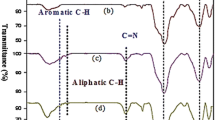
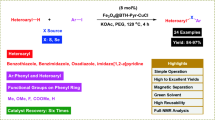
Abstract
In this paper, we report the fabrication and characterization of a stable heterogeneous nanostructure catalyst of copper immobilized on Fe3O4@SiO2@l-Arginine, for the oxidation of sulfides and oxidative coupling of thiols. The prepared nanocatalyst has been characterized by different techniques such as FTIR, XRD, SEM, TEM and TGA. These nanoparticles were the effective catalyst for selective oxidation of sulfides and oxidative coupling of thiols using 30% H2O2. The suggested method offers several prominent advantages such as mild condition, use of magnetically reusable catalyst, simple workup procedure, good to high yields of products and great selectivity.













Similar content being viewed by others
References
S. Gao, N. Koshizaki, H. Tokuhisa, E. Koyama, T. Sasaki, J.K. Kim, J. Ryu, D.S. Kim, Y. Shimizu, Highly stable Au nanoparticles with tunable spacing and their potential application in surface plasmon resonance biosensors. Adv. Func. Mater. 20, 78–86 (2010). https://doi.org/10.1002/adfm.200901232
T.K. Sau, A.L. Rogach, F. Jäckel, T.A. Klar, J. Feldmann, Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 22, 1805–1825 (2010). https://doi.org/10.1002/adma.200902557
D.Y. Wu, X.M. Liu, Y.F. Huang, B. Ren, X. Xu, Z.Q. Tian, Surface catalytic coupling reaction of p-mercaptoaniline linking to silver nanostructures responsible for abnormal SERS enhancement: a DFT study. J. Phys. Chem. C 113, 18212–18222 (2009). https://doi.org/10.1021/jp9050929
S. Li, S.R. Zhai, Q.D. An, M.H. Li, Y. Song, X.W. Song, Designed synthesis of multifunctional Fe3O4@SiO2–NH2@CS–Co(II) towards efficient oxidation of ethylbenzene. Mater. Res. Bull. 60, 665–673 (2014). https://doi.org/10.1016/j.materresbull.2014.09.042
J. Zhang, S. Zhai, S. Li, Z. Xiao, Y. Song, Q. An, G. Tian, Pb(II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem. Eng. J. 215, 461–471 (2013). https://doi.org/10.1016/j.cej.2012.11.043
Y. Chen, F. Zhang, Y. Fang, X. Zhu, W. Zhen, R. Wang, J. Ma, Phosphotungstic acid containing ionic liquid immobilized on magnetic mesoporous silica rod catalyst for the oxidation of dibenzothiophene with H2O2. Catal. Commun. 38, 54–58 (2013). https://doi.org/10.1016/j.catcom.2013.04.005
Y. Jiang, C. Guo, H. Xia, I. Mahmood, C. Liu, H. Liu, Magnetic nanoparticles supported ionic liquids for lipase immobilization: enzyme activity in catalyzing esterification. J. Mol. Catal. Enzym. 58, 103–109 (2009). https://doi.org/10.1016/j.molcatb.2008.12.001
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Magnetically recoverable nanocatalysts. Chem. Rev. 111, 3036–3075 (2011). https://doi.org/10.1021/cr100230z
J. Wang, B. Xu, H. Sun, G. Song, Palladium nanoparticles supported on functional ionic liquid modified magnetic nanoparticles as recyclable catalyst for room temperature Suzuki reaction. Tetrahedron Lett. 54, 238–241 (2013). https://doi.org/10.1016/j.tetlet.2012.11.009
B. Tahmasbi, A. Ghorbani-Choghamarani, Magnetic MCM-41 nanoparticles as a support for the immobilization of a palladium organometallic catalyst and its application in C–C coupling reactions. New J. Chem. 43, 14485–14501 (2019). https://doi.org/10.1039/C9NJ02727K
