Abstract
Purpose of Review
The prevalence of obesity continues to rise steadily. While obesity management typically relies on dietary and lifestyle modifications, individual responses to these interventions vary widely. Clinical guidelines for overweight and obesity stress the importance of personalized approaches to care. This review aims to underscore the role of precision nutrition in delivering tailored interventions for obesity management.
Recent Findings
Recent technological strides have expanded our ability to detect obesity-related genetic polymorphisms, with machine learning algorithms proving pivotal in analyzing intricate genomic data. Machine learning algorithms can also predict postprandial glucose, triglyceride, and insulin levels, facilitating customized dietary interventions and ultimately leading to successful weight loss. Additionally, given that adherence to dietary recommendations is one of the key predictors of weight loss success, employing more objective methods for dietary assessment and monitoring can enhance sustained long-term compliance.
Summary
Biomarkers of food intake hold promise for a more objective dietary assessment. Acknowledging the multifaceted nature of obesity, precision nutrition stands poised to transform obesity management by tailoring dietary interventions to individuals' genetic backgrounds, gut microbiota, metabolic profiles, and behavioral patterns. However, there is insufficient evidence demonstrating the superiority of precision nutrition over traditional dietary recommendations. The integration of precision nutrition into routine clinical practice requires further validation through randomized controlled trials and the accumulation of a larger body of evidence to strengthen its foundation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a global epidemic that puts a tremendous burden on public health on account of its association with the increased risk of non-communicable diseases (NCDs). The European region, which consists of 53 countries in Europe and Central Asia, has high rates of overweight and obesity that affect almost 60% of adults and 30% of children, according to the WHO European Regional Obesity Report 2022. Unfortunately, the WHO Global Action Plan for preventing the rise in obesity prevalence has not been achieved by any European nation. The prevalence of obesity continues to increase exponentially regardless of age, sex, ethnicity, or socioeconomic status, and it shows no significant signs of decreasing soon [1•].
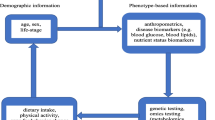
Obesity management strategies include a calorie-restricted diet, physical activity, behavior therapy, pharmacotherapy, and bariatric surgery. Clinical practice guidelines for overweight and obesity management highlight the importance of customized interventions and patient-centered care [2••]. Precision nutrition aims to personalize dietary recommendations based not only on phenotype but also on genotype or additional molecular factors such as gene expression, microbiome, proteome, and metabolome [3,4,5]. This novel approach can be considered to occur at three levels; Level 1 is the traditional diet based on general guidelines for populations by age, sex, and social determinants, Level 2 adds in phenotypic information about the individual’s current nutritional status (e.g. anthropometry, biochemical and metabolic analysis, physical activity), and Level 3 is genotype-oriented nutrition based on common or rare gene variations [6•].
All this information raises the question of how precision nutrition will affect obesity management. By synthesizing the existing literature, this review aims to contribute to the understanding of precision nutrition’s transformative potential in shaping the future of obesity management, with a focus on prioritizing personalized approaches.
Methods of Literature Search
For this narrative review, we searched the PubMed, Science Direct, and Web of Science scientific databases and the gray literature including Google Scholar for English-language articles published between 2000–2023, with full-text availability. Data extraction was independently conducted by two researchers (HGUG and NR). The search approach involved of one or a combination of the following search terms, using the “AND” and “OR” operators: “precision nutrition”[TIAB], “personalized nutrition”[TIAB], “personalised nutrition”[TIAB], “obesity”[MeSH], “overweight”[MeSH], “weight loss”[MeSH], “body weight maintenance”[MeSH], “weight management”[TIAB], “polymorphism”[MeSH], “single nucleotide polymorphism”[MeSH], “gene variation”[TIAB], “biomarkers of food intake”[TIAB], “food intake biomarkers”[TIAB], “multiomics”[MeSH], “dietary assessment”[TIAB], “dietary monitoring”[TIAB], “nutritional status”[MeSH], “interindividual differences”[TIAB], “metabotype”[TIAB], “epigenomics”[MeSH], “genomics”[MeSH], “metabolomics”[MeSH], and “microbiomics”[TIAB]. To broaden the search, keywords with the asterisk (*) operator were employed. Additionally, hand-searching of reference lists from retrieved articles was also conducted. The process of selecting articles for inclusion involved thorough examination and discussion among the researchers to ensure relevance to the review's focus. Any discrepancies in article selection were resolved through consensus. After removing duplicates, a total of 85 peer-reviewed articles (excluding methodologic literature) were identified. These articles were then screened, and 13 were selected for detailed review and discussion, focusing on their contributions to understanding the connections between obesity and precision nutrition.
