Abstract
Lead compounds containing nitrogen pharmacophores from natural resources have garnered interest among researchers due to their potential for drug development. However, the extractions of the active metabolites are usually labor-intensive and time-consuming. In this study, halogenated vanillin derivatives featuring azo dyes (N=N) (1a-1 h) and Schiff base (C=N) (2a-2 h) have been synthesized via diazonium coupling and nucleophilic substitution reaction, respectively. The comparative effect of N=N and C=N moieties was evaluated for antibacterial properties against Staphylococcus aureus and Escherichia coli via disc diffusion method. Incorporating C=N (8–13 mm) into the vanillin network showed excellent inhibition against S. aureus compared to N=N (7–8 mm) and the standard ampicillin (12 mm). While the halogenated vanillin featuring N=N (7–9 mm) and C=N (7–8 mm) moieties showed excellent zone of inhibitions against E. coli compared to the parent vanillin. The in-silico screening using AutoDock Vina, showed 2c-h (inhibition zone > 10 mm) with a high binding affinity against DNA gyrase enzyme with binding energy ranging from − 7.3 to − 7.9 kcal/mol, similar to re-docking of ampicillin − 7.6 kcal/mol and co-crystalize compounds BPH651 with − 7.5 kcal/mol. This research contributes a significant milestone in drug design, especially for the development of new antibacterial drugs with outstanding properties.
Graphical abstract

Article Highlights
-
A synergistic effect of functional groups in vanillin-Schiff base (C=N) and vanillin-azo dyes (N=N) contributed to the hydrogen bond interaction with the biological receptors.
-
Structure–Activity relationship of halogenated vanillin derivatives supported the inhibition activity against Staphylococcus aureus and Escherichia coli.
-
The incorporation of nitrogen chromophores and halogen into vanillin moieties improved binding interactions and binding affinity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The rise of pathogenic microbe resistance has prompted researchers to search for effective pharmaceutical drugs. The prescription against nonbacterial diseases, such as viral infections and uncontrolled consumption of drugs has led to microbial resistance [1]. It has become challenging for researchers due to the rising expenses in drug development with a limited time [2,3,4]. Traditional medicine has utilized natural resources such as penicillin [5], coumarin [3], and aspirin [4] to relieve pain and fever. Modifying active scaffolds from natural product-based compounds can potentially develop novel drugs with enhanced efficacy against drug-resistant microbes with less cost and time.
Chemical modification of compounds derived from natural sources offers a promising approach as it enhances the likelihood of diverse interactions with biological targets and improves their biological activity [2, 3]. Vanillin is an example of an active scaffold derived from the natural product Vanilla planifolia, a common food-grade additive that is widely used in food [6] and pharmaceutical products [7]. However, its extraction process is labor-intensive and lower in yield [8]. Many studies reported on the chemical alteration of vanillin's structure to enhance its biological properties for anticancer [9, 10], antiviral [11], antifungal [12] and antibacterial [13]. Trimethoprim is an example of a known pharmaceutical drug containing vanillin to treat upper respiratory tract infections [14].
Many active chromophores with nitrogen moieties, including azo dyes (N=N) and Schiff base (C=N) have been exclusively integrated into the vanillin network to increase their biological activities. The presence of an active moiety of azomethine (C=N) in Schiff base contributed to a broad range of biological effects in the pharmaceutical industry such as anticancer [15], antimicrobial [16] and antibacterial properties [17]. Schiff bases have also been utilised as ligands to form metal complexes due to their stronger coordinative ability, which makes them useful for catalytic reactions [16], fluorescent chemosensors [15] and dyes [17]. While, Azo dyes have been widely used in textile, fiber, cosmetics, leather and paints [3, 17].
There are many drugs containing azo dyes (N=N) that have been commercially used to treat bacterial infections such as sulfasalazine and phenazopyridine [18]. The presence of an azo group (N=N) that is easily protonated under acidic conditions contributes to improving the biological activity of a compound [3]. Nevertheless, a synergistic incorporation of nitrogen moieties and halogen substituents in the vanillin is scarcely reported. The presence of halogen moieties can enhance the lipophilic characteristics of molecules, facilitating their ability to penetrate the outer membrane of bacteria [19, 20]. The inclusion of halogens into the molecular framework of vanillin, which bears nitrogen chromophores, is anticipated to synergistically enhance the antimicrobial effectiveness of the parent vanillin.
