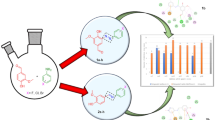
Abstract
New spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine derivatives (3–23) were investigated. Then there is in vitro antimicrobial potency against possible organisms Staphylococcus aurous ATCC-47,077, Bacillus cereus ATCC-12228, Escherichia coli ATCC-25922, Salmonella typhiATCC-15566, and Candida albicans ATCC-10231 were tested utilizing commercially available antibiotics ampicillin as a reference drug. A preliminary antimicrobial test represented that derivatives: (Aldoses) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (16–19) and (Acetyl aldoses) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro [cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (20–23) exhibited higher antifungal, and antibacterial performance with minimum inhibitory concentrations values of (39–67 µg/ml) toward all pathogenic strains compared to common reference drug ampicillin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The design, synthesis, and manufacture of molecules with therapeutic benefits for humans are one of the fundamental goals of medicinal organic chemistry [1,2,3,4]. Chemical libraries based on favored structures have been available thanks to advances in combinatorial chemistry during the last decade [5,6,7]. Spiro-compounds are a type of naturally founding chemical with significant biological features (Fig. 1) [8,9,10]. Because of their structure–activity relationship, are a very appealing target for combinatorial library synthesis [11,12,13]. It is a significant category of nitrogen and sulfur-containing heterocycles that are frequently used as major building blocks in the field of pharmaceuticals and pharmacological agents [14,15,16,17]. Recently; an efficient and additive-free synthesis of heterocycles via green strategy in excellent yields and short reaction time as an environmental procedure [18,19,20,21,22]
The spiro-thiazolidinone nucleus is also recognized as the “wonder nucleus” since it produces a set of derivatives with various biological activities [23,24,25]. The existence of an N–C-S linkage in the compounds has been found to have antimicrobial and antithyroid properties [24]. The antifungal performance of several 4-thiazolidinones has been evaluated [26]. Antitubercular [27], antioxidant [28], analgesic, [29] anticonvulsant [30], anti-inflammatory [31], antihyperglycemic [32], diuretic [33], antihistaminic [34], antidiabetic [35, 36], cyclooxygenase inhibitors [37], and lipoxygenase inhibitors[37] properties were also discovered. The presence of nitrogen and sulfur atoms is highlighting the importance of thiazolidinone which is widely used as a key building block in the scope of pharmaceutical agents and drugs [38, 39].
Carbohydrates have a unique property: the electrophilic nature of the anomeric core allows the sugar to link with diverse molecules through presenting a functional group that acts as a nucleophile [40, 41]. In nature, the OH; NH2 group usually performs this linkage; however, sulfur or carbon nucleophile may also be involved, resulting in S-glycosides and C-glycosides, respectively [42]. The attention to S- and C-glycosides is attributed to the metabolic stability of the glycosidic bond, which cannot be hydrolyzed by glycosidases enzyme [43]. The metabolic stability of S- and C-glycosides is also exploited in the generation of glycomimetic drugs resistant against in vivo hydrolysis. [44]
The most popular strategy for treating diseases caused by resistant bacteria is antimicrobial therapy [45]. As a result, there is a lot of demand and benefit in the discovery of novel antimicrobial compounds [46, 47]. Furthermore, some S- and C-glycosides are significant pharmacophore that is integrated into various bioactive compounds, especially in antimicrobial therapy [48, 49]. In the past decade, many heterocyclic compounds and their corresponding glycosides have been synthesized to obtain a novel antimicrobials drug, which would be able to treat infections caused by resistant bacteria and fungi strains. [50, 51]
Because of the above-mentioned, the main subject of our study is to synthesize the biologically important scaffold based on new spiro(cyclohexane-thiazolidine) derivatives and its corresponding glycosides with the assessment of their antibacterial and antifungal activity.
Results and discussion
Chemistry
Continuing our interesting research on the design and investigation of a broad range of applicable heterocyclic derivatives, the 4-(4-Fluorophenyl)-1-thia-4-azaspiro[4.5]decan-3-one 1 was prepared in agreement with our previous method (Fig. 2, c.f. experimental). Compound 1 was allowed to react with malononitrile through a one-pot three-component reaction in the presence of both 4-methylbenzaldehyde and ammonium acetate yielded the corresponding 5'-Amino-3'-(4-fluorophenyl)-7'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (2). The chemical structure of spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]carbonitrile derivative 2 is assigned with all spectroscopic data; the new C≡N group displays signals at 2216 cm−1and 114.16 ppm in both IR and 13C-NMR spectrum, respectively. Also, the new NH2 group shows a peak at 3132 cm−1 in IR spectra and a broad signal at 8.66 ppm exchangeable with D2O in 1H-NMR spectra. Besides presence signal attributed to CH3 group at 2.37 and 21.23 ppm in both 1H and13C-NMR spectrum, respectively (Fig. 2, c.f. experimental).
Spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]carbonitrile derivative 2 was utilized as an important framework for the preparation of further new fused heterocyclic compounds. It reacted with sodium nitrite in the presence of hydrochloric acid to produce the corresponding spirothiazolo triazine derivative 3; where its IR and NMR spectrum revealed the disappearance of signal characteristic to both NH2 and CN groups; besides displaying the molecular weight with isotopic pattern peak; m/z at 477 [M+], 479 [M+ + 2] (Fig. 2, c.f. experimental). Further treatment of spiro[cyclo hexane-1,2'-thiazolo[4,5-b]pyridine]carbonitrile derivative 2 with various reagents namely: malononitrile, diethyl malonate, ethyl cyanoacetate and benzylidene malononitrile produced a new spiro[cyclohexane-1,2'-thiazolo[4,5-b] [1, 8] naphthyridine]-7'-carbonitrile derivatives 4–7, respectively (Fig. 2). The IR spectra for previous derivatives 4–7 represented that 6',8'-diamino-3'-(4-fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b][1,8]naphthyridine]-7'-carbonitrile(4) show the existence of new amino group at 3435 cm−1 besides the presented amino and cyano groups at 3162, 2219 cm−1, respectively, Ethyl 8'-amino-3'-(4-fluorophenyl)-6'-oxo-9'-(p-tolyl)-5',6'-dihydro-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b] [1, 8] naphthyridine]-7'-carboxylate (5) show the presence of two carbonyl groups at 1734 cm−1 for ester and 1670 cm−1 for amide, while 8'-Amino-3'-(4-fluorophenyl)-6'-oxo-9'-(p-tolyl)-5',6'-dihydro-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b] [1, 8] naphthyridine]-7'-carbonitrile (6) show a peak represented for new amide group at 3116 and 1681 cm−1 for NH and C = O groups, respectively. The 1H-NMR spectra for latter derivatives showed that compound 4 showed two broad singlet signals characteristic for two amino groups at 8.40 and 8.65 ppm, while compound 5 showed an ethyl ester group at ppm = 1.31 (t, J = 7.49, 3H, CH2CH3) and 4.26 (q, J = 7.50, 2H, CH2 CH3). Also, compound 6 given signal peak characteristic for the amide NH group which is exchangeable with D2O involved with aromatic proton peaks at 7.19–7.98 (m, 9H, 8Ar-H + NH amide) (Fig. 2, c.f. experimental).
Moreover, the solvent effect in the organic heterocyclization can be illustrated when spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]carbonitrile derivative 2 is allowed to react with malononitrile in polar protic solvent (EtOH) to produce 2-(8'-Amino-3'-(4-fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'thiazolo[5',4':5,6]pyrido[2,3-d] pyrimidin]-6'-yl)acetonitrile (8) rather than derivative 4 which previously obtained when the reaction done in a polar aprotic solvent (DMF), where NMR spectra displayed singlet signals in 1H-NMR at δ 4.56 ppm for new CH2-CN group and in 13C-NMR at δ 27.96 ppm. Additionally; in mass, the spectrum represented the molecular ion peak at m/z (%) 496 (M+, 59%) and its base peak at m/z 456 (M+-CH2CN, 100%) (Fig. 3, c.f. experimental).