Overview of Precision Nutrition
The aphorism "What is food to one, is to others bitter poison" uttered by Lucretius (99-55 BC), a Roman philosopher, was one of the first written sources to imply interindividual variability in response to dietary factors and their effects on human health [7]. Later, Roger J. Williams (1893–1988), who discovered pantothenic acid, noticed the differences among healthy individuals long before the relationship between nutrition and metabolism was established. While Williams' work provided early clues for precision medicine, it also demonstrated the need to address interindividual variability in response to nutrients, drugs, and vaccines [8]. Studies on the hypothesis that genetic differences contribute to interindividual nutrient, food, diet, and lifestyle responses have led to the emergence of precision nutrition concepts.
Current views on precision nutrition are based on omics technology, which aims to reveal all biological molecules involved in the structure, function, and dynamics of a cell, organism, or all organisms in a particular environment [6•]. These include a comprehensive analysis of genes (genomics), DNA modifications (epigenomics), messenger RNA (mRNA) or transcripts (transcriptomics), proteins (proteomics), metabolites (metabolomics), lipids (lipidomics), foods (foodomics), and microbiota (microbiomics, metagenomics) [9]. Significant connections exist between bioactive food components and cellular processes, with changes apparent at different molecular levels, encompassing DNA (nutrigenetics), pre-transcriptional modifications (such as methylation and epigenetics) affecting mRNA (nutrigenomics), and proteins (proteomics) [10]. Since single-omics technology alone cannot represent the whole picture of nutrition and obesity, the use of “multi-omics” technology has been accepted to provide a more comprehensive view [9]. Recent advances in multi-omics technology have shed light on the molecular and cellular effects of nutrients on individuals, enabling personalized nutritional interventions that not only consider physiological, ethnic, cultural, and economic factors but also take into account genomics, foodomics and microbiomics [10].
Nevertheless, using these technologies for each individual is expensive and time-consuming. Metabotyping, clustering individuals into three subgroups based on their similar metabolic phenotypes (anthropometric, biochemical, metabolomics, and microbiomics data), is a more feasible and cost-effective option for precision nutrition strategy at the group level (Fig. 1) [11,12,13,14].
Precision nutrition and “metabotyping”. The figure was modified with text and after adaptation of images from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com)
Genetics of Obesity
The genome is defined by all the genetic material, which consists of 23 pairs of chromosomes in the nucleus and a small circular chromosome in the mitochondria called mitochondrial DNA (mtDNA). Despite phenotypic diversity, the human genomes are substantially 99.9% identical, implying that only minor differences in DNA sequence exist [15]. Understanding the human genome has been a journey toward gaining a better understanding of interindividual differences. The success of the Human Genome Project, an international research project aimed at identifying the entire human DNA sequence through the sequencing of 3 billion base pairs, led to more rapid and less expensive DNA sequencing techniques.
The minor differences in DNA are commonly referred to as mutations or polymorphisms. Mutations, which are permanent alterations in the DNA sequence, occur at < 1% frequency in the population and most certainly cause disease by impacting phenotype [16]. Polymorphism refers to the common variant in a specific DNA sequence or the presence of two or more alleles of the gene in a locus in the population at a frequency of approximately ≥ 1%. Polymorphism does not directly cause disease but may act as a predisposing factor [17]. Single nucleotide polymorphism (SNP), the most common type of polymorphism, involves a change in a single base pair. Although the human genome normally contains approximately 3–10 million SNPs, most SNPs do not affect phenotype [18]. Some SNPs, which are in coding regions of DNA, can affect protein synthesis and function, altering nutrient requirements and metabolism [6•].
