Abstract
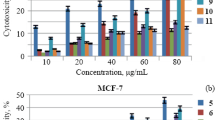
The derivatization of three hydroxycinnamamides becomes trans-N-benzylhydroxycinnamamides, and their potential assay as anticancer agents has been carried out. N-benzyl-p-coumaramide (5a), N-benzylcaffeamide (5b), and N-benzylferulamide (5c) were obtained from p-coumaric, caffeic, and ferulic acid, respectively, with benzylamine via four reaction steps, i.e., acetylation, chlorination, amidation, and deacetylation. All products characterize using FTIR, 1H-NMR, and 13C-NMR spectroscopy, and their cytotoxicity were tested against P388 leukemia murine cells by MTT method. Although compound 5b and 5c have no and low anticancer activity with IC50 sequentially of 674.38 and 179.56 µg/mL, compound 5a showed potentially use as an anticancer agent with IC50 of 16.15 µg/mL Molecular modelling studies were performed to understand the interactions with the activity against murine leukemia P388 cells.



Similar content being viewed by others
References
L.H. Boudreau, J. Maillet, L.M. Leblanc, T.M. Jean-franc, Caffeic acid phenethyl ester and its amide analogue are potent inhibitors of leukotriene biosynthesis in human polymorphonuclear leukocytes. PLoS ONE 7(2), 31833 (2012). https://doi.org/10.1371/journal.pone.0031833
M.G. Chochkova, E.Y. Chorbadzhiyska, G.I. Ivanova, H. Najdenski, M. Ninova, T.S. Milkova, Antimicrobial and radical scavenging activities of N-hydroxycinnamoyl-L-antimicrobial and radical scavenging activities of N-Hydroxycinnamoyl-L-cysteine and -L-proline ethyl esters. Nat. Prod. J. 2(1), 1–5 (2012). https://doi.org/10.2174/2210315511202010050
M.G. Chochkova, P.P. Petrova, B.M. Stoykova, G.I. Ivanova, M. Sticha, G. Dibo, T.S. Milkova, Structure-activity relationships of n-cinnamoyl and hydroxycinnamoyl amides on α-glucosidase inhibition. J. Chem. 2017, 1–5 (2017)
C. Cui, Z.P. Wang, X.J. Du, L.Z. Wang, S.J. Yu, X.H. Liu, Z.M. Li, W.G. Zhao, Synthesis and antiviral activity of hydrogenated ferulic acid derivatives. J. Chem. 2013, 1–5 (2013)
D. Systemes, Biovia discovery studio visualizer (Dassault Systemes, San Diego, 2019)
M. De Armas-ricard, E. Ruiz-reyes, O. Ramírez-rodríguez, Caffeates and caffeamides: synthetic methodologies and their antioxidant properties. Int. J. Med. Chem. 2019, 1–15 (2019)
H.D.R. Firdaus, T. Naid, N.H. Soekamto, S. Sumarna, M.F. Islam, Synthesis of piperidine and morpholine amides of ferulic acid and their bioactivity against P-388 leukemia cells. Int. J. ChemTech Res. 10(1), 27–33 (2017)
S. Firdaus, D. Husain, H. Rasyid, Sukarti, Methylation of p-coumaric acid with dimethyl sulfate and sodium hydoxide as catalyst. Proc. First Int. Conf. Sci. 1, 339–344 (2014)
Firdaus, Seniwati, N. Alamsyah, S. Paramita, Synthesis and activity of N-(o-tolyl)caffeamide and N-(o-tolyl)-p-coumaramide against P388 leukemia murine cells. J. Phys. Conf. Ser. 1341(3), 032005 (2019)
S.N.H. Firdaus, S. Firdausiah, H. Rasyid, N. Asmi, M. Waelulu, Novel hydroxycinnamamide from morpholine and pyrrolidine: synthesis, characterization, docking study, and anticancer activity against P388 leukemia murine cells. J. Appl. Pharm. Sci. 11(1), 40–48 (2021). https://doi.org/10.7324/JAPS.2021.110104
Firdaus, N.H. Soekamto, Seniwati, M.F. Islam, Sultan, Phenethyl ester and amide of ferulic acids: synthesis and bioactivity against p388 leukemia murine cells. J. Phys. Conf. Ser. 979, 012016 (2018)
L. Georgiev, M. Chochkova, G. Ivanova, H. Najdenski, M. Ninova, T. Milkova, Radical scavenging and antimicrobial activities of cinnamoyl amides of biogenic monoamines. La Riv. Ital. Delle Sostanze Grasse 89(2), 91 (2012)
