Abstract
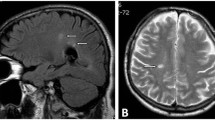
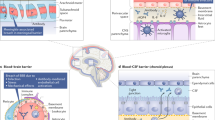
Rheumatoid arthritis (RA) is a chronic disease, the etiology of which has yet to be clarified, which causes activation of proinflammatory pathways that bring about joint and systemic inflammation. Although peripheral nervous system anomalies are observed widely in RA, very few case reports on changes in the central nervous system (CNS) have been published. In recent years, the pathophysiology of CNS involvement that can occur in RA has attracted a great deal of attention. Emphasis has focused on the possibility that CNS involvement occurs due to blood–brain barrier (BBB) damage associated with chronic inflammation. The present study was performed to investigate the possible effects of BBB dysfunction and tumor necrosis factor (TNF) blocker therapy on BBB function, which may cause CNS damage in patients with RA. 58 RA patients [47 (81.0%) females, 11 (19.0%) males] and 34 healthy controls [24 (70.6%) females, 10 (29.4%) males] were included in the study. All RA patients were on synthetic DMARD therapy at the beginning. Thirty patients continued DMARD therapy, and 28 patients with high disease activity were started on TNF blocker therapy. All demographic characteristics of the patients were recorded. Disease activity was evaluated using the Disease Activity Score 28-joint count C reactive protein. The Mini-Mental State Examination was used to evaluate cognitive function, and the Fazekas scale was used to assess cranial lesions visualized by magnetic resonance imaging (MRI). Patients’ peripheral blood S100β, glial fibrillary acidic protein (GFAP), claudin, interleukin (IL)-17, and IL-1β levels were measured at the beginning of the study and after 6 months. Demographic characteristics (including sex, age, and body mass index) were similar in the RA and control groups. S100β and GFAP levels were significantly higher in the patient group than in the control group. In the group that was started on TNF blocker therapy, S100β and GFAP levels were significantly decreased 6 months after commencement of treatment. No difference was observed between the RA and control groups in terms of hyperintense lesions seen on cranial MRI. The S100β levels increased with lesions in the deep white matter seen on cranial MRI in patients with RA. In conclusion, next to decreasing disease activity and joint erosions by suppressing inflammation, anti-TNF therapy in RA can also suppress potential CNS involvement linked to BBB (blood–brain barrier) dysfunction. Further studies with broader participation and longer patient follow-up are needed to reinforce this hypothesis.
Similar content being viewed by others
References
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
Watson P, Fekete J, Deck J (1977) Central nervous system vasculitis in rheumatoid arthritis. Can J Neurol Sci 4:269–272
Paci R, Giuffrida CM, Marangolo M, Ventura F (1983) Di Paola F (1983) Neuroradiologic picture of cerebral vasculitis in rheumatoid arthritis. Neuroradiology 25:343–345
Ishizuka T, Suzuki K, Hara M, Kitani A, Kawagoe M, Nakamura H, Tsuchiya K (1992) Detection of intracranial lesions of rheumatoid vasculitis with magnetic resonance imaging (MRI). Ryumachi 32:19–26
Chowdhry V, Kumar N, Lachance DH, Salomao DR, Luthra HS (2005) An unusual presentation of rheumatoid meningitis. Neuroimaging 15:286–288
Caballol Pons N, Montalà N, Valverde J, Brell M, Ferrer I, Martínez-Yélamos S (2010) Isolated cerebral vasculitis associated with rheumatoid arthritis. Joint Bone Spine 77:361–363
Banks WA, Robinson SM (2010) Minimal penetration of lipopolysaccharide across the murine blood–brain barrier. Brain Behav Immun 24:102–109
Pan W (2002) Kastin, A.J.TNFa transport across the blood–brain barrier is abolished in receptor knockout mice. Exp Neurol 174:193–200
Nishioku TL, Furusho K, Tomita A, Ohishi H, Dohgu S, Shuto H, Yamauchi A, Kataoka Y (2011) Potential role for S100A4 in the disruption of the blood–brain barrier in collagen-induced arthritic mice, an animal model of rheumatoid arthritis. Neuroscience. 189:286–292
Nishioku TL, Dohgu S, Takata F, Eto T, Ishikawa N, Kodama KB, Nakagawa S, Yamauchi A, Kataoka Y (2009) Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 29:309-316
Nishioku T, Yamauchi A, Takata F, Watanabe T, Furusho K, Shuto H, Dohgu S, Kataoka Y (2010) Disruption of the blood–brain barrier in collagen-induced arthritic mice. Neurosci Lett 482:208–211
Ambartsumian N, Klingelhofer J, Grigorian M, Christensen C, Kriajevska M, Tulchinsky E, Georgiev G, Berezin V, Bock E, Rygaard J, Cao R, Cao Y, Lukanidin E (2001) The metastasis associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene 20:4685–4695
Bao LL, Zhu Y, Elhassan AM, Wu Q, Xiao B, Zhu J, Lindgren JU (2001) Adjuvant induced arthritis: IL-1 beta, IL-6 and TNF-alpha are up-regulated in the spinal cord. Neuroreport 12:3905–3908