Additionally; treatment of spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]carbonitrile derivative 2 with formamide, thiourea, benzonitrile, phenylisothiocyanat, formic acid, chloroacetyl chloride and thioacetamide the newly synthesized spiro[cyclohexane-1,2'-thiazolo[5',4':5,6] pyrido[2,3-d]pyrimidin]-8'-amine derivatives 9–15 were given, respectively (Fig. 3). The 1H-NMR spectrum of compounds 9 displayed a new singlet signal related to pyrimidine-H at 8.20 ppm; compound 10 showed a broad singlet signal exchangeable with D2O at δ = 7.02 for -NHCS- group in pyrimidine ring; compound 11 showed new signals for substituted phenyl group in pyrimidine ring, compound 12 observe two signals exchangeable with D2O specific for two NH groups in pyrimidine ring at 9.19 ppm for C = NH and at aromatic-H range 7.21–8.41 ppm for NHCS; compound 13 showed a characteristic signal for pyrimidine-H at δ 8.28 ppm compound 14 displayed new signal related to CH2Cl group substituted in pyrimidine ring at δ 5.17 ppm and compound 15 presented singlet signal at δ 2.32 ppm for new methyl group substituted pyrimidine ring (Fig. 3; c.f. experimental). While the IR and 13C-NMR spectrum of compounds 13 and 14 showed a new characteristic band attributed to the carbonyl group in pyrimidine ring at 1685, 1682 cm−1 and 162.20, 162.19 ppm, respectively; on the other hand, the 13C-NMR spectra for compounds 10, 12 and 15 displayed new signals for C = S group in pyrimidine ring at 172.19, 171.21 and 169.32, respectively (Fig. 3; c.f. experimental).
Finally, the reaction of spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]carbonitrile derivative 2 with aldoses namely; D-xylose, D-arabinose, D-glucose, and D-galactose in presences of Schiff's based reaction condition produced the corresponding sugars amides 16–19, respectively. The IR and 1H-NMR spectrum of latter derivatives represented the disappearance of the characteristic signal of the NH2 group besides the appearance of signal characteristics for both OH groups and sugars chain moieties. Acetylating sugars amides derivatives 16–19 leads to the formation of the corresponding acetylated derivatives 20–23, respectively; where IR and NMR spectrum of last derivatives 20–23 exhibited characteristic signals attributed to acetyl groups (Fig. 4; c.f. experimental).
Anti-microbial activity
The antimicrobial performance for novel derivatives based on spirothiazolidine backbone, which was prepared in excellent yields, against gram-positive bacteria Staphylococcus aurous ATCC- 47,077 (St.), Bacillus cereus ATCC-12228 (B.C.), and gram-negative bacteria species Escherichia coli ATCC-25922 (E.C.), Salmonella typhi ATCC-15566 (Salm.), in addition to fungi strain namely; Candida albicans ATCC-10231 (C. Alb.) were tested. The results are shown in Table 1.
As shown in Table 1, compounds 16–23 showed higher performance than the reference drug commonly utilized toward all strains according to the following activity order 16–19 > 20–23 > ampicillin, whereas the potency of derivatives 8–15 against all strains was the same as ampicillin (8–15 ≈ ampicillin) according to the following activity order; 8–11 > 13–15 ≥ ampicillin. Moreover, the estimation of the potency revealed that compounds 4–7 exhibited moderate performance against all strains when compared with the reference ampicillin drug with potency order; ampicillin ≥ 4–7. Additionally, all newly prepared compounds were more selective toward fungi strain Candida albicans ATCC-10231 (C. Alb.)
The minimal inhibitory concentration (MIC) of all new prepared analogs 1–23 was tested by utilizing different concentrations of each compound and determining the lowest concentration showing pathogen growth inhibition. The results are illustrated in Table 2 based on the type of prepared derivative, and microbe strain. For the same compound, the MIC values changed depending on the pathogen.
Structure Activity Relationship (SAR)
The structure–activity relationship (SAR) of spirothiazolopyridine can be established from the data of the antimicrobial potency shown in Table 1. Analysis of the SAR of the spirothiazolopyridine derivatives 1–23 explains the link between the activity and the structure, which offers clues for structural modifications that can enhance antimicrobial performance. SAR analysis is important in understanding antibacterial and antifungal activities for newly prepared derivatives 1–23. First: Introduce open-chain sugar moiety shows a significant role to increase antimicrobial performance more than the common reference drug; ampicillin and the activity increased by increasing the number of –OH groups rather than –OAc groups in the open-chain sugar nucleus according to the following order: 19≈18 (5 free polar hydroxyl groups) > 17≈16 (4 free polar hydroxyl groups) > 23≈22 (5 free acetylated hydroxyl groups) > 21≈20 (4 free acetylated hydroxyl groups) > Ampicillin (Fig. 5a). Second: The presence of pyrimidine ring fused to spirothiazolopyridine backbone leads to an increase in the antimicrobial potency for derivatives 8–15 more than common reference drugs. The substitutions on position 4 and 2 in the pyrimidine nucleus play an essential role to enhance the activity; where the presence of group with high nucleophilicity (NH2 > C = S > C = O > C = NH) in position 4 is preferred beside presence of electron-donating group in position 2 according to the following derivatives order (10 > 9 > 8 > 11) > (15 > 14 > 13 > 12) ≥ Ampicillin (Fig. 5b). Third: The presence of pyridine ring fused to spirothiazolopyridine framework leads to a decrease in the antimicrobial potency for derivatives 4–7 less than common reference drugs. The substitutions on positions 2, 3, and 4 in the pyridine ring effect directly the antimicrobial activity, the existence of an amino group in position 4 is a fundamental group with the presence of an electron-withdrawing group in position 3 (CN > CO2C2H5) and presence of an electron-donating group in position 2 (NH2 > OH > C6H5); the derivatives 4–7 order can be represented as Ampicillin > (4 > 6 > 5 > 7) (Fig. 5c).
Conclusion
In the present work, we discovered a new antimicrobial candidate that might be utilized alone or in combination with research methods for therapeutic, preventive, and growth promoter purposes. A series of novel spirothiazolopyridine derivatives were designed and prepared. Most of the analogs exhibited excellent antibacterial and antifungal activity against all tested species. Some of the compounds displayed lower MIC values than the positive control on some of the tested species. We carried out the first SAR investigation into the antimicrobial activity of all prepared compounds. The SAR results showed that the N-nucleoside and S-nucleoside produced in spirothiazolopyridine skeleton have significant effects on the activity. All of the results revealed that the compounds are potential antimicrobial agents, which could be further optimized and developed as a new antibiotic against pathogenic that cause serious infections in public health.
Experimental
Chemistry
General
Melting points were measured using an Electro-Thermal IA 9100 digital melting point apparatus (Büchi, Flawil, Switzerland) and are uncorrected. Infrared spectra were recorded on a PerkinElmer 1600 FTIR (PerkinElmer, Waltham, MA, USA) discs. NMR spectra were determined on a Jeol-Ex-300 NMR spectrometer (JEOL, Tokyo, Japan) and chemical shifts were expressed as part per million; (δ values, ppm) against TMS as internal reference, National Research Centre, Cairo, Egypt. 1H and 13C chemical shifts were referred to the solvent signal (DMSO) at 2.50 and 39.52 ppm, respectively. Data are presented as follows: chemical shift, integration, multiplicity (br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet) and coupling constant in Hertz (Hz).The mass spectra were run at 70 eV with a Finnigan SSQ 7000 spectrometer (Thermo Electron Corporation, Madison, WI, USA) using EI and the values of m/z are indicated in Dalton. Elemental analyses were performed on a PerkinElmer 2400 analyzer (PerkinElmer) and were found within the accepted range (± 0.30) of the calculated values. Reaction monitoring and verification of the purity of the compounds was done by TLC on silica gel pre-coated aluminum sheets (type 60 F254, Merck, Darmstadt, Germany). All chemical reagents and solvents were purchased from Aldrich (Munich, Germany).
4-(4-Fluorophenyl)-1-thia-4-azaspiro[4.5]decan-3-one (1)[52]
A mixture of cyclohexanone (0.01 mol), p-fluoroaniline (0.01 mol) and thioglycolic acid (0.01 mol) in dry toluene (50 mL) was refluxed for 10 h. The solution was concentrated and the formed solid was filtered off; dried and crystallized from dioxane/methanol (v; 1:1) to obtain compound 1 as a pale yellow needle. Yield 70%; m.p.143–145 °C; IR (KBr, υ, cm−1): 1668 (C = O). 1H-NMR (DMSO): δ (ppm) 1.45–1.70 (m, 6H, 3CH2), 1.79–1.98 (m, 4H, 2CH2), 3.52 (s, 2H, SCH2CO), 7.01 (d, J = 8.01, 2H, Ar–H), 7.38 (d, J = 8.02, 2H, Ar–H). 13C NMR (DMSO, 75 MHz): δ (ppm) 23.40, 24.39, 25.11, 43.70, 74.13, 115.4, 123.15, 138.2, 162.12, 169.18. MS, m/z (%): 265 (M+, 100). Analysis calc. for C14H16FNOS (265.35): C, 63.37; H, 6.08; N, 5.28; S, 12.08. Found: C, 63.11; H, 5.82; N, 5.08; S, 11.86.
