Manag 12:187–196. doi:10.1080/14634980902907466
Kirschner AKT, Schauer S, Steinberger B et al (2011) Interaction of Vibrio cholerae non-O1/non-O139 with copepods, cladocerans and competing bacteria in the large Alkaline Lake Neusiedler See, Austria. Microb Ecol 61:496–506. doi:10.1007/s00248-010-9764-9
Koirala SR, Gentry RW, Perfect E et al (2008) Temporal variation and persistence of bacteria in streams. J Environ Qual 37:1559–1566. doi:10.2134/jeq2007.0310
Korajkic A, Brownell MJ, Harwood VJ (2011) Investigation of human sewage pollution and pathogen analysis at Florida Gulf coast beaches. J Appl Microbiol 110:174–183. doi:10.1111/j.1365-2672.2010.04869.x
Kothary MH, Babu US (2001) Infective dose of foodborne pathogens in volunteers: a review. J Food Saf 21:49–73. doi:10.1111/j.1745-4565.2001.tb00307.x
Labelle RL, Gerba CP, Goyal SM et al (1980) Relationships between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl Environ Microbiol 39:588–596
le Roux WJ, van Blerk GN (2011) The use of a high resolution melt real-time polymerase chain reaction (PCR) assay for the environmental monitoring of Vibrio cholerae. African J Microbiol Res 5:3520–3526. doi:10.5897/AJMR11.695
Leclerc H, Mossel DA, Edberg SC, Struijk CB (2001) Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Annu Rev Microbiol 55:201–234. doi:10.1146/annurev.micro.55.1.201
Lemarchand K, Masson L, Brousseau R (2004) Molecular biology and DNA microarray technology for microbial quality monitoring of water. Crit Rev Microbiol 30:145–172. doi:10.1080/10408410490435142
Malorny B, Hoorfar J, Bunge C, Helmuth R (2003) Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl Environ Microbiol 69:290–296. doi:10.1128/AEM.69.1.290-296.2003
Mcfeters GA, Bissonnette GK, Jezeski JJ et al (1974) Comparative survival of indicator bacteria and enteric pathogens in well water. Appl Mcrobiol 27:823–829
Molobela IP, Sinha P (2011) Management of water resources in South Africa: a review. African J Environ Sci Technol 5:993–1002. doi:10.5897/AJEST11.136
Monis PT, Thompson RCA (2003) Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect Genet Evol 3:233–244. doi:10.1016/j.meegid.2003.08.003
Mostowy S, Boucontet L, Moya MJM et al (2013) The zebrafish as a new model for the in vivo study of Shigella flexneri interaction with phagocytes and bacterial autophagy. PLoS Pathog 9:e1003588. doi:10.1371/journal.ppat.1003588
Mulamattathil SG, Bezuidenhout C, Mbewe M, Ateba CN (2014) Isolation of environmental bacteria from surface and drinking water in Mafikeng, South Africa, and characterization using their antibiotic resistance profiles. J Pathog. doi:10.1155/2014/371208
Nandi B, Nandy RK, Mukhopadhyay S et al (2000) Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J Clin Microbiol 38:4145–4151
Ndlovu T, Le Roux M, Khan W, Khan S (2015) Co-detection of virulent Escherichia coli genes in surface water sources. PLoS ONE 10:e0116808. doi:10.1371/journal.pone.0116808
Olsvik O, Sørum H, Birkness K et al (1985) Plasmid characterization of Salmonella typhimurium transmitted from animals to humans. J Clin Microbiol 22:336–338
Páll E, Niculae M, Kiss T et al (2013) Human impact on the microbiological water quality of the rivers. J Med Microbiol 62:1635–1640. doi:10.1099/jmm.0.055749-0
Pan B, Wang W, Xie Y et al (2006) Detection, serology classification and drug susceptibility of Shigella from experimental monkeys. Guangxi Agric Sci 37:331–332
Pandey PK, Soupir ML, Rehmann CR (2011) A model for predicting resuspension of Escherichia coli from streambed sediments. Water Res 46:115–126. doi:10.1016/j.watres.2011.10.019
Paulse AN, Jackson VA, Khan W (2009) Comparison of microbial contamination at various sites along the Plankenburg—and Diep Rivers, Western Cape, South Africa. Water SA 35:469–478
Pianetti A, Bruscolini F, Sabatini L, Colantoni P (2004) Microbial characteristics of marine sediments in bathing area along Pesaro-Gabicce coast (Italy): a preliminary study. J Appl Microbiol 97:682–689. doi:10.1111/j.1365-2672.2004.02352.x
Rabbani GH, Albert MJ, Rahman H et al (1995) Development of an improved animal model of shigellosis in the adult rabbit by colonic infection with Shigella flexneri 2a. Infect Immun 63:4350–4357
Rawlings TK, Ruiz GM, Colwell RR (2007) Association of Vibrio cholerae O1 El Tor and O139 Bengal with the copepods Acartia tonsa and Eurytemora affinis. Appl Environ Microbiol 73:7926–7933. doi:10.1128/AEM.01238-07
