Abstract
Purpose of Review
The diagnosis and treatment of behavioral variant frontotemporal dementia is challenging and often delayed because of overlapping symptoms with more common dementia syndromes or primary psychiatric illnesses. The purpose of this paper is to explore the relevant presentation, diagnostic workup, pathophysiology, and both pharmacologic and non-pharmacologic management.
Recent Findings
Behavioral variant frontotemporal dementia is a highly heritable disorder. The gradual accumulation of diseased protein culminates in the destruction of those brain circuits responsible for much of one’s emotional and social functioning.
Summary
Behavioral variant frontotemporal dementia is a progressive neurodegenerative disorder with a far-reaching impact on patients and caregivers. Patients often present with emotional blunting, lack of empathy, apathy, and behavioral disinhibition. Non-pharmacologic interventions and caregiver support are the cornerstone of treatment. The use of cholinesterase inhibitors and memantine is not supported by the evidence. While current pharmacologic therapies target only certain symptoms, there are disease modifying agents currently in or nearing the clinical research stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is a significant clinical concern for those practicing geriatrics and primary care. Due to the variety of possible presentations and conditions, diagnosis may be challenging. Frontotemporal dementia (FTD) is a leading cause of dementia in those under the age of 65 [1]. Frontotemporal dementia is commonly divided into three syndromes: behavioral variant FTD (bvFTD), nonfluent/agrammatic primary progressive aphasia (nfvPPA) and semantic variant primary progressive aphasia (svPPA) [2, 3]. The nfvPPA and svPPA variants present with a prominent decline in language abilities.
Behavioral variant FTD, the most common of the FTDs, is the focus of this review. Behavioral variant FTD is characterized by striking changes in personality, along with disinhibition, impaired judgment and social habits, changes in conduct and temperament, increased stereotypic behaviors, and declines in executive functioning [4•]. The mean age of onset is 58 years old and the mean survival from time of diagnosis is 4.5 years [5, 6]. In patients with early-onset dementia, the prevalence of FTD is between 3% to 26% [1]. In the US population of adults aged 45–64, the prevalence ranges from 15 to 22 per 100,000 [1].
Diagnosis
Given the overlap with other neurodegenerative and psychiatric disorders, the diagnosis of bvFTD is often challenging and delayed. Early symptoms of bvFTD may be misdiagnosed as a mid-life crisis, depression, or late-onset primary bipolar and psychotic disorders. Patients with bvFTD often present with emotional blunting and apathy, which are often both clinically and neuroanatomically distinct from depression. Many patients are ostracized by family and friends, lose their jobs, and endure multiple referrals before their disease is recognized as a neurodegenerative disorder. It does not help that these patients often have no insight into their symptoms. Even when patients are identified as suffering from a neurological problem, they are often misdiagnosed with Alzheimer’s disease (AD) or other causes of dementia [7, 8]. Early and accurate diagnosis of bvFTD is crucial, as it allows for appropriate treatment decisions as well as adequate counseling of families and caregivers. Diagnosis is made based on presentation, course, family history, neuroimaging, and cognitive testing. The differential may include related disorders such as vascular cognitive impairment, Alzheimer’s Disease (AD), corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), amyotrophic-lateral sclerosis (ALS), depression and other idiopathic psychiatric disorder [9].
Definitive diagnosis of bvFTD requires histopathological confirmation at autopsy. Seeing as this is of little benefit to living patients, guidelines for diagnosing bvFTD with varying degrees of confidence exist. The first step is the clinical recognition of progressive deterioration in behavior and/or cognition. The International Behavioral Variant FTD Criteria Consortium guidelines provide diagnostic criterion for bvFTD (Refer to Table 1). For a diagnosis of ‘possible’ bvFTD, patients must fulfill three of six features: disinhibition, apathy, loss of sympathy/empathy, compulsive/perseverative behaviors, hyperorality or dietary changes and executive function deficits [2]. To meet criteria for ‘probable’ bvFTD, a patient must exhibit functional disability with characteristic neuroimaging findings, in addition to the features noted for ‘possible’ bvFTD (see neuroimaging section below) [2].