This work reports the formation of halogenated vanillin-azo dyes (1a-1 h) from a series of vanillin derivatives comprising nitrogen chromophores and halogens at ortho, meta, and para positions by the diazo coupling reaction. Another series of halogenated vanillin-Schiff base derivatives (2a-2 h) was prepared using a nucleophilic substitution reaction. The in vitro antibacterial activities against E. coli ATCC 25922 and S. aureus S48/81 were comparatively evaluated for potential antimicrobial drugs, using ampicillin as the reference drug. The structure–activity relationship of the molecules and evaluation of the binding affinity via in-silico molecular docking on DNA gyrase enzyme (PDB ID: 4DUH) as a potential therapeutic target were performed to postulate the molecular basis of the antibacterial action of the synthesized compounds.
2 Results and discussion
2.1 Chemistry
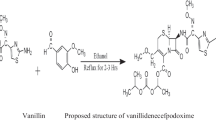
A series of vanillin-azo dyes 1a-1 h containing halogen and nitrogen moieties (N=N) as an active pharmacophore was prepared from the reaction of vanillin with anilines featuring halogens. The diazo coupling reaction was conducted in the presence of NaOH. A comparative nitrogen active pharmacophores of vanillin-Schiff base derivatives (C=N) 2a-2 h featuring halogens were synthesized via nucleophilic substitution reaction of halogenated aniline with vanillin in ethanol at the ambient temperature. The schematic diagram for the synthesis of 1a-1 h and 2a-2 h is shown in Scheme 1.
The chemical structures of 1a-1 h & 2a-2 h were elucidated via FTIR, 1H and 13C NMR spectroscopy (Figure S2, S3 & S4). The characterization 1a-1 h using FTIR depicted a strong absorption peak at 1490–1464 cm−1 indicating the formation of v(N=N) [3]. The v(C=O) peaks were observed at 1649–1685 cm−1 [21] while v(O–H) presence as a broad peak at 3426–3453 cm−1 [22]. The 1H and 13C NMR spectroscopy of 1a-1 h showed the presence of aromatic protons at 7.33–8.13 ppm for the aromatic protons in the compound. The peaks at 3.90–3.91 ppm represent the -OCH3 [22] while the resonance at 9.88–9.91 ppm is attributed to the aldehyde peak [23]. The 13C NMR peak at 190.8–192.2 ppm and 56.1–56.8 ppm attributed to the C=O and –OCH3 [23], respectively, which confirmed the formation of 1a-1 h.
The FTIR spectra of 2a-2 h showed the disappearance of v(C=O) at 1650–1680 cm−1 and the appearance of strong absorption at 1617–1622 cm−1 for v(C=N) [24], indicating the formation of the imine group. The broad peak at 3055–3175 cm−1 corresponded to v(O–H). 1H and 13C NMR spectroscopy of 2a-2 h, depicted peaks at 6.50–7.62 ppm for the Ar–H in 2a-2 h, while the peak at 3.89–3.93 ppm attributed to the –OCH3. The disappearance of the aldehyde peak at 9–10 ppm and the appearance of a singlet peak at 8.42–8.45 ppm represented the imine peak [24], which confirmed the formation of 2a-2 h. The 13C NMR spectra depicted peaks at 160.6–162.3 ppm and 55.4–55.5 ppm for C=N and –OCH3, respectively.
2.2 Antibacterial activities of 1a-1 h and 2a-2 h
The antibacterial activity of the halogenated vanillin-azo series (1a-1 h) and halogenated vanillin-Schiff base series (2a-2 h) were carried out against S. aureus and E. coli using the turbidimetric kinetic method. However, several compounds encountered solubility restrictions and dissolution in the culture media caused the precipitation of the drug[2, 21]. This occurrence has interfered with the measurement of biological activities. It was envisaged that the relatively lipophilic molecular characteristics of the compounds may restrict their solubility in the culture media [2].