M. Esmaeilpour, A.R. Sardarian, J. Javidi, Schiff base complex of metal ions supported on superparamagnetic Fe3O4@SiO2 nanoparticles: an efficient, selective and recyclable catalyst for synthesis of 1,1-diacetates from aldehydes under solvent-free conditions. Appl. Catal. A Gen. 445, 359–367 (2012). https://doi.org/10.1016/j.apcata.2012.09.010
A. Ghorbani-Choghamarani, B. Tahmasbi, N. Noori, S. Faryadi, Pd–S-methylisothiourea supported on magnetic nanoparticles as an efficient and reusable nanocatalyst for Heck and Suzuki reactions. C. R. Chim. 20, 132–139 (2017). https://doi.org/10.1016/j.crci.2016.06.010
N.T. Phan, H.V. Le, Superparamagnetic nanoparticles-supported phosphine-free palladium catalyst for the Sonogashira coupling reaction. J. Mol. Catal. A: Chem. 334, 130–138 (2011). https://doi.org/10.1016/j.molcata.2010.11.009
J. Andrez, G. Bozoklu, G. Nocton, J. Pecaut, R. Scopelliti, L. Dubois, M. Mazzanti, Lanthanide(II) complexes supported by N,O-donor tripodal ligands: synthesis, structure, and ligand-dependent redox behavior. Chem. Eur. J. 21, 15188–15200 (2015). https://doi.org/10.1002/chem.201502204
R.N. Baig, R.S. Varma, Organic synthesis via magnetic attraction: benign and sustainable protocols using magnetic nanoferrites. Green Chem. 15, 398–417 (2013). https://doi.org/10.1039/C2GC36455G
K. Azizi, M. Karimi, H.R. Shaterian, A. Heydari, Ultrasound irradiation for the green synthesis of chromenes using l-arginine-functionalized magnetic nanoparticles as a recyclable organocatalyst. RSC Adv. 4, 42220–42225 (2014). https://doi.org/10.1039/C4RA06198E
M. Nasr-Esfahani, S.J. Hoseini, M. Montazerozohori, R. Mehrabi, H. Nasrabadi, Magnetic Fe3O4 nanoparticles: efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and Hantzsch 1,4-dihydropyridines under solvent-free conditions. J. Mol. Catal. A: Chem. 382, 99–105 (2014). https://doi.org/10.1016/j.molcata.2013.11.010
A. Ghorbani-Choghamarani, P. Moradi, B. Tahmasbi, Nickel(II) immobilized on dithizone–boehmite nanoparticles: as a highly eicient and recyclable nanocatalyst for the synthesis of polyhydroquinolines and sulfoxidation reaction. J. Iran. Chem. Soc. 16, 511–521 (2019). https://doi.org/10.1007/s13738-018-1526-5
A. Akdag, T. Webb, S. Worley, Oxidation of thiols to disulfides with monochloro poly(styrenehydantoin) beads. Tetrahedron Lett. 47, 3509–3510 (2006). https://doi.org/10.1016/j.tetlet.2006.03.105
S. Kumar, S. Verma, S.L. Jain, B. Sain, Thiourea dioxide (TUD): a robust organocatalyst for oxidation of sulfides to sulfoxides with TBHP under mild reaction conditions. Tetrahedron Lett. 52, 3393–3396 (2011). https://doi.org/10.1016/j.tetlet.2011.04.088
B. Li, A.H. Liu, L.N. He, Z.Z. Yang, J. Gao, K.H. Chen, Iron-catalyzed selective oxidation of sulfides to sulfoxides with the polyethylene glycol/O2 system. Green Chem. 14, 130–135 (2012). https://doi.org/10.1039/C1GC15821J
R. Ozen, F. Aydin, Oxidation of thiols to disulfides with molecular oxygen in subcritical water. Monatshefte für Chemie/Chem. Mon. Monatsh. Chem. 137, 307–310 (2006). https://doi.org/10.1007/s00706-005-0430-8