5'-Amino-3'-(4-fluorophenyl)-7'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo [4,5-b]pyridine]-6'-carbonitrile (2)[52]
Compound 1 (0.01 mol); p-tolualdehyde (0.01 mol); ammonium acetate (0.02 mol) and malononitrile (0.01 mol) in glacial acetic acid (40 mL) was refluxed for 24 h. The reaction mixture was cooled and poured onto water. The formed solid was filtered off; dried and crystallized from dioxane to give compound 2 as a deep yellow powder. Yield 65%; m.p. 137–139 °C; IR (KBr, υ, cm−1): 3132 (NH2), 2216 (CN). 1H-NMR (DMSO): δ (ppm) 1.47–1.71 (m, 6H, 3CH2), 1.78–1.97 (m, 4H, 2CH2), 2.37 (s, 3H, CH3), 7.20–7.86 (m, 8H, Ar–H), 8.66 (brs, 2H, NH2, D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.23, 23.60, 25.12, 38.14, 74.15, 82.21, 114.16, 115.31, 116.25, 121.34, 126.51, 127.25, 131.33, 135.11, 144.12, 151.12, 158.31, 161.60, 163.21. MS, m/z (%): 430 (M+, 70). Analysis calc. for C25H23FN4S (430.55): C, 69.74; H, 5.38; N, 13.01; S, 7.45.Found: C, 69.52; H, 5.11; N, 12.79; S, 7.20.
4'-chloro-8'-(4-fluorophenyl)-5'-(p-tolyl)-8'H-spiro[cyclohexane-1,7'-thiazolo [5',4':5,6]pyrido[2,3-d][1,2,3]triazine] (3)
A solution of sodium nitrite (0.01 mol) in water (10 mL) was added to a cold solution (0 °C) of compound 2 (0.005 mol) in acetic acid (30 mL) and concentrated hydrochloric acid (15 mL) with stirring in ice bath. After completion of the addition, the ice bath was removed and stirring continued for an additional 2 h at room temperature. The crude product formed was collected by filtration and purification by recrystallized from ethanol to obtain compound 3 as a yellow crystals. Yield 77%; m.p. 148–150 °C. 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.49‒2.02 (m, 10H, 5CH2), 2.38 (s, 3H, CH3), 7.23–7.90 (m, 8H, Ar–H). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.09, 23.01, 25.16, 35.02, 76.12, 119.98, 122.31, 124.31, 129.21, 130.55, 131.21, 133.23, 136.88, 141.24, 141.38, 148.31, 154.76, 156.91, 162.21. MS, m/z (%): 477 (M+, 57), 479 (M+ + 2, 17). Anal. Calcd. for C25H21ClFN5S (477.99): C, 62.82; H, 4.43; Cl, 7.42; N, 14.65; S, 6.71.Found: C, 62.57; H, 4.20; Cl, 7.21; N, 14.39; S, 6.44.
6',8'-diamino-3'-(4-fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b][1,8]naphthyridine]-7'-carbonitrile (4)
Compound 2 (0.01 mol) and malononitrile (0.01 mol) in 10 mL DMF containing piperidine (0.1 mL) was heated under reflux for 4 h. The reaction was left to cool at room temperature and then poured onto ice-cold water with stirring. The crude product formed was collected by filtration and purification by recrystallized from acetic acid to give compound 4 as a brown powder, yield 92%, m.p. 160–162 °C; IR (KBr, υ, cm−1): 3435 (NH2), 3161 (NH2), 2219 (CN). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.48–2.03 (m, 10H, 5CH2), 2.39 (s, 3H, CH3), 7.24–7.92 (m, 8H, Ar–H), 8.40 (brs, 2H, NH2; D2O exchangeable), 8.65 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.12, 23.17, 25.35, 35.19, 78.18, 87.95, 119.49, 120.21, 121.52, 123.24, 129.35, 129.71, 132.33, 133.77, 136.89, 137.51, 145.67, 147.09, 148.41, 151.11, 161.53, 163.07. MS, m/z (%): 496 (M+, 80); Anal. Calcd. for C28H25FN6S (496.61): C, 67.72; H, 5.07; N, 16.92; S, 6.46. Found: C, 67.49; H, 4.95; N, 16.71; S, 6.23.
Ethyl 8'-amino-3'-(4-fluorophenyl)-6'-oxo-9'-(p-tolyl)-5',6'-dihydro-3'H-spiro [cyclohexane-1,2'-thiazolo[4,5-b][1,8]naphthyridine]-7'-carboxylate (5).
Compound 2 (0.01 mol) and diethylmalonate (0.01 mol) was added to 20 mL freshly prepared sodium ethoxide solution [prepared by adding 1 g sodium metal into absolute ethanol (20 mL)] and the mixture was refluxed for 4 h, and left to cool overnight. The solid product formed was collected by filtration, washed with ethanol and recrystallized from ethanol to obtain compound 5 as a white powder. Yield 92%; m.p.173–175 °C; IR (KBr, υ, cm−1): 3272 (NH2), 3164 (NH), 1734 (C = O; ester), 1670 (C = O; amide). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.31 (t, J = 7.49, 3H, CH2CH3), 1.47–2.02 (m, 10H, 5CH2), 2.37 (s, 3H, CH3), 4.26 (q, J = 7.50, 2H, CH2 CH3), 7.22–7.97 (m, 9H, 8 Ar–H + NH; D2O exchangeable), 8.65 (brs, 2H, NH2; D2O exchangeable).13C NMR (DMSO, 75 MHz): δ (ppm) 14.82, 21.34, 22.49, 25.73, 34.11, 61.47, 75.31, 92.17, 98.56, 119.91, 122.83, 124.63, 129.72, 130.19, 134.87, 135.79, 136.11, 136.82, 151.90, 158.06, 159.70, 162.27, 167.36, 169.49. MS, m/z (%): 544 (M+, 57); 471 (M+-CO2Et, 100). Anal. Calcd. for C30H29FN4O3S (544.65): C, 66.16; H, 5.37; N, 10.29; S, 5.89. Found: C, 65.91; H, 5.15; N, 10.02; S, 5.61.
8'-Amino-3'-(4-fluorophenyl)-6'-oxo-9'-(p-tolyl)-5',6'-dihydro-3'H-spiro[cyclo hexane-1,2'-thiazolo[4,5-b][1,8]naphthyridine]-7'-carbonitrile (6).
Compound 2 (0.01 mol) and ethyl cyanoacetate (0.01 mol) in ethanol (10 mL) containing piperidine (0.1 mL) was heated under reflux for 4 h. The reaction was left to cool at room temperature and the formed was collected by filtration and purification by recrystallized from acetic acid to give compound 6 as a pale yellow crystal, yield 92%, m.p.158–160 °C; IR (KBr, υ, cm−1): 3242 (NH2); 3110 (NH); 2217 (CN); 1681 (C = O). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.48–2.00 (m, 10H, 5CH2), 2.39 (s, 3H, CH3), 7.19–7.98 (m, 9H, 8Ar-H + NH; D2O exchangeable), 8.41 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.11, 22.44, 25.67, 34.45, 69.25, 76.42, 98.71, 114.70, 119.85, 122.34, 124.25, 129.63, 130.25, 134.15, 135.42, 136.34, 137.19, 149.28, 153.91, 161.25, 167.27. MS, m/z (%): 497 (M+, 77). Anal. Calcd. for C28H24FN5OS (497.59): C, 67.59; H, 4.86; N, 14.07; S, 6.44. Found: C, 67.28; H, 4.62; N, 13.81; S, 6.21.
8'-Amino-3'-(4-fluorophenyl)-6'-phenyl-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b][1,8]naphthyridine]-7'-carbonitrile (7)
Equimolar amounts of compound 2 and benzylidenemalononitrile (0.01 mol) was refluxed in dioxane (25 mL) containing piperidine (0.1 mL) for 5 h. Then allow the reaction mixture to cool and the solid product formed was filtered off and purification by recrystallized from dioxane/dimethylformamide mixture (v; 3:1) to give compound 7 as a brown crystal. Yield 92%; m.p.170–172 °C; IR (KBr, υ, cm−1): 3290 (NH2), 2221 (CN). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.50–2.03 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 7.17–8.22 (m, 13H, Ar–H), 8.43 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.62, 23.21, 25.58, 34.09, 75.96, 87.19,109.40, 114.58, 119.21, 122.54, 127.26, 128.51, 129.14, 129.92, 130.24, 133.01, 135.57, 136.43, 136.51, 136.88, 137.84, 146.97, 149.40, 151.31, 161.07, 162.68. MS, m/z (%): 557 (M+, 91), 480 (M+-Ph, 100); Anal. Calcd. for C34H28FN5S (557.69): C, 73.23; H, 5.06; N, 12.56; S, 5.75. Found: C, 73.01; H, 4.82; N, 12.22; S, 5.49.