Kirby-Bauer disc diffusion assay was alternatively used in this study to evaluate the inhibitory efficacy of the compounds against E. coli and S. aureus (Figure S1 and Fig. 1). Both series of halogenated vanillin derivatives featuring C=N moiety (2a-2 h) and N=N (1a-1 h) showed moderate to excellent zone inhibition of S. aureus and E. coli. The incorporation of C=N into the vanillin network demonstrated superior inhibition against S. aureus (zone of inhibition 8–13 mm) compared to N=N (7–8 mm) and the standard ampicillin (12 mm). In comparison to the parent vanillin, the halogenated vanillin bearing C=N and N=N moieties showed excellent zones of inhibitions against E. coli with 7–8 mm and 7–9 mm, respectively. It is also worth mentioning that low lipid solubility can reduce antimicrobial efficacy due to their impermeability towards the bacterial surface such as S. aureus [25]. The introduction of halogen has remarkably increased the lipophilic characteristic of the synthesized compounds, enabling them to penetrate the outer membrane of the bacteria [26].
The remarkable inhibition activity of 2a-2 h was aligned with the recent similar findings [27] on the superior antimicrobial properties of Schiff bases compared to azo dyes due to their stronger binding affinity and better hydrogen bonding interactions with specific protein sites, effectively disrupting bacterial growth [17]. However, the antimicrobial activity of vanillin-bearing Schiff base moieties was relatively lower against E. coli due to the rigid lipopolysaccharide bilayer at the outer membrane of bacteria which therefore reduced the ability of the compound for penetration [19]. Interestingly, the inhibition activity against E. coli could only be observed on compounds featuring bromine (2f, 2 g, & 2 h). This could be attributed to the polarizability of the bromine atom which possesses a larger atomic size. The high polarization effect of bromine compared to fluorine and chlorine influenced the binding interactions of the modified scaffolds with the target protein, which in turn impacted the overall antibacterial efficacy [20].
The meta-halogenated vanillin derivatives (1b, 1d, 1 g) exhibited significant activity against S. aureus, consistent with previous findings demonstrating the antimicrobial efficacy of organic compounds containing halogen substitutions at the meta position [4, 28]. Nevertheless, meta-substituted chlorine (1d) showed no inhibition against E. coli due to the less lipophilic [20] compared to meta-substituted bromine (1 g). Excellent inhibition of meta-fluoro (1b) was due to the formation of hydrogen bonds with the target receptor [29]. The presence of the active moiety N=N that is easily protonated under acidic conditions contributes to the antibacterial properties of the compound [30,31,32]. Additional interaction of the carbonyl and phenyl ring with the phosphates moieties on the bacteria's surface has also increased the antibacterial properties of a compound [33, 34]. Besides, the presence of halogen atoms as substituents has also significantly increased the biological activity of the compounds by maintaining the non-hydrophobic interaction between halogen and the amino acid of the bacteria, [21]. It is worth mentioning that the integration of halogen substituents may result in steric hindrance of the molecule and increase its capacity to interact efficiently with the bacteria [34]. Less inhibition on both series against E. coli was reported due to the thicker outer membrane of the bacteria containing lipopolysaccharide which could reduce the compound’s ability to penetrate [19].
A synergistic effect of functional groups presence on vanillin-Schiff base (C=N, –OH) and vanillin-azo dyes (N=N, C=O, –OH) has contributed to the antibacterial of the compounds via hydrogen bond interaction with the biological receptors compared to the parent vanillin alone. Among all the synthesized compounds, the meta-halogenated vanillin showed good inhibition against both Gram-positive and Gram-negative bacteria. This is due to the synergistic effects of hydrophilic-lipophilic characteristics in the molecule thus contributing to the antibacterial properties [35]. The aromatic rings in the molecular network have also played a significant role in enhancing the lipophilic properties which also can potentially interact with the hydrophobic areas of bacterial enzymes [21].
2.3 Molecular docking studies
Molecular docking screening was used to postulate the molecular basis of the antibacterial action of the synthesized compounds. DNA gyrase enzyme (PDB ID: 4DUH) was chosen as a potential therapeutic target to examine the antibacterial action. One of the topoisomerase classes (topoisomerase II) is represented by the DNA Gyrase enzyme. The DNA is wound and unwound by these enzymes during transcription and replication. Due to its ability to change the topological state of DNA and act as a model for other DNA topoisomerases, the gyrase enzyme is anticipated to be a crucial intracellular target for antibacterial drugs [36]. Re-docking was performed to the crystal structures of the DNA gyrase enzyme using the inhibitors BPH651 to validate the docking parameter used in this work for the docking outputs to be replicated. The orientation of the inhibitor's optimal docking pose was superimposed with the binding site of the inhibitor's original crystal structure. The conformation (1.0292) was set for BPH651 and BPH651 (Fig. 2). In a re-docking study, both docked inhibitor conformations showed the same orientation with the inhibitor, indicating that AutoDock Vina has excellent accuracy in predicting the ligand the binding interactions with the DNA gyrase enzyme [37].
