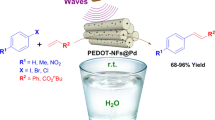
Abstract
Self-assembled peptide nanofibers have attracted extensive attention; they offer unique templating possibilities, which allow the synthesis of nanostructured materials with high surface areas, and also act as organocatalysts for various transformations in organic chemistry. In the present work, peptide nanofibers as hydrogen-bonding organocatalysts have been developed as efficient organocatalysts for three-component Groebke condensation reactions of aldehydes, isocyanides, and 2-aminopyridines in PEG to afford the corresponding 3-aminoimidazo [1, 2-a] pyridines in high yields without any additives. The key advantages of catalytic systems are (1) using peptide nanofibers as powerful hydrogen-bonding organocatalysts for the synthesis of 3-aminoimidazo [1, 2-a] pyridines, (2) having high catalytic activity, and (3) performing the reactions which can be carried out in PEG, as green solvent instead of the usually used organic solvents. This catalyst could be recycled and reused at least for four times without noteworthy loss of its activity.
Graphic abstract




Similar content being viewed by others
References
P. Liu, L.S. Fang, X.S. Lei, G.Q. Lin, Tetrahedron Lett. 51, 4505–4608 (2010)
G. Song, Y. Zhang, X. Li, Organometallics 27, 1936–1943 (2008)
A. Hu, G.T. Yee, W. Lin, J. Am. Chem. Soc. 127, 12486–12487 (2005)
A. Gueiffier, S. Mavel, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J.C. Teulade, M. Witvrouw, J. Balzarini, E.D. Clercq, J.P. Chapat, J. Med. Chem. 41, 5108–5112 (1998)
N. Chernyak, V. Gevorgyan, Angew. Chem. Int. Ed. 49, 2743–2746 (2010)
E.F. DiMauro, J.M. Kennedy, J. Org. Chem. 72, 1013–1016 (2007)
G.B. Blackburn, P. Fleming, K. Shiosaki, S. Tsai, Tetrahedron Lett. 39, 3635–3638 (1998)
A. Shaabani, E. Soleimani, A. Sarvary, A.H. Rezayan, A. Maleki, Chin. J. Chem. 27, 369–371 (2009)
A. Shaabani, E. Soleimani, A. Maleki, Monatshefte Chem. 138, 73–76 (2007)
A. Shaabani, E. Soleimani, A. Maleki, Synth. Commun. 38, 1090–1095 (2008)
A. Shaabani, E. Soleimani, A. Maleki, Tetrahedron Lett. 47, 3031–3034 (2006)
C. Blackburn, B. Guan, Tetrahedron Lett. 41, 1495–1500 (2000)
M.A. Zolfigol, N. Bahrami-Nejad, F. Afsharnadery, S. Bagheri, J. Mol. Liq. 221, 851–859 (2016)
M.A. Zolfigol, M. Navazeni, M. Yari, R. Ayazi-Nasrabadi, RSC Adv. 6, 92862–92868 (2016)
K. Amiri, A. Rostami, S. Samadi, A. Rostami, Catal. Commun. 86, 108–112 (2016)
A. Sengupta, C.L. Su, C.L. Bao, C.T. Nai, K.P. Loh, ChemCatChem 6, 2507–2511 (2014)
A. Ghorbani-Choghamarani, Z. Taherinia, RSC Adv. 6, 59410–59421 (2016)
A. Ghorbani-Choghamarani, L. Shiri, G. Azadi, RSC Adv. 6, 32653–32660 (2016)
D. Astruc, F. Lu, J.R. Aranzaes, Angew. Chem. Int. Ed. 44, 7852–7872 (2005)
M. Hajjami, A. Ghorbani-Choghamarani, R. Ghafouri-Nejad, B. Tahmasbi, New J. Chem. 40, 3066–3074 (2015)
S. Rayati, S. Shokoohi, E. Bohloulbandi, J. Iran. Chem. Soc. 13, 1983–1991 (2016)
J. Kofoed, J.L. Reymond, Curr. Opin. Chem. Biol. 9, 656–664 (2005)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, RSC Adv. 6, 56549–56561 (2016)
I. Maity, D.B. Rasale, A.K. Das, RSC Adv. 4, 2984–2988 (2014)
L. Shao, W. Ji, P. Dong, M. Zeng, C. Qi, X.M. Zhang, J. Appl. Catal. 267, 413–417 (2012)
R. Sahay, J. Sundaramurthy, P.S. Kumar, V. Thavasi, S.G. Mhaisalkar, S. Ramakrishna, Solid State Chem. 186, 261–267 (2012)
R.S. Varma, D. Kumar, Tetrahedron Lett. 40, 7665–7669 (1999)
M. Adib, E. Sheikhi, N. Rezaei, Tetrahedron Lett. 52, 3191–3194 (2011)
A. Shaabani, E. Soleimani, A. Maleki, J. Moghimi-Rad, Synth. Commun. 38, 1090–1095 (2008)
A. Habibi, Z. Tarameshloo, S. Rostamizadeh, A. M. Amani, Lett. Org. 9, 155–159 (2012)
S. Sadjadi, M. Eskandari, Monatshefte Chem. 143, 653–656 (2012)
M.L. Bode, D. Gravestock, S. Moleele, S.C.W. van der Westhuyzen, S.C. Pelly, P.A. Steenkamp, L.A. Nkabinde, Bioorg. Med. Chem. 19, 4227–4237 (2011)
A. Habibi, Z. Tarameshloo, S. Rostamizadeh, A.M. Amani, Lett. Org. Chem. 9, 155–159 (2012)
S. Rostamnia, A. Hassankhani, RSC Adv. 3, 1826–18629 (2013)
S. Vidyacharan, A.H. Shinde, B. Satpathi, D.S. Sharada, Green Chem. 16, 1168–1175 (2014)
Acknowledgements
Authors thank the research facilities of Ilam University, Ilam, Iran, for financial support of this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbani-Choghamarani, A., Taherinia, Z. Eco-friendly synthesis of 3-aminoimidazo [1, 2-a] pyridines via a one-pot three-component reaction in PEG catalyzed by peptide nanofibers: as hydrogen-bonding organocatalyst. J IRAN CHEM SOC 17, 59–65 (2020). https://doi.org/10.1007/s13738-019-01744-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01744-w