2-(8'-Amino-3'-(4-fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[5',4':5,6]pyrido[2,3-d]pyrimidin]-6'-yl)acetonitrile (8).
Compound 2 (0.01 mol) and malononitrile (0.01 mol) was added to ethanol (20 mL) and allowed to reflux for 4 h, then left to cool overnight. The solid product formed was collected by filtration, washed with ethanol and purification by recrystallized from ethanol to obtain compound 8 as a white powder. Yield 82%; m.p. 260–262 °C; IR (KBr, υ, cm−1): 3341 (NH2); 2223(CN). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.47–2.00 (m, 10H, 5CH2), 2.39 (s, 3H, CH3), 4.26 (s, 2H, CH2), 7.20–7.99 (m, 8H, Ar–H), 8.38 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.42, 23.36, 24.51, 25.63, 27.96, 34.99, 76.42, 96.84, 114.58, 117.34, 122.52, 129.74, 129.98, 132.89, 136.48, 137.41, 139.31, 143.18, 149.17, 158.41, 161.39, 162.07, 163.05.MS, m/z (%): 496 (M+, 59); 456 (M+-CH2CN, 100); Anal. Calcd. For C28H25FN6S (496.61): C, 67.72; H, 5.07; N, 16.92; S, 6.46. Found: C, 67.48; H, 4.81; N, 16.67; S, 6.21.
3'-(4-Fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[5',4':5,6] pyrido[2,3-d]pyrimidin]-8'-amine (9)
Compound 2 (0.01 mol) in formamide (10 mL) was refluxed for 5 h, and then left to cool overnight. The formed solid was collected by filtration and purification by recrystallized from dioxane to obtain compound 9 as a gray crystal. Yield 88%; m.p.150–152 °C; IR (KBr, υ, cm−1): 3244 (NH2). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.48‒2.00 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 7.21–7.99 (m, 8H, Ar–H), 8.20 (s, 1H, CH-pyrimidine), 8.55 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.12, 22.63, 25.58, 34.14, 75.42, 101.25, 119.88, 122.24, 129.21, 126.74, 132.14, 135.82, 136.11, 140.11, 144.25, 147.02, 153.14, 156.04, 161.46, 163.27. MS, m/z (%): 457 (M+, 87); Anal. Calcd. For C26H24FN5S (457.57): C, 68.25; H, 5.29; N, 15.31; S, 7.01. Found: C, 68.01; H, 5.05; F; N, 15.09; S, 6.81.
8'-Amino-3'-(4-fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[5',4':5,6]pyrido[2,3-d]pyrimidine]-6'(5'H)-thione (10)
Compound 2 (0.01 mol) and thiourea(0.01 mol) were mixed in a mortar, and then the mixture was allowed to fused at 180 °C in an oil bath for 2 h. The molten product was boiled 10 min with water, cooled and the formed solid was collected by filtration and purification by recrystallized from DMF/H2O (v; 1:1) to obtain compound 10 as a brown powder. Yield 92%; m.p.175–177 °C; IR (KBr, υ, cm−1): 3271 (NH2); 3173 (NH). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.48–2.00 (m, 10H, 5CH2), 2.35 (s, 3H, CH3), 7.02 (brs, 1H, NH; D2O exchangeable), 7.21–8.00 (m, 8H, Ar–H), 8.37 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.01, 22.12, 25.62, 34.25, 76.15, 99.46, 119.80, 122.24, 129.04, 131.01, 134.00 135.08, 137.00, 141.24, 146.30, 149.11, 154.10, 161.50, 162.77, 172.19. MS, m/z (%): 489 (M+, 66); Anal. Calcd. for C26H24FN5S2 (489.63):C, 63.78; H, 4.94; N, 14.30; S, 13.10. Found: C, 63.51; H, 4.69; N, 14.10; S, 12.89.
3'-(4-Fluorophenyl)-6'-phenyl-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo [5',4':5,6]pyrido[2,3-d]pyrimidin]-8'-amine (11)
Compound 2 (0.01 mol) in 20 mL freshly prepared sodium ethoxide solution [prepared by adding 1 g sodium metal into absolute ethanol (20 mL)] and benzonitrile (0.01 mol) in 2-propanol (10 mL) was refluxed for 20 h. On cooling the precipitate formed was collected by filtration and purification by recrystallized from ethanol to give compound 11 as a yellow powder. Yield 92%; m.p. 220–222 °C; IR (KBr, υ, cm−1): 3371 (NH2). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.49‒2.01 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 7.19–8.23 (m, 13H, Ar–H), 8.41 (brs, 2H, NH2; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.32, 22.17, 25.37, 34.29, 75.14, 96.92, 119.28, 121.35, 125.55, 126.34, 129.74, 130.00, 132.89, 134.88, 136.14, 137.05, 138.02, 141.32, 144.02, 147.00, 151.16, 154.61, 157.07, 162.11. MS, m/z (%): 533 (M+, 47), 456 (M+-Ph, 100); Anal. Calcd. For C32H28FN5S (533.67): C, 72.02; H, 5.29; N, 13.12; S, 6.01. Found: C, 71.79; H, 5.07; N, 12.85; S, 5.81.
3'-(4-Fluorophenyl)-8'-imino-7'-phenyl-9'-(p-tolyl)-7',8'-dihydro-3'H-spiro [cyclohexane-1,2'-thiazolo[5',4':5,6]pyrido[2,3-d]pyrimidine]-6'(5'H)-thione (12).
Compound 2 (0.01 mol) and phenyl isothiocyanate (0.01 mol) in 20 mL freshly prepared sodium ethoxide solution [prepared by adding 1 g sodium metal into absolute ethanol (20 mL)] was refluxed for 20 h. The solvent was distilled off and the obtained solid was collected and purification by recrystallized from tetrahydrofurane (THF) to give compound 12 as a white powder. Yield 90%; m.p.145–147 °C; IR (KBr, υ, cm−1): 3197 (NH); 3117 (NH). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.50‒2.04 (m, 10H, 5CH2), 2.35 (s, 3H, CH3), 7.21–8.41 (m, 14H, 13Ar-H + NH; D2O exchangeable), 9.15 (brs, 1H, NH; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.19, 23.11, 24.58, 34.09, 75.35, 94.96, 119.09, 123.45, 127.01, 128.02, 129.74, 130.11, 130.79, 132.71, 134.19, 135.88, 137.00, 141.02, 142.11, 143.20, 155.00, 161.14, 162.70, 171.21. MS, m/z (%): 565 (M+, 39); 455 (M+-[Ph + SH], 100); Anal. Calcd. For C32H28FN5S2 (565.73): C, 67.94; H, 4.99; N, 12.38; S, 11.33. Found: C, 67.69; H, 4.71; N, 12.14; S, 11.09.
3'-(4-Fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[5',4':5,6] pyrido[2,3-d]pyrimidin]-8'(7'H)-one (13)
Compound 2 (0.01 mol) was refluxed in formic acid (20 mL) for 4 h, and left to cool overnight. The solid product formed was collected by filtration and purification by recrystallized from ethanol to give compound 13 as a brown crystal. Yield 92%; m.p. 166–168 °C; IR (KBr, υ, cm−1): 3110 (NH); 1685 (C = O). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.49‒2.00 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 7.21–7.97 (m, 8H, Ar–H), 8.28 (s, 1H, pyrimidine-H), 9.11 (brs, 1H, NH; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.17, 22.17, 25.18, 34.21, 74.21, 119.21, 120.25, 121.05, 122.55, 128.74, 131.11, 135.00, 136.88, 137.11, 139.02, 143.21, 146.80, 149.28, 161.76, 162.20. MS, m/z (%): 458 (M+, 69); Anal. Calcd. For C26H23FN4OS (458.56): C, 68.10; H, 5.06; N, 12.22; O, 3.49; S, 6.99. Found: C, 67.85; H, 4.81; N, 12.01; O, 3.21; S, 6.71.
6'-(Chloromethyl)-3'-(4-fluorophenyl)-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[5',4':5,6]pyrido[2,3-d]pyrimidin]-8'(7'H)-one(14).