Molecular docking simulation of binding interaction with the DNA gyrase enzyme using stick model. A The alignment of the redocked BPH651 (green stick model) and co-crystallized BPH651 (yellow stick model) within the binding site of DNA gyrase enzyme. B Superimposition of redocking BPH651 (green stick model) and co-crystallized BPH651 (yellow stick model). C The alignment of 2c (blue stick model), 2d (brown stick model), 2e (purple tick model), 2f (orange stick model), 2g (turquoise stick model), 2h (pink stick model), ampicillin (silver stick model) and BPH651 (yellow stick model) within the binding site of DNA gyrase enzyme. D 2c (blue stick model), 2d (brown stick model), 2e (purple tick model), 2f (Orange stick model), 2g (turquoise stick model), 2h (pink stick model), ampicillin (grey stick model) and BPH651 (yellow stick model) in the hydrophobic pocket of DNA gyrase enzyme
To investigate the mechanism of action and the inhibition of the DNA gyrase enzyme at the molecular level, a molecular docking study was performed on all compounds (1a-1 h and 2a-2 h) (Table S1). Halogenated vanillin-Schiff Base 2c-2 h with inhibition zone > 10 mm showed excellent binding affinity for the enzyme with a binding energy of − 7.3 to − 7.9 kcal/mol, similar to re-dock of ampicillin − 7.6 kcal/mol and co-crystalize compounds BPH651 with − 7.5 kcal/mol. The hydrogen bond interaction of 2c-2 h with amino acid in binding pocket enzyme makes a key contribution to the inhibitory activity. Compounds 2c-2 h show hydrogen bonds with ASN46 similar to the co-crystalize ligand and ampicillin. Notable, the Schiff base derivatives contribute to the binding interaction between nitrogen and amino acids in the enzyme, thereby leading to bacterial inhibition activity. The hydrophobicity of 2c-2 h and the hydrophobic pocket in the enzyme's binding side are other factors that contribute to the excellent interaction of the ligand and receptor. The active compounds in this study showed similar amino acid interaction with BPH65 and ampicillin. Examples of the amino acid are ASN46, PRO79, ILE94, LYS103 and VAL120.
3 Conclusion
In conclusion, the incorporation of azo 1a-h and Schiff base 2a-h on the molecular network of vanillin has surpassed the antibacterial efficacy compared to the parent vanillin. Compounds 2a-2 h exhibited pronounced effectiveness against S. aureus as compared to 1a-1 h. Among newly synthesized compounds, compound 2d exhibited better inhibition against S. aureus compared to the reference drug, ampicillin. The incorporation of halogenated azo and Schiff base groups into the vanillin structure allowed for the synergistic effects of several functional groups, which enhanced binding interactions with the bacterial surface. This finding concludes that the addition of nitrogen and halogen elements enhances the antibacterial properties of vanillin, rendering its derivatives promising candidates for further exploration and potential drug development. Further research and optimization of these derivatives hold the potential to yield antibacterial medications with wide-ranging applications in the medical field.
4 Experimental section
The chemicals were analytical grade with no purification. The melting point (Stuart MP3) was used with an open tube capillary. 1H NMR and 13C NMR spectra were recorded using JEOL ECA 500 at 500 MHz (1H) and 126 MHz (13C) with the chemical shift relative to DMSO-d6/acetone-d6 as standard reference (in δ ppm).
4.1 Synthesis series of halogenated vanillin-azo (1a-1 h): general procedure
Halogenated vanillin-azo was synthesized following the procedure reported by Mortadza & Ngaini [23]. A solution of NaNO2 (2 M, 5 mL) was added to an aniline derivative (5 mmol) in HCl solution (2 M, 10 mL) at 0–5 °C. The mixture was slowly added into vanillin (5 mmol) in NaOH(0.4 g, 10 mmol) in distilled water (10 mL) and continued stirring for 1 h. The mixture was acidified with HCl (2 M). The precipitate was filtered, washed and recrystallized in ethanol to give 1a-1 h. The supporting data (1a-1 h) is accessible in the supplementary files Data S1.
