Abstract
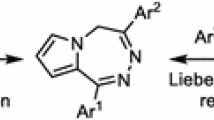
An efficient one-pot synthesis of new types of pyrrolo/pyrido[1,2-a][1,3]diazepines by using the seven-membered ring HKA, an activated methylene compound, and either arylglyoxal monohydrates or salicylaldehyde is described. This method has the advantages of mild reaction conditions and absence of catalyst and provides an entry point to pyrrolo/pyrido and diazepine ring structures.
Graphical Abstract










Similar content being viewed by others
References
A.B. Movahed, M.H. Mosslemin, Chem. Sci. Trans. 1, 365 (2012)
A. Alizadeh, A. Rezvanian, Y. Deng, Tetrahedron 66, 9933 (2010)
M. Driowya, A. Saber, H. Marzag, L. Demange, K. Bougrin, R. Benhida, Molecules 21, 1032 (2016)
A.S. Tatyana, V.B. Alexander, K.V. Vladimir, A.N. Tatyana, D.K. Gennady, Synlett 7(1106), 2 (2007)
A.R. Katritzky, R. Jain, R. Akhmedova, Y.-J. Xu, Arkivoc ix, 4 (2003)
J.H. Lin, H.G. Ramjit, S.M. Pitzenberger, E.H. Ulm, Chem. Abstr. 113, 191344 (1990)
T. Hara, Y. Shikayama, K. Ito, T. Mori, H. Fujimori, T. Sunami, Y. Hashimoto, Y. Ishimoto, Chem. Abstr. 104, 186459 (1986)
F. Corelli, S. Massa, G. Stefancich, G. Ortenzi, M. Artico, G. Pantaleoni, G. Palumbo, D. Fanini, R. Giorgi, Eur. J. Med. Chem. 21, 445 (1986)
A.V. Ivashchenko, V.Y. Vvedensky, A.P. Ilyn, V.M. Kysel, A.V. Khvat, Y.A. Kuzovkova, S.A. Kutepov, I.G. Dmitrieva, D.A. Zolotarev, S.Y. Tkachenko, I.M. Okun, D.V. Kravchenko, V.V. Kobak, A.S. Trifilenkov, Y.S. Mishunina, M.V. Loseva, E.A. Rizhova, V.Z. Parchinsky, S.A. Tsirulnikov, A.S. Kyselev, Chem. Abstr. 143, 452852 (2005)
L. Meerpoel, J. Van Gestel, F. Van Gerven, F. Woestenborghs, P. Marichal, V. Sipido, T. Gilkerson, R. Nash, D. Corens, R.D. Richards, Bioorg. Med. Chem. Lett. 15, 3453 (2005)
X.C. Li, K.S. Babu, M.R. Jacob, S.I. Khan, A.K. Agarwal, A.M. Clark, ACS Med. Chem. Lett. 2, 391 (2011)
A.P. Ilyn, A.S. Trifilenkov, J.A. Kuzovkova, S.A. Kutepov, A.V. Nikitin, A.V.J. Ivachtchenko, J. Org. Chem. 70, 1478 (2005)
L. Han, Y. Feng, M. Luo, Z. Yuan, X. Shao, X. Xu, Z. Li, Tetrahedron Lett. 57, 2727 (2016)
K.-M. Wang, S.-J. Yan, J. Lin, Eur. J. Org. Chem. 2014, 1129 (2014)
P.-H. Yang, Res. Chem. Intermed. 42, 5617 (2016)
C.-Y. Yu, P.-H. Yang, M.-X. Zhao, Z.-T. Huang, Synlett 12, 1835 (2006)
B. Zhou, Z.-C. Liu, W.-W. Qu, R. Yang, X.-R. Lin, S.-J. Yan, J. Lin, Green Chem. 16, 4359 (2014)
F. Sun, X. Shao, Z. Li, RSC Adv. 6, 15382 (2016)
M. Bayat, F.S. Hosseini, B. Notashm, Tetrahedron 73, 1196 (2017)
M. Bayat, F.S. Hosseini, B. Notashm, Tetrahedron Lett. 57, 5439 (2016)
M. Bayat, F.S. Hosseini, Tetrahedron Lett. 58, 1616 (2017)
M. Bayat, M. Rezaei, Monatsh. Chem. (2017). https://doi.org/10.1007/s00706-017-2033-6
M. Bayat, S. Nasri, Tetrahedron Lett. 58, 3107 (2017)
A.A. Mohammadi, S. Taheri, A. Amouzegar, J. Heterocycl. Chem. 53, 805 (2016)
S. Kazemi Movahed, M. Dabiri, A. Bazgir, Helv. Chim. Acta 96, 525 (2013)
A. Alizadeh, T. Firuzyar, A. Mikaeili, J. Heterocycl. Chem. 50, 676 (2013)
T.M. Lin, D.C. Evans, M.W. Warrick, R.P.J. Pioch, Pharmacol. Exp. Ther. 239, 406 (1986)
S. Kagabu, K. Moriya, K. Shibuya, Y. Hattori, S. Tsuboi, K. Shiokawa, Biosci. Biotechnol. Biochem. 56, 362 (1992)
X.-S. Shao, Z.P. Xu, X.F. Zhao, X.Y. Xu, L.M. Tao, Z. Li, X.H.J. Qian, Agric. Food Chem. 58, 2690 (2010)
X.-B. Chen, X.-M. Liu, R. Huang, S.-J. Yan, J. Lin, Eur. J. Org. Chem. 2013, 4607 (2013)
M. Bayat, M. Rezaei, J. Heterocycl. Chem. 54, 2748 (2017)
G. Brahmachari, ACS Sustain. Chem. Eng. 3, 2350 (2015)
X.-B. Chen, X.Y. Wang, D.D. Zhu, S.J. Yan, J. Lin, Tetrahedron 70, 2014 (1047)
A. Alizadeh, A.H. Vahabi, A. Bazgir, H.R. Khavasi, Z. Zhu, L.-G. Zhu, Tetrahedron 72, 1342 (2016)
I. Savych, S.A. Ejaz, S.J.A. Shah, V.O. Iaroshenko, A. Villinger, V.Y. Sosnovskikh, J. Iqbal, A. Abbasi, P. Langer, Eur. J. Org. Chem. 2017, 186 (2017)
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayat, M., Rezaei, M. Synthesis of new types of pyrrolo/pyrido[1,2-a][1,3]diazepines based on seven-membered ring HKA via a one-pot three-component reaction. J IRAN CHEM SOC 15, 769–777 (2018). https://doi.org/10.1007/s13738-017-1275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1275-x