Distinguishing between dementia types is a challenge. Alzheimer’s disease and other etiologies of dementia often present with behavioral components in addition to cognitive decline, thus, the presence of these symptoms alone does not rule in or out any particular diagnosis [10]. Obtaining clinical history from family verifying the gradual and progressive behavioral change is the most essential step toward making a diagnosis of bvFTD (Refer to Table 2). Behavioral variant FTD tends to present with significant shifts in the fundamentals of one’s personality, all of which are extremely challenging for caregivers to understand and manage. Neuropsychological testing may be helpful in distinguishing bvFTD symptoms from other dementia syndromes (see neuropsychological testing section below) [11].
Neurological examination is an important component in suspected bvFTD. Although there are no specific findings in bvFTD, motor-neuron disease signs (muscle atrophy, fasciculations, spasticity) occur in up to 12.5% of patients; parkinsonism is found in as many as 20% of cases [12, 13]. Frontal release signs, also known as primitive reflexes, may be elicited, though these are not specific for a single cause of dementia. Cranial nerve, motor, sensory, and the remaining reflex examinations are typically normal.
There are no characteristic laboratory findings for bvFTD. Standard laboratory studies include complete blood count (CBC), basic metabolic panel (BMP), thyroid studies (TSH), vitamin B12 levels, vitamin D, and folate. Depending on the individual patient’s risk factors, workup up may also include labs for inflammatory markers (c-reactive protein (CRP), erythrocyte sedimentation rate (ESR), antinuclear antibody (ANA)), Lyme antibodies, HIV, and syphilis studies. These tests are done to rule out treatable and reversible causes of cognitive deficits like nutritional deficiencies, endocrine disorders, metabolic conditions, infections, and collagen vascular diseases. Screening for sleep apnea and other sleep disorders, substance use disorders, and excessive use of caffeine may also be considered.
Neuroimaging
Neuroimaging may further support a diagnosis of bvFTD. Structural imaging modalities include computed tomography (CT) and magnetic resonance imaging (MRI), each having their advantages and disadvantages. CT scans depend on a degree of x-ray radiation and provide less image resolution when compared to MRI. In contrast, MRI does not employ radiation but requires a longer scan time and is a more expensive. If available and without contraindication, MRI without contrast is the preferred imaging modality for suspected bvFTD [14].
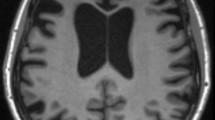
Frontotemporal dementia is named for the characteristic neurodegeneration in the frontal and temporal lobes observed on imaging or at autopsy. Depending on the extent of neurodegeneration, atrophy is most prominent on MRI in the frontal and anterior temporal lobes [Fig. 1, A & B]. The thalamus and cerebellum may show significant degenerative changes as well, although these findings are more likely visualized via nuclear imaging [15•, 16]. An important caveat is that functional impairment in neurocircuits due to decreasing synaptic density and aberrant neuronal metabolic processes often leads to clinical symptoms of bvFTD prior to obvious atrophic changes on imaging. Therefore, the absence of overt volume loss on MRI or CT does not rule out bvFTD.
When the diagnosis remains elusive, nuclear imaging such as fluorodeoxyglucose (FDG) - positron emission tomography (PET) or single-photon emission tomography (SPECT) may be used to elucidate abnormal patterns of brain function [Fig. 1, C]. Using tracers that emit radioactive signal as they decay, nuclear imaging can detect diminished levels of metabolic activity or perfusion within the brain [14]. In bvFTD, FDG-PET imaging shows asymmetrical hypometabolism in the frontal and/or anterior temporal lobes and SPECT scans show hypoperfusion of the frontal and/or anterior temporal regions.
Barriers to nuclear imaging include limited access and cost. In the United States, Medicare only covers the cost for nuclear imaging in circumstances when the patient meets criteria for both bvFTD and AD and imaging would provide diagnostic clarification.