4.2 Synthesis of halogenated vanillin-Schiff base (2a-2 h): General procedure
The synthesis of halogenated vanillin-Schiff base was adapted from previous literature by Chigurupati [24]. Halogenated aniline (0.5 mmol) and vanillin (0.5 mmol) were stirred in ethanol at room temperature for 10 h. Distilled water was added and the mixture was cooled to obtain the crude solid, filtered, washed, and recrystallized using ethanol: hexane (1:2) to afford 2a-2 h. Data for 2a-2 h is accessible in the supplementary files Data S2.
4.3 Antibacterial studies
Kirby-Bauer disc diffusion method was employed for the antibacterial evaluation of 1a-1 h and 2a-2 h against Gram-positive and Gram-negative bacteria (S. aureus and E. coli) [21]. DMSO served as a negative control and reference drug, ampicillin was chosen. Compounds 1a-1 h and 2a-2 h in DMSO (100 ppm) were introduced to the sterile paper discs and put on the surface of microorganism-inoculated media. The inhibition zone diameter was measured after 24 h and recorded.
4.4 Molecular docking studies
In-silico screening using molecular docking was applied to all the synthetic derivatives to postulate the molecular mechanism behind the antibacterial characteristics of the compounds. The 2D structures of each chemical were created using Accelrys, San Diego, USA (Discovery Studio® 4.0). The Avogadro program was used to optimize the ligand structures (shape and energy) using the MMFF-94 force field’s steepest descent and conjugate gradient approach (5,000 steps) [38]. The structure of the selected macromolecules was obtained from the Protein Data Bank (PDB). DNA gyrase enzyme (PDB ID: 4DUH) was chosen as a potential therapeutic target. The target protein contains an inhibitor with a resolution of less than 3.0 [39]. The hydrogen atoms were introduced into protein structures employing AutoDockTools [40]. AutoDock Vina was used to perform the docking [41]. Discovery Studio® 4.0 (Accelrys, San Diego, USA) was used to visualize the binding interactions where the highest binding affinity was selected.
Data availability
All data generated or analyzed during this study are included in this published article as supplementary data.
References
Rossiter SE, Fletcher MH, Wuest WM. Natural products as platforms to overcome antibiotic resistance. Chem Rev. 2017;117(19):12415–74. https://doi.org/10.1021/acs.chemrev.7b00283.
Farooq S, Ngaini Z. Synthesis, molecular docking and antimicrobial activity of α, β-unsaturated ketone exchange moiety for chalcone and pyrazoline derivatives. ChemistrySelect. 2020;5(32):9974–9. https://doi.org/10.1002/slct.202002278.
Ngaini Z, Mortadza NA. Synthesis of halogenated azo-aspirin analogues from natural product derivatives as the potential antibacterial agents. Nat Prod Res. 2019;33(24):3507–14. https://doi.org/10.1080/14786419.2018.1486310.
Nordin NA, et al. In vitro cytotoxicity evaluation of thiourea derivatives bearing Salix sp. constituent against HK-1 cell lines. Nat Prod Res. 2020;34(11):1505–14. https://doi.org/10.1080/14786419.2018.1517120.
Kaur H, Lim SM, Ramasamy K, Vasudevan M, Shah SAA, Narasimhan B. Diazenyl schiff bases: synthesis, spectral analysis, antimicrobial studies and cytotoxic activity on human colorectal carcinoma cell line (HCT-116). Arab J Chem. 2020;13(1):377–92. https://doi.org/10.1016/j.arabjc.2017.05.004.
Raymond Mohanraj DG, Alagumuthu M, Subramaniam P, Bakthavachalam D, Arumugam S, Chellam S. Antimicrobial effects of vanillin-based pyridyl-benzylidene-5-fluoroindolins. J Heterocycl Chem. 2021;58(7):1515–24. https://doi.org/10.1002/jhet.4277.
Anand A, Wahal N, Mehta M, Satija S. Vanillin: a comprehesive review of pharmacological activities. Plant Arch. 2019;19(2):1000–4.
Vijayalakshmi S, Disalva X, Srivastava C, Arun A. Vanilla-natural vs artificial: a review. Res J Pharm Technol. 2019;12(6):3068. https://doi.org/10.5958/0974-360x.2019.00520.1.
Ma W, et al. College of chemistry and chemical engineering, Lanzhou University, 222 Tianshui South Macau institute for applied research in medicine and health, state key laboratory of declaration of comperting interest the authors declare no conflicts of interests. Eur J Pharm Sci. 2020. https://doi.org/10.1016/j.ejps.2020.105464.
