Abstract
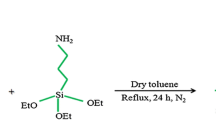
MPTMS-functionalized silica immobilized with biphenyl-2,2′-dioic acid, an efficient heterogeneous catalyst, was synthesized and characterized by various spectroscopic techniques such as FTIR, TGA, TEM and SEM. The catalytic activity of the synthesized catalyst has been demonstrated for the one-pot Knoevenagel/Michael condensation reaction of aromatic amines and 5,5-dimethyl-1,3-cyclohexanedione with various aldehydes. This method results in the synthesis of high-yielding acridine-1,8-dione derivatives and also provides simple and facile recyclability of the catalyst. The synthesized derivatives were characterized by IR, 1H-NMR, 13C-NMR and mass spectroscopy. Molecular structures of three acridine-1,8-dione derivatives were determined by single-crystal X-ray diffraction which determines that 3,3,6,6-tetramethyl-9,10-diphenyl-1,8-dioxodecahydroacridine crystallizes in the monoclinic with space group P2 1 /c, 3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methylphenyl)-1,8-dioxodecahydroacridine crystallizes in the monoclinic with space group P2 1 /n and 3,3,6,6-tetramethyl-9-(4-chlorophenyl)-10-(4-methoxyphenyl)-1,8-dioxodecahydroacridine crystallizes in the monoclinic with space group P2 1 /n.
Graphical Abstract
The one-pot tandem synthesis of acridine-1,8-dione derivatives was carried out successfully with good yields under the catalytic influence of MPTMS-modified silica immobilized with biphenyl-2,2′-dioic acid which has proved to be an efficient heterogeneous catalyst with good recyclable property.












Similar content being viewed by others
References
S. Ray, M. Ray, A. Bhaumik, A. Dutta, C. Mukhopadhyay, Green Chem. 15, 1910 (2013)
P.K. Jal, S. Patel, B.K. Mishra, Talanta 62, 1005 (2004)
M.A. Zolfigol, Tetrahedron 57, 9509 (2001)
K. Niknam, B. Karami, M.A. Zolfigol, Catal. Commun. 8, 1427 (2007)
K. Niknam, D. Saberi, Appl. Catal. A 366, 220 (2009)
S. Tayebi, M. Baghernejad, D. Saberi, K. Niknam, Chin. J. Catal. 32, 1477 (2011)
B. Karimi, M. Khalkhali, J. Mol. Catal. A Chem. 232, 113 (2005)
B. Karimi, D. Zareyee, J. Mater. Chem. 19, 8665 (2009)
K. Niknam, D. Saberi, M.N. Sefat, Tetrahedron Lett. 51, 2959 (2010)
K. Niknam, F. Panahi, D. Saberi, M. Mohagheghnejad, J. Heterocycl. Chem. 47, 292 (2010)
F. Rashedian, D. Saberi, K. Niknam, J. Chin. Chem. Soc. 57, 998 (2010)
M.N. Sefat, A. Deris, K. Niknam, Chin. J. Chem. 29, 2361 (2011)
K. Niknam, A. Deris, F. Naeimi, F. Majleci, Tetrahedron Lett. 52, 4642 (2011)
K. Niknam, A. Jamali, Chin. J. Catal. 33, 1840 (2012)
K. Niknam, N. Borazjani, R. Rashidian, A. Jamali, Chin. J. Catal. 34, 2245 (2013)
K. Niknam, A. Jamali, M. Tajaddod, A. Deris, Chin. J. Catal. 33, 1312 (2012)
K. Niknam, A. Deris, F. Panahi, M.R.H. Nezhad, J. Iran. Chem. Soc. 10, 1291 (2013)
P. Sreekanth, S.W. Kim, T. Hyeon, B.M. Kim, Adv. Synth. Catal. 345, 936 (2003)
G.D. Brykina, L.S. Krysina, V.M. Ivanov, Zh. Anal. Khim. 43, 1547 (1988)
R.K. Iler, The chemistry of silica (Wiley, New York, 1979)
U. Pyell, G. Stork, Fresenius J. Anal. Chem. 343, 576 (1992)
D. Kour, D.R. Patil, M.B. Deshmukh, R. Kant, Eur. Chem. Bull. 3, 552 (2014)
Q.H. To, Y.R. Lee, S.H. Kim, Bull. Korean Chem. Soc. 33, 1170 (2012)
Y.M. Shchekotikhin, T.G. Nikolaeva, G.M. Shub, A.P. Krivenko, Pharmaceut. Chem. J. 35, 206 (2001)
N. Pyrko, Russ. J. Org. Chem. 44, 1215 (2008)
M. Shaikh, S.G. Konda, A.V. Mehare, G.G. Mandawad, S.S. Chobe, B.S. Dawan, Der Pharma Chemica 2, 25 (2010)