S. Samanta, S. Ray, A.B. Ghosh, P. Biswas, 3,6-Di(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) mediated metal-free mild oxidation of thiols to disulfides in aqueous medium. RSC Adv. 6, 39356–39363 (2016). https://doi.org/10.1039/C6RA01509C
A. Ghorbani-Choghamarani, M. Hajjami, B. Tahmasbi, N. Noori, Boehmite silica sulfuric acid: as a new acidic material and reusable heterogeneous nanocatalyst for the various organic oxidation reactions. J. Iran. Chem. Soc. 13, 2193–2202 (2016). https://doi.org/10.1007/s13738-016-0937-4
D. Habibi, M.A. Zolfigol, M. Safaiee, A. Shamsian, A. Ghorbani-Choghamarani, Catalytic oxidation of sulfides to sulfoxides using sodium perborate and/or sodium percarbonate and silica sulfuric acid in the presence of KBr. Catal. Commun. 10, 1257–1260 (2009). https://doi.org/10.1016/j.catcom.2008.12.066
K.J. Liu, J.H. Deng, J. Yang, S.F. Gong, Y.W. Lin, J.Y. He, Z. Cao, W.M. He, Selective oxidation of (hetero)sulfides with molecular oxygen under clean conditions. Green Chem. 22, 433–438 (2020). https://doi.org/10.1039/c9gc03713f
M.A. Zolfigol, A. Khazaei, M. Safaiee, M. Mokhlesi, R. Rostamian, M. Bagheri, M. Shiri, H. Gerhardus Kruger, Application of silica vanadic acid as a heterogeneous, selective and highly reusable catalyst for oxidation of Sulfides at room temperature. J. Mol. Catal. A: Chem. 370, 80–86 (2013). https://doi.org/10.1016/j.molcata.2012.12.015
N. Noori, M. Nikoorazm, A. Ghorbani-Choghamarani, Oxo-vanadium immobilized on l-cysteine-modified MCM-41 as catalyst for the oxidation of sulfides and oxidative coupling of thiols. Microporous Mesoporous Mater. 234, 166–175 (2016). https://doi.org/10.1016/j.micromeso.2016.06.036
A. Bayat, M. Shakourian-Fard, M.M. Hashemi, Selective oxidation of sulfides to sulfoxides by a molybdate-based catalyst using 30% hydrogen peroxide. Catal. Commun. 52, 16–21 (2014). https://doi.org/10.1016/j.catcom.2014.03.026
Y.L. Hu, X.B. Liu, D. Fang, Efficient and convenient oxidation of sulfides to sulfones using H2O2 catalyzed by V2O5 in ionic liquid [C12mim][HSO4]. Catal. Sci. Technol. 4, 38–42 (2014). https://doi.org/10.1039/C3CY00719G
M. Nikoorazm, A. Ghorbani-Choghamarani, N. Noori, Preparation and characterization of functionalized Cu(II) Schiff base complex on mesoporous MCM-41 and its application as effective catalyst for the oxidation of sulfides and oxidative coupling of thiols. J. Porous Mater. 22, 877–885 (2015). https://doi.org/10.1007/s10934-015-9961-5
B. Atashkar, A. Rostami, H. Gholami, B. Tahmasbi, Magnetic nanoparticles Fe3O4-supported guanidine as an efficient nanocatalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free conditions. Res. Chem. Intermed. 41, 3675–3681 (2015). https://doi.org/10.1007/s11164-013-1480-x
B. Tahmasbi, A. Ghorbani-Choghamarani, First report of the direct supporting of palladium–arginine complex on boehmite nanoparticles and application in the synthesis of 5-substituted tetrazoles. Appl. Organomet. Chem. 31, e3644 (2017). https://doi.org/10.1002/aoc.3644