Li M, Lang Y, Gu M, Shi J, Chen BPC, Yu L. Vanillin derivative VND3207 activates DNA-PKcs conferring protection against radiation-induced intestinal epithelial cells injury in vitro and in vivo. Toxicol Appl Pharmacol. 2019. https://doi.org/10.1016/j.taap.2019.114855.
Hariono M, Abdullah N, Damodaran KV, Kamarulzaman EE. Potential new H1N1 neuraminidase inhibitors from ferulic acid and vanillin: molecular modelling, synthesis and in vitro assay. Nat Publ Gr. 2016. https://doi.org/10.1038/srep38692.
Kim JH, et al. A vanillin derivative causes mitochondrial dysfunction and triggers oxidative stress in cryptococcus neoformans. PloS one. 2014. https://doi.org/10.1371/journal.pone.0089122.
Pattrick CA, Webb JP, Green J, Chaudhuri RR, Collins MO, Kelly DJ. Proteomic profiling, transcription factor modeling, and genomics of evolved tolerant strains elucidate mechanisms of vanillin toxicity in Escherichia coli. Appl Environ Sci. 2019;4(4):1–29. https://doi.org/10.1128/msystems.00163-19.
Gulsia O. Vanillin: one drug, many cures. Resonance. 2020;25(7):981–6.
Hassan AM, Said AO, Heakal BH, Younis A, Aboulthana WM, Mady MF. Green synthesis, characterization, antimicrobial and anticancer screening of new metal complexes incorporating schiff base. ACS Omega. 2022;7(36):32418–31. https://doi.org/10.1021/acsomega.2c03911.
Vhanale B, Kadam D, Shinde A. Synthesis, spectral studies, antioxidant and antibacterial evaluation of aromatic nitro and halogenated tetradentate Schiff bases. Heliyon. 2022;8: e09650. https://doi.org/10.1016/j.heliyon.2022.e09650.
Muhammad-Ali MA, Jasim EQ, Al-Saadoon AH. Synthesis, antibacterial evaluation, and docking studies of some azo compounds and schiff bases derived from sulfonamide. J Med Chem Sci. 2023;6(9):2128–39. https://doi.org/10.26655/JMCHEMSCI.2023.9.19.
Adu JK, et al. Synthesis and in vitro antimicrobial and anthelminthic evaluation of naphtholic and phenolic azo dyes. J Trop Med. 2020;2020:1–8. https://doi.org/10.1155/2020/4850492.
Zgurskaya HI, Löpez CA, Gnanakaran S. Permeability barrier of gram-negative cell envelopes and approaches to bypass it. ACS Infect Dis. 2015;1(11):512–22. https://doi.org/10.1021/acsinfecdis.5b00097.
Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem. 2013;56(4):1363–88. https://doi.org/10.1021/jm3012068.
Abd Halim AN, Ngaini Z. Synthesis and characterization of halogenated bis(acylthiourea) derivatives and their antibacterial activities. Phosphor Sulfur Silicon Relat Elem. 2017;192(9):1012–7. https://doi.org/10.1080/10426507.2017.1315421.
Sumrra SH, et al. Synthesis, characterization, and biological screening of metal complexes of novel sulfonamide derivatives: experimental and theoretical analysis of sulfonamide crystal. Appl Organomet Chem. 2020;34(7):1–19. https://doi.org/10.1002/aoc.5623.
Mortadza NA, Ngaini Z. Microwave-assisted and conventional synthesis of halogenated coumarin-azo derivatives and structural-activity relationship study for antimicrobial potential. Malaysian J Anal Sci. 2023;27(2):342–52.
Chigurupati S. Designing new vanillin schiff bases and their antibacterial studies. J Med Bioeng. 2015;4(5):363–6. https://doi.org/10.12720/jomb.4.5.363-366.
Hassan AU, et al. New organosulfur metallic compounds as potent drugs: synthesis, molecular modeling, spectral, antimicrobial, drug likeness and DFT analysis. Mol Divers. 2022;26(1):51–72. https://doi.org/10.1007/s11030-020-10157-4.
Sumrra SH, et al. Metal incorporated aminothiazole-derived compounds: Synthesis, density function theory analysis, in vitro antibacterial and antioxidant evaluation. R Soc Open Sci. 2021. https://doi.org/10.1098/rsos.210910.