Neuropsychological Testing
Every patient should receive a cognitive performance screening test. The authors of this review do refer for more extensive neuropsychological testing when there are atypical features, young age of onset, and when the correct diagnosis remains unclear. The classic testing pattern for bvFTD includes deficits in executive function (difficulty with organizing, sequencing, mental flexibility, shifting between tasks seamlessly, decision making), impaired working memory/concentration, as well as difficulty with encoding and retrieval. There is considerable overlap between AD and bvFTD in neuropsychological testing, however patients with bvFTD may be better at recalling delayed items with cues than those with AD. Patients with bvFTD demonstrate significantly higher levels of emotional blunting, apathy, and loss of empathy than patients with AD [9]. One may observe early impairment of facial emotion recognition as a feature; recognition of negative emotions, such as anger and disgust, becomes severely impaired [17]. Additionally, those with bvFTD have diminished capacity to infer what another person may be thinking or feeling [18]. Assessment of social cognitive abilities, specifically emotion recognition, is helpful in differentiating bvFTD from other forms of dementia [17, 18].
Neuropathology and Etiologies
There are important distinctions between the frontotemporal dementias, a group of clinical syndromes with a constellation of signs and symptoms that fulfill defined criteria, and frontotemporal lobar degeneration (FTLD), a pathological descriptor characterizing neuronal loss, gliosis, and focal atrophy within the frontal and temporal lobes. Frontotemporal lobar degeneration may underpin a number of clinical dementia syndromes, including bvFTD, nfvPPA, and svPPA, as well as dementias historically conceptualized as the atypical parkinsonian syndromes, i.e. CBD and PSP [1].
As mentioned, bvFTD is a disorder manifesting in behavioral, cognitive, and personality changes because of degeneration in several regions of the brain that comprise larger scale neural networks. There is close clinicopathologic correlation between clinical observation and the loss of neurons in regions which subserve their respective function. Pathologic changes in the following structures and their associated clinical findings include: anterior cingulate cortex (ACC)/medial prefrontal cortex (mPFC) and apathy; orbitofrontal cortex (OFC)/frontoinsular cortex and disinhibition; frontoinsular cortex/anterior temporal lobes and loss of compassion; ventral striato-pallidum and stereotypies/compulsions; frontoinsular cortex/ventral striatum/hypothalamus and aberrant eating behavior; dorsolateral prefrontal cortex (dlPFC) and executive dysfunction [19•].
Neurodegenerative diseases, including those responsible for bvFTD, are often the result of an endogenous protein that aggregates, propagates, and ultimately jeopardizes normal neuronal function leading to cell death and inflammation. Several types of these irregular and diseased proteins, or proteinopathies, contribute to the bvFTD clinical syndrome. Most commonly, bvFTD results from abnormal accumulation of diseased tau or a protein named TAR DNA-binding protein 43 (TDP-43). The bvFTD syndrome can result from other, rarer, proteinopathies. There is even a non-amnestic variant of AD, the behavioral/dysexecutive or frontal variant as it is sometimes called, that may present remarkably like bvFTD [20]. Emerging evidence suggests that inflammation and immune dysfunction play a role in bvFTD [21].
Genetics
Behavioral variant FTD is the most heritable of the frontotemporal dementia variants [22]. Between 30 and 50% of patients with bvFTD have a positive family history, with an autosomal dominant mode of inheritance in 10–27% of cases [22,23,24,25,26]. Mutations in MAPT, GRN and C9orf72 account for the majority of familial FTDs [27, 28]. Genetic testing may be considered both in patients with bvFTD and in those with a family history of neurological disorders including bvFTD, AD, parkinsonism, motor neuron disease (MND) or late-onset psychosis [29, 30]. Patients with bvFTD, particularly those with C9ORF72 expanded repeat mutations, may also exhibit psychotic features early in the disease course, including visual or auditory hallucinations and delusions [28].
Treatment
Given the lack of US Food and Drug Administration (FDA) approved pharmacotherapies for bvFTD, current recommendations emphasize conservative, supportive non-pharmacological interventions with a focus on quality of life. A multi-disciplinary team approach, consisting of a physician (neurologist, geriatric psychiatrist, or geriatrician), a nurse, neuropsychologist, social worker, occupational therapist, genetic counselor, and pharmacist is crucial for the provision of a comprehensive treatment plan [19•, 31•]. In the absence of social and community support, caregiver distress is almost inevitable and contributes to major adverse consequences, most notably poor health outcomes for both caregiver and patient [32]. Given the younger age and more rapid decline, advance care planning is imperative early in the course of the illness.