M. Nikoorazm, N. Noori, S. Faryadi, B. Tahmasbi, A palladium complex immobilized onto mesoporous silica: a highly efficient and reusable catalytic system for carbon–carbon bond formation and anilines synthesis. Transit. Met. Chem. 42, 469–481 (2017). https://doi.org/10.1007/s11243-017-0151-y
P. Moradi, M. Hajjami, B. Tahmasbi, Fabricated copper catalyst on biochar nanoparticles for the synthesis of tetrazoles as antimicrobial agents. Polyhedron 175, 114169 (2020). https://doi.org/10.1016/j.poly.2019.114169
L. Shiri, B. Tahmasbi, Tribromide ion immobilized on magnetic nanoparticles as an efficient catalyst for the rapid and chemoselective oxidation of sulfides to sulfoxides. Phosphorus, Sulfur Silicon Relat. Elem. 192, 53–57 (2017). https://doi.org/10.1080/10426507.2016.1224878
A. Ghorbani-Choghamarani, B. Tahmasbi, R.H.E. Hudson, A. Heidari, Supported organometallic palladium catalyst into mesoporous channels of magnetic MCM-41 nanoparticles for phosphine-free C–C coupling reactions. Microporous Mesoporous Mater. 284, 366–377 (2019). https://doi.org/10.1016/j.micromeso.2019.04.061
C. Han, Z. Li, W. Li, S. Chou, S. Dou, Controlled synthesis of copper telluride nanostructures for long-cycling anodes in lithium ion batteries. J. Mater. Chem. A 2, 11683–11690 (2014). https://doi.org/10.1039/C4TA01579G
Q. Li, S.W. Zhang, Y. Zhang, C. Chen, Magnetic properties in a partially oxidized nanocomposite of Cu–CuCl. Nanotechnology 17, 4981 (2006). https://doi.org/10.1088/0957-4484/17/19/034
M. Nikoorazm, A. Ghorbani-Choghamarani, A. Panahi, B. Tahmasbi, N. Noori, Pd(0)-Schiff-base@MCM-41 as high-efficient and reusable catalyst for C–C coupling reactions. J. Iran. Chem. Soc. 15, 181–189 (2018). https://doi.org/10.1007/s13738-017-1222-x
B. Tahmasbi, A. Ghorbani-Choghamarani, P. Moradi, Palladium fabricated on boehmite as an organic–inorganic hybrid nanocatalyst for C–C cross coupling and homoselective cycloaddition reactions. New J. Chem. 44, 3717–3727 (2020). https://doi.org/10.1039/c9nj06129k
A. Ghorbani-Choghamaranai, P. Moradi, B. Tahmasbi, Modification of boehmite nanoparticles with Adenine for the immobilization of Cu(II) as organic–inorganic hybrid nanocatalyst in organic reactions. Polyhedron 163, 98–107 (2019). https://doi.org/10.1016/j.poly.2019.02.004
P. Moradi, M. Hajjami, F. Valizadeh-Kakhki, Biochar as heterogeneous support for immobilization of Pd as efficient and reusable biocatalyst in C–C coupling reactions. Appl. Organomet. Chem. 33, e5205 (2019). https://doi.org/10.1002/aoc.5205
M. Nikoorazm, Z. Rezaei, B. Tahmasbi, Two Schif-base complexes of copper and zirconium oxide supported on mesoporous MCM-41 as an organic–inorganic hybrid catalysts in the chemo and homoselective oxidation of sulfides and synthesis of tetrazoles. J. Porous Mater. 27, 671–689 (2020). https://doi.org/10.1007/s10934-019-00835-6
S. Hussain, D. Talukdar, S.K. Bharadwaj, M.K. Chaudhuri, VO2F(dmpz)2: a new catalyst for selective oxidation of organic sulfides to sulfoxides with H2O2. Tetrahedron Lett. 53, 6512–6515 (2012). https://doi.org/10.1016/j.tetlet.2012.09.067