K. Palani, D. Thirumalai, P. Ambalavanan, M.N. Ponnuswamy, V.T.J. Ramakrishnan, Chem. Crystallogr. 35, 751 (2005)
D. Carole, D. Michel, C. Julien, D. Florence, N.J. Anna, D. Severine, T. Gerard, G. Pierre, Bioorg. Med. Chem. 13, 5560 (2005)
M. Wainwright, J. Antimicrob. Chemother. 47, 1 (2001)
C. Srivastava, Nizamuddin. Indian J. Heterocycl. Chem. 13, 261 (2004)
M. Kidwai, D. Bhatnagar, Tetrahedron Lett. 51, 2700 (2010)
O.A. Abd-Allah, A.A. Abdelhamid, S.K. Mohamed, Med. Chem. S. 2, 2161 (2015)
S. Tu, X. Zhang, F. Shi, T. Li, Q. Wang, X. Zhu, J. Zhang, J. Xu, J. Heterocycl. Chem. 42, 1155 (2005)
S. Gallo, S. Atifi, A. Mohamoud, C.S. Rouvier, K. Wolfart, J. Molnar, J. Barbe, Eur. J. Med. Chem. 38, 19 (2003)
S.A. Moallem, N. Dehghani, S. Mehri, M. Shahsavand, F.Hadizadeh Alibolandi, Res. Pharm. Sci. 10, 214 (2015)
K. Venkatesan, S.S. Pujari, K.V. Srinivasan, Synth. Commun. 39, 228 (2009)
P. Yang, Q. Yang, X. Qian, L. Tong, X. Li, J. Photochem. Photobiol. B:Biol. 84, 221 (2006)
J.J. Xia, K.H. Zhang, Molecules 17, 5339 (2012)
M.G. Gunduz, A.E. Dogan, R. Simsek, K. Erol, C. Safak, Med. Chem. Res. 18, 317 (2009)
S. Balalaie, F. Chadegani, F. Darviche, H.R. Bijanzadeh, Chin. J. Chem. 27, 1953 (2009)
L.B. Li, S.J. Ji, Y. Liu, Chin. J. Chem. 26, 979 (2008)
J.N. Sangshetti, P.P. Dharmadhikari, R.S. Chouthe, B. Fatema, Arab. J. Chem. (2012). doi:10.1016/j.arabjc.2012.06.005
M. Kaya, Y. Yildirir, G.Y. Celik, Med. Chem. Res. 20(293), 293 (2011)
W. Shen, L.M. Wang, H. Tian, J. Tang, J.J. Yu, J. Fluorine Chem. 130, 522 (2009)
B. Das, P. Thirupathi, I. Mahender, V.S. Reddy, Y.K. Rao, J. Mol. Catal. A Chem. 247, 233 (2006)
T.S. Jin, J.S. Zhang, T.T. Guo, A.Q. Wang, T.S. Li, Synthesis 12, 2001 (2004)
X.S. Wang, M.M. Zhang, Z.S. Zeng, D.Q. Shi, S.J. Tu, X.Y. Wei, Z.M. Zong, ARKIVOC (ii), 117 (2006)
M.A. Pasha, R.R. Khan, R.K.B, Can. Chem. Trans. 4, 90 (2016)
S. Chandrasekhar, Y.S. Rao, L. Sreelakshmi, B. Mahipal, C.R. Reddy, Synthesis 11, 1737 (2008)
S. Rahmani, A. Amoozadeh, J. Nano Struct. 4, 91 (2014)
A. Khazaei, R.H. Zare, Z. Mohammadi, V. Khakyzadeh, J. Afsar, J. Chin. Chem. Soc. 63, 165 (2016)
H. Alinezhad, S.M. Tavakkoli, Sci. World J. 2013, 1 (2013)
G.M. Ziarani, A. Badiei, M.H.S. Mousavi, Arab. J. Chem. 7, 335 (2014)
M.N. Esfahani, Z. Rafiee, H. Kashi, J. Iran. Chem. Soc. (2016). doi. 10.1007/s13738-016-0860-8
P. Sharma, M. Gupta, Green Chem. 17, 1100 (2015)
Y.B. Shen, G.W. Wang, ARKIVOC (xvi), 1 (2008)
D.Q. Shi, S.N. Ni, F. Yang, J.W. Shi, G.L. Dou, X.Y. Li, X.S. Wang, J. Heterocycl. Chem. 45, 653 (2008)
G.M. Sheldrick, Acta Cryst. A64, 112 (2008)
K. Brandenburg, Diamond. Version 2.1. Crystal Impact GbR, Bonn, Germany, (1998)
D. Kumar, M. Sonawane, B. Pujala, V.K. Jain, S. Bhagat, A.K. Chakraborti, Green Chem. 15, 2872 (2013)
Acknowledgements
We are grateful to Head, Department of Physics and Electronics, University of Jammu, for recording single-crystal X-ray data of products and also thankful to Department of Chemistry, University of Jammu, for providing necessary facilities for accomplishing the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaid, R., Gupta, M. & Gupta, V.K. Immobilization of organofunctionalized silica (SiMPTMS) with biphenyl-2,2′-dioic acid and investigation of its catalytic property for one-pot tandem synthesis of acridine-1,8-dione derivatives. J IRAN CHEM SOC 14, 2199–2210 (2017). https://doi.org/10.1007/s13738-017-1156-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1156-3