Compound 2 (0.01 mol) and chloroacetyl chloride (0.01 mol) in DMF (20 mL) was stirred at room temperature for 2 h. The reaction mixture was poured onto ice water and the obtained solid was collected by filtration and purification by recrystallized from ethanol to give compound 14 as a yellow crystal. Yield 85%; m.p.171–173 °C; IR (KBr, υ, cm−1): 3142 (NH); 1682 (C = O). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.47‒2.00 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 5.17 (s, 2H, CH2Cl), 7.21–7.97 (m, 8H, Ar–H); 9.55 (brs, 1H, NH; D2O exchangeable). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.71, 22.24, 25.21, 35.09, 47.17, 75.01., 100.22, 119.35, 120.05, 123.15, 128.05, 130.09, 133.89, 135.88, 137.25, 139.15, 143.02, 147.21, 160.16, 161.70, 162.19. MS, m/z (%): 507 (M+, 57), 509 ((M+ + 2, 18), 458 (M+-CH2Cl, 100); Anal. Calcd. for C27H24ClFN4OS (507.02): C, 63.96; H, 4.77; Cl, 6.99; N, 11.05; S, 6.32. Found: C, 63.71; H, 4.39; Cl, 6.42; N, 11.80; S, 6.05.
3'-(4-Fluorophenyl)-6'-methyl-9'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo [5',4':5,6]pyrido[2,3-d]pyrimidin]-8'(7'H)-one (15)
A mixture of compound 2 (0.01 mol) and thioacetamide (0.01 mol) in trifluoroacetic acid (10 mL) was refluxed for 7 h. The reaction mixture was cooled and poured onto ice water. The obtained solid was collected by filtration and purification by recrystallized from ethanol to give compound 15 as a brown crystal. Yield 82%; m.p. 156–158 °C; IR (KBr, υ, cm−1): 3139 (NH). 1H NMR (DMSO-d6, 300 MHz): δ (ppm) 1.48‒2.02 (m, 10H, 5CH2), 2.32 (s, 3H, CH3), 2.37 (s, 3H, CH3), 7.21–7.98 (m, 8H, Ar–H), 9.39 (brs, 1H, NH; D2O exchangeable).13C NMR (DMSO, 75 MHz): δ (ppm) 20.02, 21.15, 22.37, 25.22, 35.19, 45.29, 119.25, 120.68, 122.45, 129.14, 131.00, 133.82, 135.88, 136.71, 138.21, 142.32, 144.67, 146.61, 161.10, 162.28, 169.32. MS, m/z (%): 472 (M+, 63), 457 (M+-Me, 100), Anal. Calcd. for C27H25FN4OS (472.58): C, 68.62; H, 5.33; N, 11.86; S, 6.78. Found: C, 68.35; H, 5.07; N, 11.61; S, 6.54.
General method for synthesis of amino-sugar derivatives 16–19.
Aldoses; D-xylose, D-arabinose, D-glucose, and D-galactose (0.05 mol) in water (1 mL) was added to compound 2 (0.01 mol) in ethanol (30 mL) /glacial acetic acid (0.5 mL). The reaction mixture was refluxed for 6 h (TLC; chloroform: methanol; 95:5), then keep the reaction mixture cooled down to room temperature. The excess ethanol was evaporated under reduced pressure, and the residue was treated with diethyl ether (15 mL). The solid formed was filtered off and crystallized from ethanol/DMF (3:1) to give the corresponding amino sugar 16–19, respectively.
(D-Xylose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro [cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (16).
Yield 77%, m.p. 242–244 °C. IR spectrum, ν, cm–1: 3463 (OH), 2116 (CN). 1H-NMR (DMSO): δ (ppm) 1.48–2.01 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 3.44–3.52 (m, 2H, H-5' + H-5′′), 3.99–4.22 (m, 1H, H-4′), 4.17–4.23 (m, 2H, H-3′ + H-4′), 4.30–4.35 (m, 1H, OH, D2O exchangeable), 4.44–4.62(m, 2H, H-2' + OH, D2O exchangeable), 5.20–5.24 (m, 1H, OH, D2O exchangeable), 7.20–7.86 (m, 9H, 8Ar-H + H-1′).13C NMR (DMSO, 75 MHz): δ (ppm) 21.16, 22.36, 25.60, 34.18, 64.43, 72.27, 72.95, 73.10, 85.63, 95.89, 114.88, 115.82, 122.56, 124.66, 129.55, 130.17, 134.11, 136.03, 137.27, 154.97, 155.03, 160.81, 162.92, 164.02. Analysis calc. for C30H31FN4O4S: C, 64.04; H, 5.55; N, 9.96; S, 5.70.Found: C, 63.76; H, 5.39; N, 9.70; S, 5.44.
D-Arabinose 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro [cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (17).
Yield 69%, m.p. 161–163 °C. IR spectrum, ν, cm–1: 3520 (OH), 2118 (CN). 1H-NMR (DMSO): δ (ppm) 1.48–2.01 (m, 10H, 5CH2), 2.36 (s, 3H, CH3), 3.35–3.43 (m, 2H, H-5' + H-5′′), 4.11–4.20 (m, 2H, H-3' + H-4′), 4.61–4.64 (m, 1H, H-2′), 4.37–4.41 (m, 1H, OH, D2O exchangeable), 4.82 (d, J = 6.25 Hz, 1H, OH, D2O exchangeable), 4.97–5.01 (m, 1H, OH, D2O exchangeable), 5.55–5.58(m, 1H, OH, D2O exchangeable), 7.20–7.86 (m, 9H, 8Ar-H + H-1′).13C NMR (DMSO, 75 MHz): δ (ppm) 21.17, 22.35, 25.61, 34.19, 64.44, 72.29, 72.95, 73.35, 85.67, 95.92, 114.91, 115.80, 122.51, 124.71, 129.50, 130.07, 134.11, 136.11, 137.31, 154.97, 155.08, 160.81, 162.75, 163.92. Analysis calc. for C30H31FN4O4S: C, 64.04; H, 5.55; N, 9.96; S, 5.70.Found: C, 63.77; H, 5.37; N, 9.71; S, 5.45.
(D-Glucose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclo hexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (18).
Yield 74%, m.p. 201–203 °C. IR spectrum, ν, cm–1: 3450 (OH), 2117 (CN). 1H-NMR (DMSO): δ (ppm) 1.49–2.01 (m, 10H, 5CH2), 2.39 (s, 3H, CH3), 3.34–3.41 (m, 2H, H-6' + H-6′′), 3.54–3.57 (m, 1H, H-5′), 3.82–3.97 (m, 2H, H-3′ + H-4′), 4.28–4.40 (m, 2H, H-2′ + OH, D2O exchangeable), 4.59 (d, 1H, J = 6.19 Hz, OH), 4.61–4.70 (m, 1H, OH, D2O exchangeable), 4.78–4.80 m (1H, OH, D2O exchangeable), 4.92–4.95 (m, 1H, OH, D2O exchangeable), 7.20–7.86 (m, 9H, 8Ar-H + H-1′). 13C NMR (DMSO, 75 MHz): δ (ppm) 21.17, 22.36, 25.62, 34.19, 64.47, 72.05, 74.35, 72.44, 73.75, 85.67, 95.93, 114.97, 115.88, 122.57, 124.71, 129.51, 130.07, 134.04, 136.07, 137.31, 154.97, 155.04, 160.81, 162.75, 163.98. Analysis calc. for C31H33FN4O5S: C, 62.82; H, 5.61; N, 9.45; S, 5.41. Found: C, 62.57; H, 5.37; N, 9.19; S, 5.15.
(D-Galactose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclo hexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (19).
Yield 67%, m.p. 217–219 °C. IR spectrum, ν, cm–1: 3461 (OH), 2121 (CN). 1H-NMR (DMSO): δ (ppm) 1.48–2.00 (m, 10H, 5CH2), 2.38 (s, 3H, CH3), 3.29–3.41 (m, 2H, H-6' + H-6′′), 3.58–3.59 (m, 1H, H-5′), 3.84–3.99 (m, 2H, H-3′ + H-4′), 4.34–4.41 (m, 2H, H-2′ + OH, D2O exchangeable), 4.61 (d, 1H, J = 6.19 Hz, OH), 4.75–4.79 (m, 1H, OH, D2O exchangeable), 4.91 ( t, J = 8.4 Hz, 1H, OH, D2O exchangeable), 5.89–4.94 (m, 1H, OH, D2O exchangeable), 7.20–7.86 (m, 9H, 8Ar-H + H-1′). 13C NMR (DMSO, 75 MHz): δ (ppm)21.14, 22.36, 25.61, 34.17, 62.47, 72.03, 72.18, 72.42, 74.77, 85.64, 95.92, 114.93, 115.86, 122.55, 124.71, 129.49, 130.09, 134.06, 136.09, 137.31, 154.99, 155.06, 160.74,162.59, 163.91. Analysis calc. for C31H33FN4O5S: C, 62.82; H, 5.61; N, 9.45; S, 5.41. Found: C, 62.55; H, 5.39; N, 9.21; S, 5.16.
