Abstract
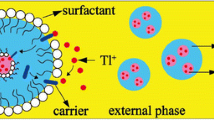
In this study, selective separation of tellurium from nitric acid leaching liquor obtained from Sarcheshmeh Copper anode slime was performed by using a bulk liquid membrane (BLM). To find the optimum operational conditions, the experiments were designed using the Taguchi method. The effect of TBP concentration in the liquid membrane phase and hydrochloric acid concentration in the donor and acceptor phases was investigated on selective extraction of tellurium. The maximum selectivity of tellurium relative to selenium and Copper was obtained equal to 10.87 and 70.05, respectively, in 4 M hydrochloric acid of the donor phase, 30% (v/v) TBP in kerosene, and the 0.5 M hydrochloric acid in acceptor phase. At these conditions, the transfer percentage (TF%) of tellurium was obtained equal to 14.04%. Moreover, to increase the selectivity and TF% of Te, the time effect and the number of separation stages (BLM) were investigated at the aforementioned optimum conditions. The results showed that TF% and the selectivity of Te to Se increase to 33.93% and 15.5, respectively, by increasing the time up to 240 min. Furthermore, using multistage BLM leads to an increase in the selectivity of tellurium than selenium and Copper up to 2549 and 4530.6, respectively, after using only four separation stages.









Similar content being viewed by others
Abbreviations
- a :
-
Ions in the acceptor phase
- BLM:
-
Bulk liquid membrane
- d :
-
Ions in the donor phase
- DOE:
-
Design of experiments
- LM:
-
Liquid membrane phase
- m :
-
Ions in the membrane phase
- MBLM:
-
Multistage bulk liquid membrane
- na :
-
Order of back-extraction reaction
- nd :
-
Order of extraction reaction
- SF:
-
Selectivity factor
- S/N:
-
Signal to noise ratio
- TF%:
-
Transfer percentage
- TBP:
-
Tributyl phosphate
- C a, t :
-
Concentration of the solute in the acceptor phase at time t (mol m−3)
- C a i :
-
Concentration of the solute i in acceptor phase (mg L−3)
- C d i :
-
Concentration of the solute i in the donor phase (mg L−3)
- C d,in :
-
Concentration of the solute in the donor phase at initial time (mol m−3)
- \(C_{{{\text{m}}i}}\) :
-
Concentration of the solute i in the membrane phase (mg L−3)
- \(k_{1}\) :
-
Rate constants of extraction process (min−1)
- \(k_{2}\) :
-
Rate constants of back-extraction process (min−1)
- n :
-
Number of experiments
- W i :
-
Weighting factor
- y i :
-
Weighted response of the ith experiment
References
Z. Albaraka, Carrier-mediated liquid membrane systems for lead (II) ion separations. Chem. Pap. 74, 77–88 (2020). https://doi.org/10.1007/s11696-019-00868-w
S.A. Allahyari, A. Charkhi, S.J. Ahmadi, A. Minuchehr, Modeling and experimental validation of the steady-state counteractive facilitated transport of Th(IV) and hydrogen ions through hollow-fiber renewal liquid membrane. Chem. Pap. 75, 325–336 (2021). https://doi.org/10.1007/s11696-020-01300-4
S.A. Allahyari, S.J. Ahmadi, A. Minuchehr, A. Charkhi, Th (IV) recovery from aqueous waste via hollow fiber renewal liquid membrane (HFRLM) in recycling mode: modelling and experimental validation. RSC Adv. 7, 7413–7423 (2017). https://doi.org/10.1039/C6RA26463H
S.A. Allahyari, A. Minuchehr, S.J. Ahmadi, A. Charkhi, Th (IV) transport from nitrate media through hollow fiber renewal liquid membrane. J. Membr. Sci. 520, 374–384 (2016). https://doi.org/10.1016/j.memsci.2016.08.009
A. Amer, Processing of Copper anodic-slimes for extraction of valuable metals. Waste Manag. 23, 763–770 (2003). https://doi.org/10.1016/S0956-053X(03)00066-7
Chakrabarty K (2010) Liquid Membrane Based Technology for Removel of Pollutants from Wastewater. Dissertation. http://gyan.iitg.ernet.in/handle/123456789/294