Pharmacological interventions for bvFTD primarily target behavioral symptoms. Limited evidence supports the use of antidepressants, i.e., selective serotonin reuptake inhibitors (SSRIs), as they may quell impulsivity, compulsivity, irritability, overeating behavior, and disinhibition [33]. Pharmacological interventions for bvFTD primarily target behavioral symptoms. Limited evidence supports the use of antidepressants, i.e., selective serotonin reuptake inhibitors (SSRIs), as they may quell impulsivity, compulsivity, irritability, overeating behavior, and disinhibition [33]. Citalopram (30–40 mg) and Paroxetine (20 mg) have been shown to be helpful for behavioral symptoms in FTD based on randomized control trials [34,35,36]. Of note, for patients older than 60 years, there exists an FDA warning of dose-dependent QTc increase with the usage of citalopram at doses greater than 20 mg. QTc data in the FDA’s original drug safety warning showed an approximate 4 ms difference between 20 mg/day and 40 mg/day [37]. But the clinical implication of this warning is unclear [38]. Studies of sertraline (50–100 mg) use in treating FTD symptoms are limited to mainly observational studies [39]. Some studies suggest the use of venlafaxine which possesses a greater noradrenergic profile, for prominent features of apathy [19•]. Although the study did not specify a particular dose range for the treatment of apathy, doses ranging anywhere from 75 mg–225 mg is recommended in clinical practice. Agitation may be treated with atypical antipsychotics such as risperidone, olanzapine and quetiapine. Though it is prudent to do so with caution given risk for cardiac complications and increased mortality [40]. A specific dose of atypical antipsychotic use in treating agitation have not been identified in the reference article. However, a starting dose of risperidone at 0.5 mg, olanzapine at 2.5 mg and quetiapine at 12.5 mg can be used, with further dose titration based on symptoms.
Acetylcholinesterase inhibitors, including donepezil, rivastigmine, and galantamine, FDA approved for treatment of AD, have been studied in bvFTD. None of the studies were adequately powered, and only one study was placebo controlled [31•]. Studies indicate that standard cholinergic agents may, in fact, exacerbate psychiatric symptoms in patients with bvFTD [41, 42]. This is likely due to a lack of consistent evidence for a cholinergic deficit in bvFTD [43].
Memantine, a N-methyl-d-aspartate receptor antagonist FDA approved for moderate to severe AD, was associated with worsening cognitive function in randomized placebo-controlled trials [42, 44].
Further research into pharmacotherapies for symptom management is much needed. Disease modifying agents targeting the underlying pathological mechanisms are in or nearing the clinical research stage [19•, 45].
Conclusion
Based on clinical history, neuropsychological testing, and MRI findings, Mrs. J was diagnosed with bvFTD. A sense of relief was expressed by Mrs. J’s daughter on receiving a diagnosis that explained her mother’s condition. Supportive counseling, advanced care planning and pharmacotherapies were explored.
The diagnosis of bvFTD is challenging as the initial presentation often overlaps with more common psychiatric syndromes and neurodegenerative diseases. A careful clinical history and thorough diagnostic evaluation, including neurological examination, laboratory studies, neuropsychological testing, and neuroimaging are indicated. Genetic testing and counseling may be appropriate in select scenarios. Early recognition is important to optimize social, community and advanced care planning resources to support patients and their families.
Change history
15 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s13670-021-00363-9
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386:1672–82. https://doi.org/10.1016/S0140-6736(15)00461-4.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. https://doi.org/10.1093/brain/awr179.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. https://doi.org/10.1212/WNL.0b013e31821103e6.
Kamath V, Chaney GS, DeRight J, Onyike CU. A meta-analysis of neuropsychological, social cognitive, and olfactory functioning in the behavioral and language variants of frontotemporal dementia. Psychol Med. 2019;49(16):2669–80. https://doi.org/10.1017/S0033291718003604This article is a meta-analysis of neuropsychological testing, social and cognitive findings and distinctions in FTD syndromes that provides an evidence-based approach to differential diagnosis and neuropsychological testing findings.
Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62. https://doi.org/10.1001/archneur.62.6.925.
Coyle-Gilchrist ITS, Dick KM, Patterson K, Rodríquez PV, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–43. https://doi.org/10.1212/WNL.0000000000002638.
Mendez MF, Selwood A, Mastri AR, Frey WH 2nd. Pick's disease versus Alzheimer's disease: a comparison of clinical characteristics. Neurology. 1993;43(2):289–92. https://doi.org/10.1212/wnl.43.2.289.
Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1999;66(2):184–8. https://doi.org/10.1136/jnnp.66.2.184.
Pressman PS, Matlock D, Ducharme S. Distinguishing Behavioral Variant Frontotemporal Dementia from Primary Psychiatric Disorders: A Review of Recently Published Consensus Recommendations From the Neuropsychiatric International Consortium for Frontotemporal Dementia. JNP. 2021:appi.neuropsych.20090238. doi:https://doi.org/10.1176/appi.neuropsych.20090238.
Mathias JL, Morphett K. Neurobehavioral differences between Alzheimer's disease and frontotemporal dementia: a meta-analysis. J Clin Exp Neuropsychol. 2010;32:682–98. https://doi.org/10.1080/13803390903427414.
Lindau M, Almkvist O, Kushi J, Boone K, Johansson SE, Wahlund LO, et al. First symptoms--frontotemporal dementia versus Alzheimer's disease. Dement Geriatr Cogn Disord. 2000;11:286–93. https://doi.org/10.1159/000017251.
Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134:2582–94. https://doi.org/10.1093/brain/awr195.
Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, et al. Demographic, neurological, and behavioral characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–65. https://doi.org/10.1093/brain/awl288.
Filippi M, Agosta F, Barkhof F, Dubois B, Fox NC, Frisoni GB, et al. EFNS task force: the use of neuroimaging in the diagnosis of dementia. Eur J Neurol. 2012;19:1487–501. https://doi.org/10.1111/j.1468-1331.2012.03859.x.
Schroeter ML, Laird AR, Chwiesko C, Deuschl C, Schneider E, Bzdok D, et al. Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses - the case of behavioral variant frontotemporal dementia. Cortex. 2014;57:22–37. https://doi.org/10.1016/j.cortex.2014.02.022This article is a recent meta-analysis that identified areas of the brain with significant atrophy and hypometabolism and linked those areas to affected neural networks and symptomology seen in bvFTD.
Chen Y, Kumfor F, Landin-Romero R, Irish M, Piguet O. The cerebellum in Frontotemporal dementia: a meta-analysis of neuroimaging studies. Neuropsychol Rev. 2019;29(4):450–64. https://doi.org/10.1007/s11065-019-09414-7.
Bora E, Dennis V, Mark W. Meta-analysis of facial emotion recognition in behavioral variant Frontotemporal dementia: comparison with Alzheimer disease and healthy controls. J Geriatr Psychiatry Neurol. 2016;29:205–11. https://doi.org/10.1177/0891988716640375.
Henry JD, Phillips LH, von Hippel C. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia. 2014;56:53–62. https://doi.org/10.1016/j.neuropsychologia.2013.12.024.
Seeley WW. Behavioral Variant Frontotemporal Dementia. Continuum (Minneap Minn). 2019;25(1):76–100. https://doi.org/10.1212/CON.0000000000000698This article describes the clinical, anatomic, genetic, and pathologic features of behavioral variant frontotemporal dementia (bvFTD) and discusses strategies to improve diagnostic accuracy.
Sawyer RP, Rodriguez-Porcel F, Hagen M, Shatz R, Espay AJ. Diagnosing the frontal variant of Alzheimer’s disease: a clinician’s yellow brick road. J Clin Mov Disord. 2017;4. https://doi.org/10.1186/s40734-017-0052-4.
Broce I, Karch CM, Wen N, et al. Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies [published correction appears in PLoS Med. 2018 Jan 29;15(1):e1002504]. PLoS Med. 2018;15(1):e1002487. Published 2018 Jan 9. doi: https://doi.org/10.1371/journal.pmed.1002487.
Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73:1451e6. https://doi.org/10.1212/WNL.0b013e3181bf997a.
Seelaar H, Kamphorst W, Rosso SM, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71:1220–6. https://doi.org/10.1212/01.wnl.0000319702.37497.72.
Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Arch Neurol. 1999;56(7):817–22. https://doi.org/10.1001/archneur.56.7.817.
Goldman JS, Farmer JM, Wood EM, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65(11):1817–9. https://doi.org/10.1212/01.wnl.0000187068.92184.63.
Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126(Pt 9):2016–22. https://doi.org/10.1093/brain/awg204.