General method for synthesis of acetylated amino sugar derivatives 20–23.
A solution of the amino sugars 16–19 (1 mmol) in acetic anhydride/acetic acid (10 mL; 1:1) was heated at 100 °C until TLC (chloroform: methanol; 96:4) showed completion of the reaction. The resulting solution was poured onto ice-cold water, and then extracted with chloroform (40 mL). Sodium hydrogen carbonate was added to the organic layer and mixture was stirred for 1 h and filtered. The chloroform layer was washed with water, dried with sodium sulfate anhydrous and evaporated till dryness to afford the corresponding acetyl sugar derivatives 20–23, respectively.
(1,2,3,4-Tetra-O-acetyl-D-xylose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclo hexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (20).
Yield 62%, m.p. 169–171 °C. IR spectrum, ν, cm–1: 2116 (CN), 1748 (C = O). 1H-NMR (DMSO): δ (ppm) 1.48–2.01 (m, 10H, 5CH2), 2.10 (s, 3H, COCH3), 2.13 (s, 3H, COCH3), 2.17(s, 3H, COCH3), 2.20 (s, 3H, COCH3), 2.37 (s, 3H, CH3), 3.84–3.92 (m, 2H, H-5' + H-5′′), 5.11–5.17 (m, 2H, H-3′ + H-4′), 5.61–5.72 (m, 1H, H-2'), 7.29–7.89 (m, 9H, 8Ar-H + H-1′).13C NMR (DMSO, 75 MHz): δ (ppm) 20.72, 21.14, 21.29, 22.37, 25.57, 34.18, 36.67, 62.44, 68.51, 68.92, 71.45, 85.60, 95.00, 114.75, 115.84, 122.55, 125.19, 129.51, 130.19, 134.33, 136.30, 137.49, 156.56, 157.11, 160.73, 165.44, 165.99, 170.88, 171.22, 171.85. Analysis calc. for C38H39FN4O8S: C, 62.45; H, 5.38; N, 7.67; S, 4.39. Found: C, 62.21; H, 5.11; N, 7.41; S, 4.10.
(1,2,3,4-Tetra-O-acetyl-D-arabinose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (21).
Yield 73%, m.p. 157–159 °C. IR spectrum, ν, cm–1: 2117 (CN), 1755 (C = O). 1H-NMR (DMSO): δ (ppm) 1.48–2.01 (m, 10H, 5CH2), 2.08 (s, 3H, COCH3), 2.011 (s, 3H, COCH3), 2.13 (s, 3H, COCH3), 2.18 (s, 3H, COCH3), 2.38 (s, 3H, CH3), 3.97(dd, 1H, J = 11.19, 2.81 Hz, H-5'), 4.16 (dd, 1H, J = 11.20, 3.20 Hz, H-5''), 4.29–4.33(m, 1H, H-4'), 5.21 (dd,0.1H, J = 7.80, 7.2 Hz, H-3'), 5.32 (dd,0.1H, J = 7.80, 7.2 Hz, H-2') 7.20–7.86 (m, 9H, 8Ar-H + H-1').13C NMR (DMSO, 75 MHz): δ (ppm) 20.70, 21.12, 21.27, 22.36, 25.56, 34.18, 62.44, 67.51, 67.92, 68.45, 85.60, 95.03, 114.75, 115.84, 122.55, 124.35, 129.51, 130.19, 134.33, 136.30, 137.49, 156.56, 157.11, 160.73, 164.98, 165.44, 165.99, 170.88, 171.22, 171.85. Analysis calc. for C38H39FN4O8S: C, 62.45; H, 5.38; N, 7.67; S, 4.39. Found: C, 62.23; H, 5.15; N, 7.40; S, 4.15.
(1,2,3,4,5-Penta-O-acetyl-D-glucose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (22).
Yield 67%, m.p. 211–213 °C. IR spectrum, ν, cm–1: 2118 (CN), 1741 (C = O). 1H-NMR (DMSO): δ (ppm) 1.47–1.99 (m, 10H, 5CH2), 2.09 (s, 3H, COCH3), 2.011 (s, 3H, COCH3), 2.14 (s, 3H, COCH3), 2.20 (s, 3H, COCH3), 2.22 (s, 3H, COCH3), 2.389 (s, 3H, CH3), 3.81–3.88 (m, 2H, H-6' + H-6′′), 4.04–4.06 (m, 1H, H-5′), 4.33–4.42 (m, 2H, H-3′ + H-4′), 4.80–4.88 (m, 1H, H-2′), 7.28–7.88 (m, 9H, 8Ar-H + H-1′).13C NMR (DMSO, 75 MHz): δ (ppm) 20.66, 21.14, 21.21, 22.31, 25.54, 34.18, 62.39, 67.15, 67.70, 67.88,69.55, 85.40, 95.33, 114.77, 115.85, 122.15, 124.35, 129.50, 130.09, 134.13, 136.10, 137.39, 154.56, 156.11, 156.88, 160.63, 163.98, 170.22, 170.88, 171.22, 171.92. Analysis calc. for C41H43FN4O10S: C, 61.34; H, 5.40; N, 6.98; S, 3.89. Found: C, 61.09; H, 5.14; N, 6.76; S, 3.74.
(1,2,3,4,5-penta-O-acetyl-D-galactose) 3'-(4-fluorophenyl)-5'-(methyleneamino)-7'-(p-tolyl)-3'H-spiro[cyclohexane-1,2'-thiazolo[4,5-b]pyridine]-6'-carbonitrile (23).
Yield 59%, m.p. 254–256 °C. IR spectrum, ν, cm–1: 2121 (CN), 1738(C = O). 1H-NMR (DMSO): δ (ppm) 1.48–2.00 (m, 10H, 5CH2), 2.09 (s, 3H, COCH3), 2.11(s, 3H, COCH3), 2.14 (s, 3H, COCH3), 2.19 (s, 3H, COCH3), 2.22 (s, 3H, COCH3), 2.37 (s, 3H, CH3), 3.79–3.83 (m, 2H, H-6 + H-6′′), 4.11–4.16 (m, 1H, H-5′), 4.34–4.66 (m, 2H, H-3′ + H-4′), 4.84–4.61 (m, 1H, H-2′), 7.27–7.87 (m, 9H, 8Ar-H + H-1′).13C NMR (DMSO, 75 MHz): δ (ppm) 20.68, 21.15, 21.24, 22.37, 25.55, 34.17, 62.40, 67.18, 67.82, 67.88, 69.51, 85.42, 95.35, 114.73, 115.84, 122.16, 124.37, 129.51, 130.08, 134.15, 136.11, 137.38, 154.58, 156.14, 156.86, 160.61, 163.95, 170.25, 170.85, 171.20, 171.95. Analysis calc. for C41H43FN4O10S: C, 61.34; H, 5.40; N, 6.98; S, 3.89. Found: C, 61.11; H, 5.17; N, 6.74; S, 3.72.
Anti-microbial activity
The antimicrobial potency of the tested samples was examined against some targeted pathogenic microorganisms obtained from the American type culture collection (ATCC; Rockville, MD, USA). The tested organisms were Staphylococcus aurous ATCC- 47,077 (St.), Bacillus cereus ATCC-12228 (B.C.), Escherichia coli ATCC-25922 (E.C.), Salmonella typhiATCC-15566 (Salm.) and Candida albicans ATCC-10231 (C. Alb.). The stock cultures of pathogens utilized in this study were kept on nutrient agar slants at 4 °C. The Agar well diffusion method was employed to study the antimicrobial activities of the samples according to the method described. Reference antibacterial drug ampicillin was evaluated for their antibacterial and antifungal action and compared with the tested samples. Seventy microliters of bacterial and yeast cells (106 CFU/mL) of each pathogen were spread on the nutrient agar plates. The wells (6 mm diameter) were dug on the inoculated agar plates and 100 µl of the samples and its derivatives suspended in DMSO, were added to the wells. The reference antibiotics disks (10 µg/disk of ampicillin) were potted onto surface of agar inoculated plates. The plates were allowed to stand at 4 °C for 2 h before incubation to allow for diffusion. The plates were incubated at 37 °C for 24 h except yeast strain that were incubated at 28 °C for 24 h then followed by the measurement of the diameter of the inhibition zone (mm), and three replicates were averaged [53,54,55].