Reeves RL, Grant SB, Mrse RD et al (2004) Scaling and management of fecal indicator bacteria in runoff from a coastal urban watershed in Southern California. Environ Sci Technol 38:2637–2648. doi:10.1021/es034797g
Santhiya G, Lakshumanan C, Selvin J, Asha D (2011) Microbiological analysis of seawater and sediments in urban shorelines: occurrence of heavy metals resistance bacteria on Chennai beaches, Bay of Bengal. Microchem J 99:197–202. doi:10.1016/j.microc.2011.05.004
Seanego KG, Moyo NAG (2013) The effect of sewage effluent on the physico-chemical and biological characteristics of the Sand River, Limpopo, South Africa. Phys Chem Earth 66:75–82. doi:10.1016/j.pce.2013.08.008
Shi R, Yang X, Chen L et al (2014) Pathogenicity of Shigella in chickens. PLoS ONE 9:e100264. doi:10.1371/journal.pone.0100264
Shibata T, Solo-Gabriele HM, Fleming LE, Elmir S (2004) Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Res 38:3119–3131. doi:10.1016/j.watres.2004.04.044
Sibanda T, Okoh AI (2013) Real-time PCR quantitative assessment of hepatitis A virus, rotaviruses and enteroviruses in the Tyume River located in the Eastern Cape Province, South Africa. Water SA 39:295–304
Signor RS (2005) Quantifying the impact of runoff events on microbiological contaminant concentrations entering surface drinking source waters. J Water Health 3:453–468. doi:10.2166/wh.2005.052
South African Department of Water Affairs (DWA) (2012) Green drop progress report. Department of Water Affairs, Pretoria
Stapleton CM, Wyer MD, Kay D et al (2007) Fate and transport of particles in estuaries, vols. I, II, III, IV Environment agency science report SC000002/SR1-4
Teklehaimanot GZ, Coetzee MAA, Momba MNB (2014) Faecal pollution loads in the wastewater effluents and receiving water bodies: a potential threat to the health of Sedibeng and Soshanguve communities, South Africa. Environ Sci Pollut Res 21:9589–9603. doi:10.1007/s11356-014-2980-y
Theron J, Morar D, du Preez M et al (2001) A sensitive seminested PCR method for the detection of Shigella in spiked environmental water samples. Water Res 35:869–874
Tyagi V, Chopra A (2006) Alternative microbial indicators of faecal pollution: current perspective. Iran J Environ Heal Sci Eng 3:205–216
Tyrrel SF, Quinton JN (2003) Overland flow transport of pathogens from agricultural land receiving faecal wastes. J Appl Microbiol 94(Suppl):87S–93S. doi:10.1046/j.1365-2672.94.s1.10.x
Venter A (2007) Prioritization of river basins in the Tshwane area with reference to faecal coliform bacteria for the purpose of the identification of candidate wetlands for rehabilitation. University of the Witwatersrand, Johannesburg
Vignesh S, Dahms H, Emmanuel KV et al (2014) Physicochemical parameters aid microbial community? A case study from marine recreational beaches, Southern India. Environ Monit Assess 186:1875–1887. doi:10.1007/s10661-013-3501-z
Walters E, Schwarzwälder K, Rutschmann P et al (2014) Influence of resuspension on the fate of fecal indicator bacteria in large-scale flumes mimicking an oligotrophic river. Water Res 48:466–477. doi:10.1016/j.watres.2013.10.002
Weaver L, Sinton L (2009) Deposition and survival of enteric microbes in aquatic sediments—a brief review. Institute of Environmental Science & Research Ltd, Christchurch
WHO (2005) Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. WHO Press, Geneva
WHO (2008) WHO guidelines for drinking-water quality, Vol 1, 3rd edn. Recommendations. doi: 10.1016/S1462-0758(00)00006-6
Zhao S, Qaiyumi S, Friedman S et al (2003) Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J Clin Microbiol 41:5366–5371. doi:10.1128/JCM.41.12.5366-5371.2003
Acknowledgments
This work received funding from the Tshwane University of Technology (TUT), Water Research commission (WRC), South Africa (Grant numbers K5/2169 and K5/2147) and the National Research Foundation (NRF). Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the TUT, WRC or NRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Abbaspour.
Rights and permissions
About this article
Cite this article
Abia, A.L.K., Ubomba-Jaswa, E. & Momba, M.N.B. Prevalence of pathogenic microorganisms and their correlation with the abundance of indicator organisms in riverbed sediments. Int. J. Environ. Sci. Technol. 13, 2905–2916 (2016). https://doi.org/10.1007/s13762-016-1116-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1116-y