Hamed Sherifa A, Selim Zahra I, Elattar Amal M, Elserogy Yasser M, Ahmed Eman A, Mohamed Hanan O (2012) Assessment of biocorrelates for brain involvement in female patients with rheumatoid arthritis. Clin Rheumatol 31:123–132
Goncalves CA, Leite MC, Nardin P (2008) Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 41:755–763
Germano AF, Tomasello F (2001) Blood–brain barrier permeability changes after subarachnoid haemorrhage: an update. Clinical implications, experimental findings, challenges and future directions. Springer, New York, pp 5–18
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376:1094–1108
Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J et al (2009) Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 68:954–960
Al-Rajeh S, Ogunniyi A, Awada A, Daif A, Zaidan R (1999) Preliminary assessment of an Arabic version of the Mini-Mental state examination. Ann Saudi Med 19:150–152
Folstein MF, Folstein SE, McHugh PH (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Kim GM, Park KY, Avery R et al (2014) Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke 45:479–485
Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 149:351–356
Waisman A, Hauptmann J, Rgen T (2015) The role of IL-17 in CNS disease. Acta Neuropathol 129:625–637
D’Mello C, Le T, Swain MG (2009) Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor—signaling during peripheral organ inflammation. J Neurosci 29:2089–2102
Mellor R (1959) Cellular origin of rheumatoid factor. J Exp Med 110:875–876
Markenson JA, McDougal JS, Tsairis P, Lockshin MD, Christian CL (1979) Rheumatoid meningitis: a localized immune process. Ann Intern Med 90:786–789
Inan AS, Masatlioglu S, Ozyurek SC, Engin D, Erdem I (2011) Unusual central nervous system involvement of rheumatoid arthritis: success- ful treatment with steroid and azathioprine. Rheumatol Int 31:1383–1385
Teeling JL, Carare RO, Glennie MJ, Perry VH (2012) Intracerebral immune complex formation induces inflammation in the brain that depends on Fc receptor interaction. Acta Neuropathol 2012(124):479–490
Rech J, Hess A, Finzel S, Kreitz S, Sergeeva M, Englbrecht M et al (2013) Association of brain functional magnetic resonance activity with response to tumor necrosis factor inhibition in rheumatoid arthritis. Arthritis Rheum 65:325–333
Skelly DT, Hennessy E, Dansereau MA, Cunningham C (2013) A systematic analysis of the peripheral and CNS effects of systemic LPS, IL. 1beta, TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS One 2013(8):e69123
Thomson CA, McColl A, Cavanagh J, Graham GJ (2014) Peripheral inflammation is associated with remote global gene expression changes in the brain. J Neuroinflammation 11:73
Chou R, Kane M, Ghimire S, Gautam S (2010) Tumor necrosis factor inhibition reduces the incidence of Alzheimer’s disease in rheumatoid arthritis patients. American College of Rheumatology, Atlanta
Detrait ER, Danis B, Lamberty Y, Foerch P (2014) Peripheral administration of anti-TNF-alpha receptor fusion protein counteracts the amyloid induced elevation of hippocampal TNF-alpha levels and memory deficits in mice. Neurochem Int 72:10–13
Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S et al (2011) Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci USA 108:3731–3736
Kaltsonoudis E, Zikou AK, Voulgari PV, Konitsiotis S, Argyropoulou MI, Drosos AA (2014) Neurological adverse events in patients receiving anti. TNF therapy: a prospective imaging and electrophysiological study. Arthritis Res Ther 16:125
Bosch X, Saiz A, Ramos-Casals M, BIOGEAS Study Group (2011) Monoclonal antibody therapy-associated neurological disorders. Nat Rev Neurol 7:165–172
Nozaki K, Silver RM, Stickler DE, Abou-Fayssal NG, Giglio P, Kamen DL et al (2011) Neurological deficits during treatment with tumor necrosis factor-alpha antagonists. Am J Med Sci 342:352–355
Andreadou E, Kemanetzoglou E, Brokalaki Ch, Evangelopoulos ME, Kilidireas C, Rombos A et al (2013) Demyelinating disease following anti-TNFα treatment: a causal or coincidental association? Report of four cases and review of the literature. Case Rep Neurol Med 2013:671935
King KS, Peshock RM, Rossetti HC, McColl RW, Ayers CR, Hulsey KM et al (2014) Effect of normal aging versus hypertension, abnormal body mass index, and diabetes mellitus on white matter hyperintensity volume. Stroke 45:255–257
Bekkelund SI, Pierre-Jerome C, Husby G, Mellgren SI (1995) Quantitative cerebral MR in rheumatoid arthritis. Am J Neuroradiol 16:767–772
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sağ, S., Sağ, M.S., Tekeoğlu, I. et al. Central nervous system involvement in rheumatoid arthritis: possible role of chronic inflammation and tnf blocker therapy. Acta Neurol Belg 120, 25–31 (2020). https://doi.org/10.1007/s13760-017-0879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-017-0879-3