M.R. Chowdhury, S.K. Sanyal, Separation by solvent extraction of tellurium (IV) and selenium (IV) with tri-n butyl phosphate: some mechanistic aspects. Hydrometallurgy 32, 189–200 (1993). https://doi.org/10.1016/0304-386X(93)90023-7
M.R. Chowdhury, S.K. Sanyal, Diluent effect on extraction of tellurium (IV) and selenium (IV) by tri-n butyl phosphate. Hydrometallurgy 34, 319–330 (1994). https://doi.org/10.1016/0304-386X(94)90069-8
R. Davarkhah, E.F. Asl, M. Samadfam, M. Tavasoli, P. Zaheri, M. Shamsipur, Selective separation of yttrium (III) through a liquid membrane system using 2-thenoyltrifluoroacetone as an extractant carrier. Chem. Pap. 72, 1487–1497 (2018). https://doi.org/10.1007/s11696-018-0407-9
M. Dehghanpoor, M. Zivdar, M. Torabi, Extraction of copper and gold from anode slime of Sarcheshmeh Copper Complex. J. S. Afr. Inst. Min. Metall. 116, 1153–1157 (2016). https://doi.org/10.17159/2411-9717/2016/v116n12a9
F.A. Devillanova, W.-W. Du Mont, Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, vol. 1 (Royal Society of Chemistry, UK, 2013)
M. Fernández, M. Segarra, F. Espiell, Selective leaching of arsenic and antimony contained in the anode slimes from Copper refining. Hydrometallurgy 41, 255–267 (1996). https://doi.org/10.1016/0304-386X(95)00061-K
J. Hait, R. Jana, S. Sanyal, Processing of Copper electrorefining anode slime: a review. Miner. Process. Extr. Metall. 118, 240–252 (2009). https://doi.org/10.1179/174328509X431463
Y.-C. Hoh, C.-C. Chang, W.-L. Cheng, I.-S. Shaw, The separation of selenium from Tellurium in hydrochloric acid media by solvent extraction with tri-butyl phosphate. Hydrometallurgy 9, 381–392 (1983). https://doi.org/10.1016/0304-386X(83)90032-4
M. Khanlarian, F. Rashchi, M. Saba, A modified sulfation-roasting-leaching process for recovering Se, Cu, and Ag from Copper anode slimes at a lower temperature. J. Environ. Manag. 235, 303–309 (2019). https://doi.org/10.1016/j.jenvman.2019.01.079
A. Kundu, B. SenGupta, M. Hashim, G. Redzwan, Taguchi optimisation approach for chromium removal in a rotating packed bed contractor. J. Taiwan Inst. Chem. Eng. 57, 91–97 (2015). https://doi.org/10.1016/j.jtice.2015.05.022
D. Li, Y. Guo, T. Deng, Y.-W. Chen, N. Belzile, Solvent extraction of tellurium from chloride solutions using tri-n-butyl phosphate: conditions and thermodynamic data. Sci. World J. (2014). https://doi.org/10.1155/2014/458705
P. Liang, W. Liming, G. Wei, Z. Nan, Study on a novel disphase supplying supported liquid membrane for transport behavior of divalent nickel ions. Chin. J. Chem. Eng. 20, 633–640 (2012). https://doi.org/10.1016/S1004-9541(11)60228-0
P. Liang, W. Liming, F. Xinglong, Separation of Eu3+ using a novel dispersion combined liquid membrane with P507 in kerosene as the carrier. Chin. J. Chem. Eng. 19, 33–39 (2011). https://doi.org/10.1016/S1004-9541(09)60173-7
J. Lin, C. Lin, The use of the orthogonal array with grey relational analysis to optimize the electrical discharge machining process with multiple performance characteristics. Int. J. Mach. Tool Manuf. 42, 237–244 (2002). https://doi.org/10.1016/S0890-6955(01)00107-9
D. Lu, Y. Chang, H. Yang, X. Feng, Sequential removal of selenium and tellurium from Copper anode slime with high nickel content. Trans. Nonferrous Met. Soc. 25, 1307–1314 (2015). https://doi.org/10.1016/S1003-6326(15)63729-3
A.A. Mhaske, P.M. Dhadke, Separation of Te (IV) and Se (IV) by extraction with Cyanex 925. Sep. Sci. Technol. 38, 3575–3589 (2003). https://doi.org/10.1081/SS-120023418
S.A. Milani, A. Charkhi, S. Eshghi, Selective transport of zirconium (IV) and niobium (V) from hydrochloric media through a bulk liquid membrane. J. Iran. Chem. Soc. 15, 1821–1829 (2018). https://doi.org/10.1007/s13738-018-1379-y
