Abstract
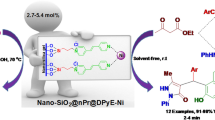
We described here, the preparation of silica bonded n-propyl-4H-1,2,4-triazol-3-amine chloride by the reaction of 3-chloropropyl silica and 3-amino-1,2,4-triazole as a novel heterogeneous catalyst. The structure of the catalyst was characterized by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), differential thermal analysis (DTA), dispersive X-ray spectroscopy (EDX), and scanning electronic microscopy (SEM). These techniques confirmed the catalyst was successfully synthesized. For evaluation of the catalytic activity and reusability of the catalyst, it was employed for the one-pot synthesis of dihydro-1H-pyrano[2,3-c]pyrazol-6-one and pyrazolopyranopyrimidine scaffolds. These compounds were prepared via the four-component catalytic reaction of hydrazine hydrate, ethyl acetoacetate, aromatic aldehydes, and Meldrum’s acid or barbituric acid (dimethylbarbituric acid). After completion of the reaction, the rapid separation and facial recycling of the catalyst were accomplished.









Similar content being viewed by others
References
Sahoo BM, Banik BK (2020) 14—Solvent-less reactions: green and sustainable approaches in medicinal chemistry. Elsevier, Nederland
Cahyana AH, Liandi AR, Yulizar Y, Romdoni Y, Wendari TP (2021) Green synthesis of CuFe2O4 nanoparticles mediated by Morus alba L. leaf extract: crystal structure, grain morphology, particle size, magnetic and catalytic properties in Mannich reaction. Ceram Int 47:21373–21380
Zhang N, Meng Y, Ning Y, Wheatley AEH, Chai F (2021) A reusable catalyst based on CuO hexapods and a CuO–Ag composite for the highly efficient reduction of nitrophenols. RSC Adv 11:13193–13200
Xu J, Huang L, He L, Ni Z, Shen J, Li X, Chen K, Li W, Zhang P (2021) A combination of heterogeneous catalysis and photocatalysis for the olefination of quinoxalin-2(1H)-ones with ketones in water: a green and efficient route to (Z)-enaminones. Green Chem 23:2123–2129
Huang F, Su Y, Tao Y, Sun W, Wang W (2018) Preparation of 5-hydroxymethylfurfural from glucose catalyzed by silica-supported phosphotungstic acid heterogeneous catalyst. Fuel 226:417–422
Yoo WJ, Ishitani H, Saito Y, Laroche B, Kobayashi S (2020) Reworking organic synthesis for the modern age: synthetic strategies based on continuous-flow addition and condensation reactions with heterogeneous catalysts. J Org Chem 85:5132–5145
Takahara I, Saito M, Inaba M, Murata K (2005) Dehydration of ethanol into ethylene over solid acid catalysts. Catal Lett 105:249–252
Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki-Miyaura reaction. Coord Chem Rev 311:1–23
Pulusu VS, Kamatala CR, Mardhanpally AK, Yelike HS, Gugulothu Y, Mukka SK, Utkoor UK, Pulusu Y (2022) Silica supported acids (SiO2-HClO4, SiO2-KHSO4) as eco-friendly reuasble catalysts for bromination of aromatic and heteroaromatic compounds using KBr under solvothermal and solvent-free conditions. SILICON. https://doi.org/10.1007/s12633-021-01489-3
Cui M, Wang D, Li Y, Zhao W, Liang C, Liu X, Fu S, Wang L, Wei X (2022) Preparation of magnetic silica supported Brønsted acidic ionic liquids for the depolymerization of lignin to aromatic monomers. New J Chem 46:4167–4178
Liu B, Sekine N, Nakagawa Y, Tamura M, Yabushita M, Tomishige K (2022) Synthesis of secondary monoalcohols from terminal vicinal alcohols over silica-supported rhenium-modified ruthenium catalyst. ACS Sustain Chem Eng 10:1220–1231
Chandrashekhar VG, Natte K, Alenad AM, Alshammari AS, Kreyenschulte C, Jagadeesh RV (2022) Reductive amination, hydrogenation and hydrodeoxygenation of 5-hydroxymethylfurfural using silica-supported cobalt-nanoparticles. ChemCatChem 14:e202101234. https://doi.org/10.1002/cctc.202101234