Lashley T, Rohrer JD, Mahoney C, et al. A pathogenic progranulin mutation and C9orf72 repeat expansion in a family with frontotemporal dementia [published correction appears in Neuropathol Appl Neurobiol. 2014 Dec;40(7):955]. Neuropathol Appl Neurobiol. 2014;40(4):502–13. https://doi.org/10.1111/nan.12100.
Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135(Pt 3):693–708. https://doi.org/10.1093/brain/awr355.
Le Ber I. Genetics of frontotemporal lobar degeneration: an up-date and diagnosis algorithm. Rev Neurol. 2013;169:811–9. https://doi.org/10.1016/j.neurol.2013.07.014.
Goldman JS, Rademakers R, Huey ED, Mayeux R, Miller BL, et al. An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology. 2011;76:475–83. https://doi.org/10.1212/01.wnl.0000187068.92184.63.
Tsai RM, Boxer AL. Therapy and clinical trials in frontotemporal dementia: past, present, and future. J Neurochem. 2016;138 Suppl 1(Suppl 1):211–21. https://doi.org/10.1111/jnc.13640This article reviews current therapies for FTD, discusses advancements in FTD pathophysiology, therapy development, and biomarker advancement, including their relation to recent clinical trials and future implications.
Wong C, Merrilees J, Ketelle R, et al. The experience of caregiving: differences between behavioral variant of frontotemporal dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2012;20(8):724–8. https://doi.org/10.1097/JGP.0b013e318233154d.
Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors [published correction appears in J Clin Psychiatry 1997 Jun;58(6):275]. J Clin Psychiatry. doi:1997;58(5):212–216.
Herrmann N, Black S.E, Chow T. “Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia.” The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 20,9 2012: 789–797. doi:https://doi.org/10.1097/JGP.0b013e31823033f3.
Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain. 2015;138(Pt 7):1961–75. https://doi.org/10.1093/brain/awv133.
Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13–9. https://doi.org/10.1159/000067021.
US Food and Drug Administration [Internet] Silver Spring (MD): c2011. FDA safety communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide) [updated 2017 Dec 14; cited 2018 Jun 26].
McCarrell JL, Bailey TA, Duncan NA, et al. A review of citalopram dose restrictions in the treatment of neuropsychiatric disorders in older adults. Ment Health Clin. 2019;9(4):280–286. Published 2019 Jul 1. doi:https://doi.org/10.9740/mhc.2019.07.280.
Mendez MF, Shapira JS, Miller BL. Stereotypical movements and frontotemporal dementia. Mov Disord. 2005;20(6):742–5. https://doi.org/10.1002/mds.20465.
Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–43. https://doi.org/10.1001/jama.294.15.1934.
Mocellin R, Scholes A, Walterfang M, Looi JCL, Velakoulis D. Clinical update on frontotemporal dementia: diagnosis and treatment. Australasian Psychiatry. 2015;23(5):481–7. https://doi.org/10.1177/1039856215582276.
Vercelletto M, Boutoleau-Bretonnière C, Volteau C, et al. Memantine in behavioral variant frontotemporal dementia: negative results. J Alzheimers Dis. 2011;23(4):749–59. https://doi.org/10.3233/JAD-2010-101632.
Chow TW. Treatment approaches to symptoms associated with frontotemporal degeneration. Curr Psychiatry Rep. 2005;7:376–80. https://doi.org/10.1007/s11920-005-0040-5.
Boxer AL, Knopman DS, Kaufer DI, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2013;12(2):149–56. https://doi.org/10.1016/S1474-4422(12)70320-4.
Lagier-Tourenne C, Baughn M, Rigo F, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A. 2013;110(47):E4530–9. https://doi.org/10.1073/pnas.1318835110.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Geriatric Psychiatry
The original online version of this article was revised: The affiliation numbers in author group should be listed as Silpa Balachandran1, Elizabeth L. Matlock2, Michelle L. Conroy2,3 and Chadrick E. Lane4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balachandran, S., Matlock, E.L., Conroy, M.L. et al. Behavioral Variant Frontotemporal Dementia: Diagnosis and Treatment Interventions. Curr Geri Rep 10, 101–107 (2021). https://doi.org/10.1007/s13670-021-00360-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-021-00360-y