Jadama A, Yuksekdanaci S, Astley D, Yasa I. Synthesis, characterization and biological activity of schiff and azo-schiff base ligands. Stud Univ Babes-Bolyai Chem. 2023;2023(1):75–89. https://doi.org/10.24193/subbchem.2023.1.06.
Kosikowska U, Wujec M, Trotsko N, Płonka W, Paneth P, Paneth A. Antibacterial activity of fluorobenzoylthiosemicarbazides and their cyclic analogues with 1,2,4-triazole scaffold. Molecules. 2021;26(1):1–18. https://doi.org/10.3390/MOLECULES26010170.
Dalvit C, Invernizzi C, Vulpetti A. Fluorine as a hydrogen-bond acceptor : experimental evidence and computational calculations. Chem Eur J. 2014;20:11058–68. https://doi.org/10.1002/chem.201402858.
Ngaini Z, Rasin F, Wan Zullkiplee WSH, Abd Halim AN. Synthesis and molecular design of mono aspirinate thiourea-azo hybrid molecules as potential antibacterial agents. Phosphorus Sulfur Silicon Relat Elem. 2020;196(3):275–82. https://doi.org/10.1080/10426507.2020.1828885.
Ho BK, et al. Synthesis and anticancer activities of 4-[(Halophenyl)diazenyl]phenol and 4-[(Halophenyl)diazenyl]phenyl aspirinate derivatives against nasopharyngeal cancer cell lines. J Chem. 2017. https://doi.org/10.1155/2017/6760413.
Mortadza NA, Ngaini Z, Arif MAM. Synthesis of silver (I) coordination of aspirinate azo ligands as potential antibacterial agents. Defect Diffus Forum. 2021;411:17–24. https://doi.org/10.4028/www.scientific.net/DDF.411.17.
Sie CZW, Ngaini Z, Suhaili N, Madiahlagan E. Synthesis of kojic ester derivatives as potential antibacterial agent. J Chem. 2018. https://doi.org/10.1155/2018/1245712.
Ngaini Z, et al. Synthesis and antibacterial study of aspirin-chalcone derivatives. Borneo J Resour Sci Technol. 2013;3(1):52–7.
Gull P, Hashmi AA. Biological activity studies on metal complexes of macrocyclic Schiff base ligand: synthesis and spectroscopic characterization. J Braz Chem Soc. 2015;26(7):1331–7. https://doi.org/10.5935/0103-5053.20150099.
Ragab A, et al. Synthesis, characterization, thermal properties, antimicrobial evaluation, ADMET study, and molecular docking simulation of new mono Cu (II) and Zn (II) complexes with 2-oxoindole derivatives. Comput Biol Med. 2022;145: 105473. https://doi.org/10.1016/j.compbiomed.2022.105473.
Al-Madhagi W, Hashim N, Awadh Ali N, Taha H, Alhadi A, Abdullah A. Bioassay-guided isolation and in silico study of antibacterial compounds from petroleum ether extract of peperomia blanda (Jacq.) Kunth. J Chem Inf Model. 2019;59(5):1858–72.
Hanwell M, Curtis D, Lonie D, Vandermeersch T, Zurek E, Hutchison G. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4(1):17.
Danev R, Belousoff M, Liang YL, Zhang X, Eisenstein F, Wootten D. Routine sub-2.5 Å cryo-EM structure determination of GPCRs. Nat Commun. 2021;12(1):4333.
Forli S, Huey R, Pique M, Sanner M, Goodsell D, Olson A. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc. 2016;11(5):905–19.
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61.
Acknowledgements
The authors would like to express their gratitude to the Ministry of Higher Education Malaysia for the financial support provided through FRGS/1/2019/STG01/UNIMAS/01/1.
Author information
Authors and Affiliations
Contributions
Conceptualization, research design and funding acquisition by Z.N. and A.N.. M. A., F. N. A. and F. S. conducted the experiment. N. H. performed the computational molecular docking analysis. M. A. and N. H. wrote the original draft of the manuscript and prepared the supplementary materials. Z.N. reviewed and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hissam, M.A., Ngaini, Z., Zamakshshari, N.H. et al. Synthesis and molecular docking simulation on the antimicrobial effects of halogenated vanillin-azo dyes and schiff base derivatives. Discov Appl Sci 6, 325 (2024). https://doi.org/10.1007/s42452-024-05830-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05830-4