B. Godlewska-żyłkiewicz, R. Swisloscka, M. Kalinowska, A. Golonko, G. Swiderslo, Z. Arciszewska, E. Nalewajko-Sielowoniuk, M. Naumowies, W. Lewandowski, Biologically active compounds of plants: structure-related antioxidant, microbiological and cytotoxic activity of selected carboxylic acid. Materials 13(19), 1–37 (2020). https://doi.org/10.3390/ma13194454
J.D. Guzman, Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 19, 19292–19349 (2014). https://doi.org/10.3390/molecules191219292
M.D. Hanwell, D.E. Curtis, D.C. Lonie, T. Vandermeersch, E. Zurek, G.R. Hutchison, Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 1–17 (2012)
R.F. Helm, J. Ralph, R.D. Hatfield, Synthesis of feruloylated methyl glycosides and p-coumaroylated. Carbohydr. Res. 229, 183–194 (1992)
R. Huey, G.M. Morris, A.J. Olson, D.S. Goodsell, A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 28(6), 1145–1152 (2007)
P.A. Jackson, J.C. Widen, D.A. Harki, K.M. Brummond, Covalent modifiers: a chemical perspective on the reactivity of α, β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J. Med. Chem. 60(3), 839–885 (2017). https://doi.org/10.1021/acs.jmedchem.6b00788
S.M. Jakoveti, B.Z. Jugovi, M.M. Gvozdenovi, D.I. Bezbradica, Synthesis of aliphatic esters of cinnamic acid as potential lipophilic antioxidants catalyzed by lipase B from Candida antarctica. Appl Biochem Biotechnol 170, 1560–1573 (2013). https://doi.org/10.1007/s12010-013-0294-z
A. Jitareanu, G. Tataringa, C. Tuchilus, M. Balan, U. Stanescu, Cinnamic acid derivatives and 4-aminoantipyrine amides—synthesis and evaluation of biological properties. Res. J. Chem. Sci. 3(3), 9–13 (2013)
A. Khatkar, A. Nanda, P. Kumar, B. Narasimhan, Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 10, 3804–3815 (2017). https://doi.org/10.1016/j.arabjc.2014.05.018
B. Korošec, M. Sova, S. Turk, N. Krasevec, M. Novak, L. Lah, J. Stojan, B. Pobobnik, S. Berne, N. Zupanec, M. Bunc, S. Gobec, R. Komel, Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP53). J. Appl. Microbiol. 116(4), 955–966 (2014). https://doi.org/10.1111/jam.12417
Y. Kuo, W. Jim, L. Su, C. Chung, C. Lin, Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 16, 10748–10766 (2015). https://doi.org/10.3390/ijms160510748
H. Kuncoro, L. Rijai, E. Julaeha, U. Supratman, Cytotoxic activity against P-388 murine Leukemia cell from Lygodium microphyllum herb. J. Farm. Galen. 3(1), 13–16 (2003)
R.A. Laskowski, M.B. Swindells, LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51(10), 2778–2786 (2011)
G.E. Magoulas, D. Papaioannou, Bioinspired syntheses of dimeric hydroxycinnamic acids (Lignans) and hybrids, using phenol oxidative coupling as key reaction, and medicinal significance thereof. Molecules 19, 19769–19835 (2014). https://doi.org/10.3390/molecules191219769
T. Matsubara, S. Asako, L. Ilies, E. Nakamura, Synthesis of anthranilic acid derivatives through iron-catalyzed ortho amination of aromatic carboxamides with N-chloroamines. J. Am. Chem. Soc. 136, 646–649 (2014)
G. Morris, R. Huey, AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30(16), 2785–2791 (2009). https://doi.org/10.1002/jcc.21256.AutoDock4
T. Nakazawa, K. Ohsawa, Metabolism of rosmarinic acid in rats. J. Nat. Prod. 2(98), 993–996 (1998)
S.B. Nimse, D. Pal, A. Mazumder, R. Mazumder, Synthesis of cinnamanilide derivatives and their antioxidant and antimicrobial activity. J. Chem. 2015, 1–6 (2015)
W. Peng, J.G. Wu, Y.B. Jiang, Y.J. Liu, T. Sun, N. Wu, C.J. Wu, Antitumor activity of 4-O-(2″-O-acetyl-6″-O-p-coumaroyl-β-d-glucopyranosyl)-p-coumaric acid against lung cancers via mitochondrial-mediated apoptosis. Chem. Interact. J. 233, 8–13 (2015). https://doi.org/10.1016/j.cbi.2015.03.014
E.F. Pettersen, T.D. Goddard, C.C. Huang, G.S. Couch, D.M. Greenblatt, E.C. Meng, T.E. Ferrin, UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