Minimum inhibition concentration (MIC):
The MIC calculation of the prepared materials was performed according to a slightly modified previous method. In brief, serial dilutions were prepared of the tested materials dissolved in DMSO. 150μL of double strength Mueller Hinton broth medium were loaded in each well of the 96 well micro liter plate followed by 150 μL of the twofold appropriate concentration and mixed well to obtain the final concentration. Overnight broth cultures of the tested bacterial and yeast strains prepared as an inoculums of 5% (V/V) (OD = 0.5 McFarland standard) was inoculated into the respective wells. For the positive growth control, the same inoculums size of each test strain was inoculated in wells that did not containing any of the tested materials. DMSO solution was tested as negative control. The plates were statically incubated at 37 °C for 24 h. 30 μL of prepared solution (0.18%) was added to each well to act as an electron acceptor and reduce to a pink, red or purple resourcing colored product by active micro-organisms (i.e., inhibition of bacterial growth was visible as a dark blue well and the presence of growth was detected by the presence of pink, red or purple color). The MIC was defined as the concentration at which the bacteria and yeast do not show visible growth with respect to the positive control [53,54,55].
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 5.0 (Graph Pad Software, LaJolla, CA). In One-way model ANOVA, the observed variance is partitioned into components due to different explanatory variables. A level of p < 0.05 was considered to be statistically significant.
References
S. Askin, H. Tahtaci, C. Türkeş, Y. Demir, A. Ece, G.A. Çiftçi, Ş Beydemir, Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo [2, 1-b][1, 3, 4] thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors. Bioorg. Chem. 113, 105009 (2021)
K. Kovaleva, O. Yarovaya, K. Ponomarev, S. Cheresiz, A. Azimirad, I. Chernyshova, A. Zakharenko, V. Konev, T. Khlebnikova, E. Mozhaytsev, Design, synthesis, and molecular docking study of new Tyrosyl-DNA Phosphodiesterase 1 (TDP1) inhibitors combining resin acids and adamantane moieties. Pharmaceuticals 14(5), 422 (2021)
R.M. Abdelhameed, M. Abu-Elghait, M. El-Shahat, Engineering titanium-organic framework decorated silver molybdate and silver vanadate as antimicrobial, anticancer agents, and photo-induced hydroxylation reactions. J. Photochem. Photobiol., A 423, 113572 (2022)
A. El-Sayed, M. El-Shahat, S. Rabie, E. Flefel, D. Abd-Elshafy, New pyrimidine and fused pyrimidine derivatives: Synthesis and anti Hepatitis A virus (HAV) evaluation. Int. J. Pharm 5(1), 69–79 (2015)
S. Jaime-Figueroa, A.D. Buhimschi, M. Toure, J. Hines, C.M. Crews, Design, synthesis and biological evaluation of Proteolysis Targeting Chimeras (PROTACs) as a BTK degraders with improved pharmacokinetic properties. Bioorg. Med. Chem. Lett. 30(3), 126877 (2020)
F. Adib, M. Peytam, M. Rahmanian-Jazi, S. Mohammadi-Khanaposhtani, H.R. Mahernia, M. Bijanzadeh, S. Jahani, M.A. Imanparast, M. Faramarzi, Mahdavi, Design, synthesis and in vitro α-glucosidase inhibition of novel coumarin-pyridines as potent antidiabetic agents. New J. Chem. 42(21), 17268–17278 (2018)
R.M. Abdelhameed, O.M. Darwesh, M. El-Shahat, Synthesis of arylidene hydrazinylpyrido [2, 3-d] pyrimidin-4-ones as potent anti-microbial agents. Heliyon 6(9), e04956 (2020)
M. Sathish, A.P. Sakla, F.M. Nachtigall, L.S. Santos, N. Shankaraiah, TCCA-mediated oxidative rearrangement of tetrahydro-β-carbolines: facile access to spirooxindoles and the total synthesis of (±)-coerulescine and (±)-horsfiline. RSC Adv. 11(27), 16537–16546 (2021)
Y.K. Xi, H. Zhang, R.X. Li, S.Y. Kang, J. Li, Y. Li, Total synthesis of spirotryprostatins through organomediated intramolecular umpolung cyclization. Chem.–A Eur. J. 25(12), 3005–3009 (2019)
H. Song, J. Song, L. Yan, W. He, P. Wang, Y. Xu, H. Wei, W. Xie, A concise synthesis of (-)-dihydrospirotryprostatin B via tandem Michael addition. Tetrahedron Lett. 85, 153486 (2021)
Y. Hui, Y. Zhang, Y. Luo, J. Li, Y. Wang, T. Gao, J. Xia, S. Wang, S. Zhang, Facile synthesis of spiro thiazolidinone via cyclic ketones, amines and thioglycolic acid by MCM-41-Schiff base-CuSO 4· 5H 2 O. Res. Chem. Intermed. 47(2), 521–532 (2021)
A.N. Al-Romaizan, Synthesis, characteristic and antimicrobial activity of some new spiro [indol-thiazolidon-2, 4-diones] and Bis (5-fluorospiro [indoline-3, 2’-thiazolidine]-2, 4’-dione) probes. Int. J. Org. Chem. 10(2), 77–87 (2020)
M. Salama, M. El-Shahat, E. Elhefny, A. El-Sayed, A novel fused pyridopyrimidine derivatives: synthesis and characterization. Int. J. Pharm. 5(1), 53–58 (2015)
A. Bodryakov, A.Y. Aliev, S. Rustamova, Biological activity of new sulfur-containing derivatives of nitrogen heterocycles, containing carbodithiotate group and quaternized nitrogen atom. Hayчнo-пpaктичecкий жypнaл 4(13), 25 (2020)
P.K. Sharma, A. Amin, M. Kumar, A review: medicinally important nitrogen sulphur containing heterocycles. The Open Med. Chem. J. 14(1), 49–64 (2020)
E.M. Flefel, W.I. El-Sofany, M. El-Shahat, A. Naqvi, E. Assirey, Synthesis, molecular docking and in vitro screening of some newly synthesized triazolopyridine, pyridotriazine and pyridine-pyrazole hybrid derivatives. Molecules 23(10), 2548 (2018)
M. El-Shahat, M. Salama, A.F. El-Farargy, M.M. Ali, D.M. Ahmed, Effective pharmacophore for CDC25 phosphatases enzyme inhibitors: newly synthesized bromothiazolopyrimidine derivatives. Mini. Rev. Med. Chem. 21(1), 118–131 (2021)
N. Azizi, M.S. Haghayegh, Greener and additive-free reactions in deep eutectic solvent: One-pot. Three-Compon. Synth. Highly Substit. Pyridines, ChemistrySelect 2(28), 8870–8873 (2017)
P.N. Patel, D.H. Desai, N.C. Patel, A.G. Deshmukh, Efficient multicomponent processes for synthesis of novel poly-nuclear hetero aryl substituted terpyridine scaffolds: Single crystal XRD study. J. Mol. Struct. 1250, 131737 (2022)
M. Edrisi, N. Azizi, Sulfonic acid-functionalized graphitic carbon nitride composite: a novel and reusable catalyst for the one-pot synthesis of polysubstituted pyridine in water under sonication. J. Iran. Chem. Soc. 17(4), 901–910 (2020)
L. Du, Y. Gong, J. Han, X. Xin, H. Luo, Y. Tian, Y. Li, B. Li, Cascade 8π electrocyclization/benzannulation to access highly substituted phenylpyridines. Org. Lett. 23(20), 7966–7971 (2021)
H.E. Emam, M. El-Shahat, M.S. Hasanin, H.B. Ahmed, Potential military cotton textiles composed of carbon quantum dots clustered from 4–(2, 4–dichlorophenyl)–6–oxo–2–thioxohexahydropyrimidine–5–carbonitrile. Cellulose 28(15), 9991–10011 (2021)
R. Singh, S. Ahmad Ganaie, A. Singh, A. Chaudhary, Carbon-SO3H catalyzed expedient synthesis of new spiro-[indeno [1, 2-b] quinoxaline-[11, 2′]-thiazolidine]-4′-ones as biologically important scaffold. Synth. Commun. 49(1), 80–93 (2019)