X.F. Wu, A general and selective zinc-catalyzed oxidation of sulfides to sulfoxides. Tetrahedron Lett. 53, 4328–4331 (2012). https://doi.org/10.1016/j.tetlet.2012.06.003
A. Shaabani, A.H. Rezayan, Silica sulfuric acid promoted selective oxidation of sulfides to sulfoxides or sulfones in the presence of aqueous H2O2. Catal. Commun. 8, 1112–1116 (2007). https://doi.org/10.1016/j.catcom.2006.10.033
M. Hajjami, L. Shiri, A. Jahanbakhshi, Zirconium oxide complex-functionalized MCM-41 nanostructure: an efficient and reusable mesoporous catalyst for oxidation of sulfides and oxidative coupling of thiols using hydrogen peroxide. Appl. Organomet. Chem. 29, 668–673 (2015). https://doi.org/10.1002/aoc.3348
S.M. Islam, A.S. Roy, P. Mondal, K. Tuhina, M. Mobarak, J. Mondal, Selective oxidation of sulfides and oxidative bromination of organic substrates catalyzed by polymer anchored Cu(II) complex. Tetrahedron Lett. 53, 127–131 (2012). https://doi.org/10.1016/j.tetlet.2011.10.138
B. Karimi, D. Zareyee, Selective, metal-free oxidation of sulfides to sulfoxides Using 30% hydrogen peroxide catalyzed with N-bromosuccinimide (NBS) under neutral buffered reaction conditions. J. Iran. Chem. Soc. 5, S103–S107 (2008). https://doi.org/10.1007/BF03246497
J. Zhang, T. Jiang, Y. Mai, X. Wang, J. Chen, B. Liao, Selective catalytic oxidation of sulfides to sulfoxides or sulfones over amorphous Nb2O5/AC catalysts in aqueous phase at room temperature. Catal. Commun. 127, 10–14 (2019). https://doi.org/10.1016/j.catcom.2019.04.013
M. Safaiee, M. Moeinimehr, M.A. Zolfigol, Pyridiniumporphyrazinato oxo-vanadium tribromomethanide as a new source of Br+ catalyst for the chemo and homoselective oxidation of sulfides and benzylic alcohols. Polyhedron 170, 138–150 (2019). https://doi.org/10.1016/j.poly.2019.05.007
A. Ghorbani-Choghamarani, M. Nikoorazm, H. Goudarziafshar, B. Tahmasbi, An efficient and new method on the oxidative coupling of thiols under mild and heterogeneous conditions. Bull. Korean Chem. Soc. 40, 1388–1390 (2009). https://doi.org/10.1002/chin.200945034
M. Hajjami, Z. Shirvandi, Z. Yousofvand, Zr(IV)-ninhydrin supported MCM-41 and MCM-48 as novel nanoreactor catalysts for the oxidation of sulfides to sulfoxides and thiols to disulfides. J. Porous Mater. 24, 1461–1472 (2017). https://doi.org/10.1007/s10934-017-0386-1
M. Nikoorazm, A. Ghorbani-Choghamarani, H. Mahdavi, S.M. Esmaeili, Efficient oxidative coupling of thiols and oxidation of sulfides using UHP in the presence of Ni or Cd salen complexes immobilized on MCM-41 mesoporous as novel and recoverable nanocatalysts. Microporous Mesoporous Mater. 211, 174–181 (2015). https://doi.org/10.1016/j.micromeso.2015.03.011
Acknowledgements
This work was supported by the research facilities of Ilam University, Ilam, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikoorazm, M., Moradi, P., Noori, N. et al. l-Arginine complex of copper on modified core–shell magnetic nanoparticles as reusable and organic–inorganic hybrid nanocatalyst for the chemoselective oxidation of organosulfur compounds. J IRAN CHEM SOC 18, 467–478 (2021). https://doi.org/10.1007/s13738-020-02040-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02040-8