K. Saritha, G. Rajitha, Synthesis, antimicrobial and antioxidant activity of N-dimethylaminophenylallylidene cinnamide derivatives. Int. Res. J. Pharm. 9(12), 120–127 (2018). https://doi.org/10.7897/2230-8407.0912304
A. Rauf, H. Parveen, Direct esterification of fatty acids with phenylalkanols by using dicyclohexylcarbodiimide. Eur. J. Lipid Sci. Technol. 106, 97–100 (2004). https://doi.org/10.1002/ejlt.200300887
S. Salahuddin, M. Hanafi, H. Hariyanti, Synthesis and anticancer activity test of 2-hydroxy-n-phenylnicotinamide. Indones. J. Chem. 13(2), 166–170 (2013)
U.Y. Shaheen, P-coumaric acid ester with potential antioxidant activity from the genus salvia. Free Radic. Antioxid. 1(1), 23–27 (2011). https://doi.org/10.5530/ax.2011.1.5
B.L. Staker, C.M. FeeseMD, Y. Pommier, D. Zembower, L. Stewart, A.B. Burgin, Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 48(7), 2336–2345 (2005). https://doi.org/10.1021/jm049146p
K. Takao, K. Toda, T. Saito, Y. Sugita, Synthesis of amide and ester derivatives of cinnamic acid and its analogs: evaluation of their free radical scavenging and monoamine oxidase and cholinesterase inhibitory activities. Chem. Pharm. Bull. 65(11), 1020–1027 (2017)
P. Tang, Boric Acid Catalyzed Amide Formation from Carboxylic Acids and Amines: N-Benzyl-4-Phenylbutyramide. Organic Syntheses (Wiley, Hoboken, 2005), pp. 262–272
O. Taofiq, A.M. González-Paramás, M.F. Barreiro, I.C.F.R. Ferreira, Hydroxycinnamic acids and their derivatives: future perspectives, a review. Molecules 22(281), 1–24 (2017). https://doi.org/10.3390/molecules22020281
J. Teixeira, A. Gaspar, E.M. Garrido, J. Garrido, F. Borges, Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed. Res. Int. 2013, 1–11 (2013)
T. Tokoroyama, Discovery of the Michael reaction. Eur. J. Org. Chem. 2010, 2009–2016 (2010). https://doi.org/10.1002/ejoc.200901130
G. Wang, Q.Y. Yu, J. Wang, S. Wang, S.Y. Chen, X.Q. Yu, Iodide-catalyzed amide synthesis from alcohols and amines. RSC Adv. 3(44), 21306–21310 (2013). https://doi.org/10.1039/C3RA43799J
Y. Xiao, X. Yang, B. Li, H. Yuan, S. Wan, Y. Xu, Design, synthesis and antifungal/insecticidal evaluation of novel cinnamide derivatives. Molecules 16, 8945–8957 (2011). https://doi.org/10.3390/molecules16118945
Y.H. Yang, T. Raku, E. Song, S.H. Park, D. Yoo, H.Y. Park, B.G. Kim, H.J. Kim, S.H. Lee, H.S. Kim, Y. Tokiwa, Lipase catalyzed reaction of L-ascorbic acid with cinnamic acid esters and substituted cinnamic acids. Biotechnol. Bioprocess Eng. 17, 50–54 (2012). https://doi.org/10.1007/s12257-011-0071-1
K. Zhou, D. Chen, B. Li, B. Zhang, F. Miao, L. Zhou, Bioactivity and structure-activity relationship of cinnamic acid esters and their derivatives as potential antifungal agents for plant protection. PLoS ONE 12(4), 0176189 (2017)
Acknowledgements
Authors thank the Ministry of Education and Culture as well as Rector of Hasanuddin University for a research grant on the scheme of Academic Supervision of Magister Student. We also thank Chemistry Laboratory and Natural Products Laboratory of Mathematics and Science Faculty, Institute Technology of Bandung for accessing the NMR measurements and the facilities in performing the antitumor analysis. We also thank the Integrated Chemistry Laboratory of Chemistry Department, Mathematics and Science Faculty, Hasanuddin University, for FTIR measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zenta, F., Soekamto, N.H., Dali, S. et al. Development trans-N-benzyl hydroxyl cinnamamide based compounds from cinnamic acids and characteristics anticancer potency. J IRAN CHEM SOC 19, 2845–2853 (2022). https://doi.org/10.1007/s13738-022-02499-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02499-7