Hamzehloueian M, Sarrafi Y, Darroudi M, Arani MA, Darestani RN, Safari F, Foroumadi A (2021) Synthesis, antibacterial and anticancer activities evaluation of new 4-thiazolidinone-indolin-2-one analogs
E.M. Flefel, W.I. El-Sofany, H.M. Awad, M. El-Shahat, First synthesis for bis-spirothiazolidine derivatives as a novel heterocyclic framework and their biological activity. Mini. Rev. Med. Chem. 20(2), 152–160 (2020)
Bawazir WAB (2019) Synthesis of some new thioethers and 4-Thiazolidinones Bearing 3-(Pyridine-4’-yl)-1, 2, 4-Triazino [5, 6-b] Indole moiety as antifungal agents
A.R. Deshmukh, S.T. Dhumal, L.U. Nawale, V.M. Khedkar, D. Sarkar, R.A. Mane, Dicationic liquid mediated synthesis of tetrazoloquinolinyl methoxy phenyl 4-thiazolidinones and their antibacterial and antitubercular evaluation. Synth. Commun. 49(4), 587–601 (2019)
Shetty Chaithra R, Bhat Ishwar K, Abhishek K, Kumar M, Revanasidappa B, Felicity Dkhar G (2020) Conventional and microwave synthesis and antioxidant evaluation of benzothiazole substituted 4-thiazolidinones
K.K. Valluri, T.R. Allaka, I. Viswanath, P. Nagaraju, Design, molecular docking studies of oxaprozin linked to 4-thiazolidinone derivatives as a potent anticancer, analgesic and antiinflammatory agents. Asian J. Res. Chem. 11(3), 617–627 (2018)
M. Mishchenko, S. Shtrygol, D. Kaminskyy, R. Lesyk, Thiazole-bearing 4-thiazolidinones as new anticonvulsant agents. Sci. Pharm. 88(1), 16 (2020)
J. Singh, P.A. Chawla, R. Bhatia, S. Singh, 2, 5-disubstituted-4-thiazolidinones: Synthesis, anti-inflammatory, free radical scavenging potentials and structural insights through molecular docking. Lett. Org. Chem. 18(12), 957–968 (2021)
A.R. Deshmukh, M.R. Bhosle, L.D. Khillare, S.T. Dhumal, A. Mishra, A.K. Srivastava, R.A. Mane, New tetrazoloquinolinyl methoxyphenyl-4-thiazolidinones: Synthesis and antihyperglycemic evaluation. Res. Chem. Intermed. 43(2), 1107–1120 (2017)
Srivastava S, Srivastava S, Srivastava S (2002) Synthesis of 5-arylidene-2-aryl-3-(1, 2, 4-triazoloacetamidyl)-1, 3-thiadiazol-4-ones as antibacterial, antifungal, analgesic and diuretic agents
M.V. Diurno, O. Mazzoni, E. Piscopo, A. Calignano, F. Giordano, A. Bolognese, Synthesis and antihistaminic activity of some thiazolidin-4-ones. J. Med. Chem. 35(15), 2910–2912 (1992)
R.V. Shingalapur, K.M. Hosamani, R.S. Keri, M.H. Hugar, Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 45(5), 1753–1759 (2010)
E.M. Flefel, W.I. El-Sofany, R.A. Al-Harbi, M. El-Shahat, Development of a novel series of anticancer and antidiabetic: Spirothiazolidines analogs. Molecules 24(13), 2511 (2019)
P. Eleftheriou, A. Geronikaki, D. Hadjipavlou-Litina, P. Vicini, O. Filz, D. Filimonov, V. Poroikov, S.S. Chaudhaery, K.K. Roy, A.K. Saxena, Fragment-based design, docking, synthesis, biological evaluation and structure–activity relationships of 2-benzo/benzisothiazolimino-5-aryliden-4-thiazolidinones as cycloxygenase/lipoxygenase inhibitors. Eur. J. Med. Chem. 47, 111–124 (2012)
Lesyk R, Zimenkovsky B, Kaminskyy D, Kryshchyshyn A, Havryluk R, Atamanyuk D, Subtel’na IY, Khyluk D (2011) Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group, Biopolymers and Cell
S. Nirwan, V. Chahal, R. Kakkar, Thiazolidinones: synthesis, reactivity, and their biological applications. J. Heterocycl. Chem. 56(4), 1239–1253 (2019)
Pigman W (2012) The carbohydrates: chemistry and biochemistry, Elsevier2012.
Ernst B, Hart GW, Sinaÿ P (2000) Carbohydrates in chemistry and biology, Wiley Online Library2000.
J. Ati, P. Lafite, R. Daniellou, Enzymatic synthesis of glycosides: from natural O-and N-glycosides to rare C-and S-glycosides. Beilstein J. Org. Chem. 13(1), 1857–1865 (2017)
Nicotra F, Airoldi C, Cardona F (2007) 1.16 Synthesis of C-and S-Glycosides, Elsevier: Oxford, 2007, pp. 647–683.
J.K. Prasain, S. Barnes, J.M. Wyss, Kudzu isoflavone C-glycosides: Analysis, biological activities, and metabolism. Food Frontiers 2(3), 383–389 (2021)
H.-M. Chen, Y. Wang, L.-H. Su, C.-H. Chiu, Nontyphoid Salmonella infection: microbiology, clinical features, and antimicrobial therapy. Pediatr. Neonatol. 54(3), 147–152 (2013)
H.M. Dardeer, A. Toghan, M.E. Zaki, R.B. Elamary, Design, synthesis and evaluation of novel antimicrobial polymers based on the inclusion of polyethylene Glycol/TiO2 Nanocomposites in Cyclodextrin as drug carriers for sulfaguanidine. Polymers 14(2), 227 (2022)
A. El-Sayed, N. Khaireldin, M. El-Shahat, E. El-Hefny, M. El-Saidi, M. Ali, A. Mahmoud, Antiproliferative activity for newly heterofunctionalized pyridine analogues. Ponte 72(7), 106–118 (2016)
V. Kren, L. Martínková, Glycosides in medicine:“The role of glycosidic residue in biological activity.” Curr. Med. Chem. 8(11), 1303–1328 (2001)
X. Li, R. Wang, Y. Wang, H. Chen, Z. Li, C. Ba, J. Zhang, Stereoselective synthesis and biological activity of novel spiro-oxazinanone-C-glycosides. Tetrahedron 64(42), 9911–9920 (2008)
G. Suresha, K. Prakasha, K.S. Kumara, W. Kapfo, D.C. Gowda, Design and synthesis of heterocyclic conjugated peptides as novel antimicrobial agents. Int. J. Pept. Res. Ther. 15(1), 25–30 (2009)
Y.I. Asiri, A.B. Muhsinah, A. Alsayari, K. Venkatesan, M. Al-Ghorbani, Y.N. Mabkhot, Design, synthesis and antimicrobial activity of novel 2-aminothiophene containing cyclic and heterocyclic moieties. Bioorg. Med. Chem. Lett. 44, 128117 (2021)
E.M. Flefel, H.H. Sayed, A.I. Hashem, E.A. Shalaby, W. El-Sofany, F.M. Abdel-Megeid, Pharmacological evaluation of some novel synthesized compounds derived from spiro (cyclohexane-1, 2′-thiazolidines). Med. Chem. Res. 23(5), 2515–2527 (2014)
M. El-Shahat, W.I. El-Sofany, A.-G.A. Soliman, M. Hasanin, Newly synthesized imidazolotriazole, imidazolotriazine, and imidazole-pyrazole hybrid derivatives as promising antimicrobial agents. J. Mol. Struct. 1250, 131727 (2022)
A.E. Rashad, A.H. Shamroukh, M.A. El-Hashash, A.F. El-Farargy, N.M. Yousif, M.A. Salama, N. Abdelwahed, M. El-Shahat, 1, 3-Bis (4-chlorophenyl)-2, 3-epoxypropanone as synthons in synthesis of some interesting potential antimicrobial agents. Org. Chem.: An Indian J. 9(7), 287–294 (2013)
R.M. Abdelhameed, H.A. El-Sayed, M. El-Shahat, A.A. El-Sayed, O.M. Darwesh, Novel triazolothiadiazole and triazolothiadiazine derivatives containing pyridine moiety: Design, synthesis, bactericidal and fungicidal activities. Curr. Bioact. Compd. 14(2), 169–179 (2018)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sofany, W.I., Flefel, E.M., Darwesh, O.M. et al. Boosting the antimicrobial performance based on new fused spirothiazolidine framework analogs. J IRAN CHEM SOC 19, 4223–4236 (2022). https://doi.org/10.1007/s13738-022-02595-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02595-8