S.A. Milani, F. Zahakifar, A. Charkhi, Continuous bulk liquid membrane technique for thorium transport: modeling and experimental validation. J. Iran. Chem. Soc. 16, 455–464 (2019). https://doi.org/10.1007/s13738-018-1516-7
B. Mokhtari, K. Pourabdollah, Emulsion liquid membrane for selective extraction of Bi (III) Chinese. J. Chem. Eng. 23, 641–645 (2015). https://doi.org/10.1016/j.cjche.2014.06.035
C. Onac, A. Kaya, D. Ataman, N.A. Gunduz, H.K. Alpoguz, The removal of Cr (VI) through polymeric supported liquid membrane by using calix [4] arene as a carrier. Chin. J. Chem. Eng. 27, 85–91 (2019). https://doi.org/10.1016/j.cjche.2018.01.029
N. Othman, N.F.M. Noah, L.Y. Shu, Z.-Y. Ooi, N. Jusoh, M. Idroas, M. Goto, Easy removing of phenol from wastewater using vegetable oil-based organic solvent in emulsion liquid membrane process. Chin. J. Chem. Eng. 25, 45–52 (2017). https://doi.org/10.1016/j.cjche.2016.06.002
H. Pasdar, B. Hedayati Saghavaz, M. Masoumi, A simple method for the recovery of selenium from Copper anode slime sample using alkaline roasting process. Int. J. New Chem. 6, 143–150 (2019). https://doi.org/10.22034/IJNC.2019.106292.1050
R. Ranjbar, M. Naderi, H. Omidvar, G. Amoabediny, Gold recovery from Copper anode slime by means of magnetite nanoparticles (MNPs). Hydrometallurgy 143, 54–59 (2014). https://doi.org/10.1016/j.hydromet.2014.01.007
B.M. Sargar, S.V. Mahamuni, M.A. Anuse, Sequential separation of selenium (IV) from tellurium (IV) by solvent extraction with Nn-octylaniline: analysis of real samples. J. Saudi Chem. Soc. 15, 177–185 (2011). https://doi.org/10.1016/j.jscs.2010.08.001
A. Sattari, M. Kavousi, E.K. Alamdari, Solvent extraction of selenium in hydrochloric acid media by using triisobutyl phosphate and triisobutyl phosphate/dodecanol mixture. Trans. Ind. Inst. Met. 70, 1103–1109 (2017). https://doi.org/10.1007/s12666-016-0904-x
A. Shabani, A. Hoseinpur, H. Yoozbashizadeh, J.V. Khaki, As, Sb, and Fe removal from industrial Copper electrolyte by solvent displacement crystallisation technique. Can. Metall. Q. 58, 253–261 (2019). https://doi.org/10.1080/00084433.2018.1549346
S. Shafiee, H.A. Ahangar, A. Saffar, Taguchi method optimization for synthesis of Fe3O4@ chitosan/Tragacanth Gum nanocomposite as a drug delivery system. Carbohydr. Polym. 222, 114982 (2019). https://doi.org/10.1016/j.carbpol.2019.114982
B. Shakib, R. Torkaman, M. Torab-Mostaedi, M. Asadollahzadeh, Exact hydrodynamic description of pilot plant Oldshue–Rushton contactor: a case study with the introduction of selenium and tellurium into reaction system. Int. J. Environ. Anal. Chem. (2020). https://doi.org/10.1080/03067319.2020.1781103
M. Shamsipur, R. Davarkhah, A.R. Khanchi, Facilitated transport of uranium (VI) across a bulk liquid membrane containing thenoyltrifluoroacetone in the presence of crown ethers as synergistic agents. Sep. Purif. Technol. 71, 63–69 (2010). https://doi.org/10.1016/j.seppur.2009.11.003
W.D. Xing, M.S. Lee, Development of a hydrometallurgical process for the recovery of gold and silver powders from anode slime containing copper, nickel, tin, and zinc. Gold Bull. 52, 69–77 (2019). https://doi.org/10.1007/s13404-019-00254-0
F. Zahakifar, A. Charkhi, M. Torab-Mostaedi, R. Davarkhah, Kinetic study of uranium transport via a bulk liquid membrane containing Alamine 336 as a carrier. J. Radioanal. Nucl. Chem. 316, 247–255 (2018). https://doi.org/10.1007/s10967-018-5739-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Yazdani Ahmad Abadi, N., Kheradmand Saadi, M., Charkhi, A. et al. Intensification of tellurium separation through the multistage bulk liquid membrane technique from nitric acid leaching liquor of Copper anode slime. J IRAN CHEM SOC 19, 413–421 (2022). https://doi.org/10.1007/s13738-021-02311-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02311-y