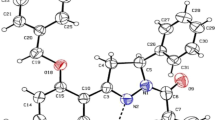
Abstract
Metiamide is a class of medications called H2-receptor antagonist or H2 blockers. It decreases the amount of acid made in the stomach. It is commonly used in the treatment for peptic ulcer disease and gastroesophageal reflux disease. In this study, the metiamide tautomer stability, structural data, HOMO and LUMO (energies and shapes), ΔΕ HOMO–LUMO gaps, UV–visible data and graphs, dipole moments, Mulliken charges, and thermodynamic and kinetic stabilities in aqueous media as a biological solvent and some of the different media (vacuum, H2O, Et-OH and DMSO) were investigated for the tautomers of metiamide by the density functional theory DFT-B3LYP/6-31G* method. The results presented that the probability of the adaptability and compatibility of which tautomer (T1–T6) are better than the other tautomers for interactions with the pattern and structural map of the H2-receptor. The diversities of the interaction points and mosaic patterns of the T3 and T4 tautomers in H2O media with the imaginary H2-receptor were investigated.




















Similar content being viewed by others
References
J.P. Kahrilas, N. Engl. J. Med. 359(16), 1700–1707 (2008)
M. Khan, J. Santana, C. Donnellan, C. Preston, P. Moayyedi, Cochrane Database Syst. Rev. 18(2), CD003244 (2007)
I.L. Salom, S.E. Silvis, A. Doscherholmen, Scand. J. Gastroenterol. 17(1), 129–131 (1982)
J. Black et al., Metiamide—an orally active histamine H2-receptor antagonist. Agents Actions 43(3–4), 91–96 (1994)
J.G. Hardman, A.W. Petri, Goodman and Gilman’s, the Pharmacological Basis of Therapeutics, 9th edn. (McGraw-Hill, New York, 1996), pp. 1092–1094
S. Mallow, J.A. Rebuck, T. Osler et al., Curr. Surg. 61(5), 452–458 (2004)
R.B. Canani, P. Cirillo, P. Roggero et al., Pediatrics 117(5), 817–820 (2006)
E. Untersmayr, N. Bakos, I. Scholl et al., FASEB J. 19(6), 656–658 (2005)
C.R. Ganellin, G.J. Durant, J.C. Emmett, Some chemical aspects of histamine H2-receptor antagonists, in Federation proceedings, vol. 35, no. 8, pp. 1924–1930)
T. Nguyen, D.A. Shapiro, S.R. George, V. Setola, D.K. Lee, R. Cheng et al., Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 59(3), 427–433 (2001)
M.J. Smit et al., Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc. Natl. Acad. Sci. 93(13), 6802–6807 (1996)
D.U. Preiss, C.F. Code, Effect of the H2-receptor antagonists (burimamide and metiamide) on gastric secretion stimulated by histamine and its methyl derivatives. J. Pharmacol. Exp. Ther. 193(2), 614–620 (1975)
L.S.P. Wickramasinghe, S.K. Basu, Br. Med. J. 289(6454), 1272 (1984)
W.S. Ruddell, A.T. Axon, J.M. Findlay et al., Lancet 1, 672–674 (1980)
E. Untersmayr, N. Bakos, I. Scholl et al., FASEB J. 19(6), 656–658 (2005)
A.A. Taherpour, A. Mozafari, S. Ranjbar, S. Taban, Struct. Chem. 26(2), 517–529 (2015)
A.A. Taherpour, R. Rahimizadeh, Med. Chem. Res. (2016). doi:10.1007/s00044-016-1612-0)
J. Clayden, N. Greeves, S. Warren, P. Wothers, Organic Chemistry, 1st edn. (Oxford University Press, Oxford, 2001), pp. 204–206, 586–588
Tagamet, Discovery of Histamine H 2 -receptor Antagonists, National Historic Chemical Landmarks (American Chemical Society, Washington, 2012)
M. Targema, N.O. Obi-Egbedi, M.D. Adeoye, Comput. Theor. Chem. 1012, 47–53 (2013)
S. Shaji, S.M. Eappen, T.M.A. Rasheed, K.P.R. Nair, Spectrochem. Acta Part A 60, 351–355 (2004)
M.M. Islam, M.D.H. Bhiuyan, T. Bredow, A.C. Try, Comput. Theor. Chem. 967, 165–170 (2011)
B. Kosar, C. Albayrak, C.C. Ersanli, M. Odabasoglu, O. Buyukgungor, Spectrochim. Acta, Part A 93, 1–9 (2012)
P.L. Praveen, D.P. Ojha, Cryst. Res. Technol. 47, 91–100 (2012)
P.M. Anbarasan, P.S. Kumar, M. Geetha, R. Govindan, S. Manimegalai, K. Velmurugan, Recent Res. Sci. Technol. 2, 8–16 (2010)
N. Sundaraganesan, J. Karpagam, S. Sebastian, J.P. Conard, Spectrochem. Acta Part A 73, 11–19 (2009)
A. Karakas, H. Unver, Spectrochim. Acta, Part A 75, 1492–1496 (2010)