Kumar H, Das R, Choithramani A, Gupta A, Khude D, Bothra G, Shard A (2021) Efficient green protocols for the preparation of pyrazolopyrimidines. ChemSelect 6:5807–5837
Kamdar NR, Haveliwala DD, Mistry PT, Patel SK (2010) Design, synthesis and in vitro evaluation of antitubercular and antimicrobial activity of some novel pyranopyrimidines. Eur J Med Chem 45:5056–5063
Rajendra Prasad Y, Lakshmana Rao A, Prasoona L, Murali K, Ravi Kumar P (2005) Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2″-hydroxy naphthalen-1″-yl)-1,5-diphenyl-2-pyrazolines. Bio Med Chem Lett 15:5030–5034
Nasr MN, Gineinah MM (2002) Pyrido [2, 3-d]pyrimidines and Pyrimido[5′, 4′:5, 6]pyrido[2, 3-d]pyrimidines as new antiviral agents: synthesis and biological activity. Arch Pharm 335:289–295
Agarwal A, Ramesh A, Goyal N, Chauhan PMS, Gupta S (2005) Dihydropyrido[2,3-d]pyrimidines as a new class of antileishmanial agents. Bio Med Chem 13:6678–6684
Ahluwalia VK, Sharma P, Goyal B, Aggarwal R (1997) Synthesis of some new 2-(pyrazol-l-yl)-4-(pyrimidin-5-yl)thiazoles. Indian J Chem B 36B:920–922
Azzam SHS, Pasha MA (2012) Simple and efficient protocol for the synthesis of novel dihydro-1H-pyrano[2,3-c]pyrazol-6-ones via a one-pot four-component reaction. Tetrahedron Lett 53:6834–6837
Heravi MM, Mousavizadeh F, Ghobadi N, Tajbakhsh M (2014) A green and convenient protocol for the synthesis of novel pyrazolopyranopyrimidines via a one-pot, four-component reaction in water. Tetrahedron Lett 55:1226–1228
Patil KT, Jamale DK, Valekar NJ, Patil PT, Warekar PP, Kolekar GB, Anbhule PV (2017) Uncatalyzed four-component synthesis of pyrazolopyranopyrimidine derivatives and their antituberculosis activities. Synth Commun 47:111–120
Wei J, Wenjun G, Gui Y, Zhang Z, Yousif QA (2020) SCMNPs@Uridine/Zn: an effi cient and reusable heterogeneous nanocatalyst for the rapid one-pot synthesis of tricyclic fused pyrazolopyranopyrimidine and 3-methyl carboxylate substituted pyrano[2,3-c]pyrazole derivatives under solvent-free condition. Pol J Chem Technol 22:20–33
Rigi F, Shaterian HR (2012) Magnetic nanoparticle supported ionic liquid assisted green synthesis of pyrazolopyranopyrimidines and 1,6-diamino-2-oxo-1,2,3,4-tetrahydropyridine-3,5-dicarbonitriles. J Chin Chem Soc 63:557–561
Shaterian HR, Rigi F (2011) Starch sulfate as an efficient and biodegradable polymer catalyst for one-pot, four-component reaction of 2H-indazolo[2,1-b]phthalazine-triones. Starch 63:340–346
Rigi F, Shaterian HR (2017) Silica-supported ionic liquids prompted one-pot four-component synthesis of pyrazolopyranopyrimidines, 3-methyl-4-aryl-4,5-dihydro-1H-pyrano[2,3-c]pyrazol-6-ones, and 1,6-diamino-2-oxo-1,2,3,4-tetrahydropyridine-3,5-dicarbonitriles. Polycycl Aromat Compd 37:314–326
Bi W, Zhou J, Row KH (2010) Separation of xylose and glucose on different silica-confined ionic liquid stationary phases. Anal Chim Act 677:162–168
Li XT, Zhao AD, Mo LP, Zhang ZH (2014) Meglumine catalyzed expeditious four-component domino protocol for synthesis of pyrazolopyranopyrimidines in aqueous medium. RSC Adv 4:51580–51588
Niknam K, Jamali A, Tajaddod M, Deris A (2012) Synthesis of 2-amino-4,6-diarylnicotinonitriles using silica-bound N-propyl triethylenetetramine sulfamic acid as a recyclable solid acid catalyst. Chin J Catal 33:1312–1317
Acknowledgements
We are thankful to the University of Sistan and Baluchestan Research Council for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no confict of interest.
Rights and permissions
About this article
Cite this article
Rigi, F., Shaterian, H.R. A Novel Triazole Bonded Silica Heterogeneous Catalyst for the One-Pot Synthesis of Pyrazolopyranopyrimidines and Dihydro-1H-pyrano[2,3-c]pyrazol-6-ones. Chemistry Africa 5, 1255–1263 (2022). https://doi.org/10.1007/s42250-022-00408-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00408-2