Y. Shao, L. Fusti-Molnar, Y. Jung, J. Kussmann, C. Ochsenfeld, S.T. Brown, A.T.B. Gilbert, L.V. Slipchenko, S.V. Levchenko, D.P. O’Neill, R.A. DiStasio Jr., R.C. Lochan, T. Wang, G.J.O. Beran, N.A. Besley, J.M. Herbert, C.Y. Lin, T. Van Voorhis, S.H. Chien, A. Sodt, R.P. Steele, V.A. Rassolov, P.E. Maslen, P.P. Korambath, R.D. Adamson, B. Austin, J. Baker, E.F.C. Byrd, H. Dachsel, R.J. Doerksen, A. Dreuw, B.D. Dunietz, A.D. Dutoi, T.R. Furlani, S.R. Gwaltney, A. Heyden, S. Hirata, C.-P. Hsu, G. Kedziora, R.Z. Khalliulin, P. Klunzinger, A.M. Lee, M.S. Lee, W. Liang, I. Lotan, N. Nair, B. Peters, E.I. Proynov, P.A. Pieniazek, Y.M. Rhee, J. Ritchie, E. Rosta, C.D. Sherrill, A.C. Simmonett, J.E. Subotnik, H.L. Woodcock III, W. Zhang, A.T. Bell, A.K. Chakraborty, D.M. Chipman, F.J. Keil, A. Warshel, W.J. Hehre, H.F. Schaefer III, J. Kong, A.I. Krylov, P.M.W. Gill, M. Head-Gordon, B.J. Deppmeier, A.J. Driessen, T.S. Hehre, J.A. Johnson, P.E. Klunzinger, J.M. Leonard, I.N. Pham, W.J. Pietro, J. Yu, SPARTAN’10, build 1.0.1 (Wavefunction Inc., Irvine, 2011)
A. Dufert, D.B. Werz, J. Org. Chem. 73, 5514–5519 (2008)
E. Kavitha, N. Sundaraganesan, S. Sebastian, Indian J. Pure Appl. Phys. 48, 20–30 (2010)
K.I. Ramachandran, G. Deepa, K. Namboori, Computational Chemistry and Molecular Modelling: Principles and Applications (Springer, Berlin, 2008)
M.F. Budyka, T.S. Zyubina, A.K. Zarkadis, J. Mol. Struct. (THEOCHEM) 594, 113–125 (2002)
Y.O. Lee, T. Pradhan, K. No, J.S. Kim, Tetrahedron 68, 1704–1711 (2012)
M. Amalanathan, I.H. Joe, V.K. Rastogi, J. Mol. Struct. 985, 48–56 (2011)
H. Dorsett, A. White, Overview of Molecular Modelling and Ab-initio Molecular Orbital Methods Suitable for Use with Energetic Materials: A Review (DSTO Aeronautical and Maritime Research Laboratory, Australia, 2000)
P.W. Atkins, R.S. Friedman, Molecular Quantum Mechanics, 4th edn. (Oxford University Press, New York, 2004)
B. Grossmann, J. Heinze, T. Moll, C. Palivan, S. Ivan, G. Gescheidt, J. Phys. Chem. B 108, 4669–4672 (2004)
G. Gece, Corros. Sci. 50, 2981–2992 (2008)
P. Atkins, J. de Paula, Physical Chemistry, 8th edn. (Oxford University Press, New York, 2006)
R.G. Mortimer, Physical Chemistry, 3rd edn. (Elsevier Inc., USA, 2008)
K. Parimala, V. Balachandran, Spectrochim. Acta A Mol. Biomol. Spectrosc. 81(1), 711–723 (2011)
http://en.wikipedia.org/w/index.php?title=Mulliken_population_analysis&oldid=572345005
R.S. Mulliken, J. Chem. Phys. 23(10), 1833–1840 (1955)
A.R. Leach, Molecular Modelling: Principles and Applications (Prentice Hall, Englewood Cliffs, 2011)
T. Schlik, Molecular Modeling and Simulation: An Interdisciplinary Guide (Interdisciplinary Applied Mathematics, 2nd edn. (Springer, Berlin, 2013)
W.S. Ohlinger, K.E. Philip, J.D. Bernard, J.H. Warren, J. Phys. Chem. A 113(10), 2165–2175 (2009)
F.M. Bickelhaupt, N.J.R. van Eikema Hommes, C. Fonseca Guerra, E.J. Baerends, Organometallics 15(13), 2923–2931 (1996)
A.E. Reed, R.B. Weinstock, F. Weinhold, J. Chem. Phys. 83(2), 735–746 (1985)
P. Salve, D. Gharge, R. Kirtawade, P. Dhabale, K. Burade, Int. J. Pharm. Technol. Res. 2(3), 2071–2074 (2010)
A.N. Mishra, A.C. Rana, Int. J. Chem. Sci. 7(3), 2208–2210 (2009)
S.C. Dash, N.N. Das, P. Mohanty, Indian J. Chem. Technol. 18, 132–136 (2011)
http://www.gsk.ca/english/docs-pdf/product-monographs/Zantac.pdf
R.K. Murray, D.A. Bender, K.M. Botham, P.J. Kennelly, V.W. Rodwell, P.A. Weil, Harpers Illustrated Biochemistry (Lange Medical Book), 2012, 29th edn. (McGraw-Hill Companies, Inc., USA). ISBN-13: 978-0071765763.h. http://www.drugs.com/pro/cimetidine-injection.html
http://www.medicinenet.com/script/main/art.asp?articlekey=10001
J. Řezáč, L. Šimová, P. Hobza, J. Chem. Theory Comput. 9(1), 364–369 (2013)
I. Shavitt, R.J. Bartlett, Many-Body Methods in Chemistry and Physics: MBPT and Coupled-Cluster Theory (Cambridge University Press, Cambridge, 2009)
Acknowledgement
The corresponding author gratefully acknowledges Theoretical and Computational Research Center of Chemistry and Nano Sciences, Faculty of Chemistry, Razi University, Kermanshah, Iran, Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran and Health Technology Center, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taherpour, A.A., Chegeni, M.M.F., Khodaei, M.M. et al. A first-principle DFT study of solvent effects on metiamide tautomers and imaginary interactions with H2-receptors. J IRAN CHEM SOC 14, 1613–1632 (2017). https://doi.org/10.1007/s13738-017-1102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1102-4