In obesogenic settings, 40% to 70% of the interindividual differences are attributed to genetic factors [19]. From the framework of genetics, obesity can be described as two categories, monogenic and polygenic obesity. Monogenic obesity is characterized as severe obesity with early-onset caused by a single gene mutation/deficiency. For example, mutations in the genes encoding melanocortin 4 receptor (MC4R), leptin receptor (LEPR), and pro-opiomelanocortin (POMC), result in monogenic obesity. While monogenic obesity is rare, polygenic obesity, caused by hundreds of variants in many genes, is common among individuals with obesity. Each gene variant has a minor impact, as opposed to monogenic obesity the environment largely determines the development of polygenic obesity [20••, 21••].
Over the last decade, the field of multifactorial disease genetics has been changed by genome-wide association studies (GWAS) which aim to determine genotype–phenotype relationships related to disease susceptibility by identifying genomic variants. Although more ethnically diverse studies with larger sample sizes are still lacking, GWAS help identify novel variant-disease or trait associations or novel mechanisms behind them [22]. GWAS have contributed to the identification of obesity-associated genes such as FTO (Fat mass and obesity-associated protein) [23, 24, 25•, 26, 27], MC4R (Melanocortin 4 receptor) [28], MTHFR (Methylenetetrahydrofolate reductase) [29], LEP (Leptin), LEPR (Leptin receptor), ADIPOQ (Adipocyte C1q and collagen domain containing), IL (Interleukin), TNF-α (Tumour necrosis factor-α) [30], BDNF (Brain-derived neurotrophic factor) [31], ADRB2 (Beta2-adrenergic receptor) [32], ADCY3 (Adenylate cyclase) [27], PPAR-γ (Peroxisome proliferator-activated receptor-gamma) [33], PCSK1 (Proprotein convertase subtilisine/kexin type 1) [34], VDR (Vitamin D receptor) [35], TMEM18 (Transmembrane protein 18) [36], and CLOCK (Circadian locomotor output cycles kaput) [37, 38•]. Among the hundreds of obesity-associated genes, FTO has been identified as the most robust predictor of polygenic obesity [21••].
These obesity-associated genes have been very important in elucidating physiological mechanisms. However, the same polymorphisms in the same gene can even show differences between the genders in the same population. Such as risk variants in FTO, a gene known to be involved in appetite control and eating behavior, is linked to a higher risk of obesity due to increased food intake and greater preference for foods with high energy and fat in children and adults but not in older adults [39, 40•]. Similarly, the LIPC (Hepatic lipase) gene C-514 T polymorphism among Chinese children was associated with higher body mass index (BMI) levels in boys, and it was found to protect against higher BMI levels in girls [41].
With the increasing number of studies and advanced technological developments, the identification of obesity-associated candidate genes is growing rapidly. Since genomic data is large and complex, analytical tools such as machine learning algorithms and deep learning methods are required to reveal unexpected genomic relationships, derive hypotheses and new models, and make predictions. Machine learning algorithms are suitable for data-driven sciences such as genomics, as they are designed to automatically detect patterns in data [42]. Associations of some SNPs with obesity from meta-analyses of case-control studies are shown in Table 1. Given the pivotal role of genetic information in precision nutrition at level 3, researchers have undertaken precision nutrition studies that integrate some of these specific genes and related SNPs to personalize interventions. This emphasis is exemplified by studies summarized in Table 3, where the utilization of SNPs emerges as a prominent strategy for personalized interventions. The likelihood that genetic data may soon be utilized to determine people with a higher risk of obesity is increasing as more variations for obesity are uncovered. Unfolding if there is a genetic vulnerability, would give the chance to act earlier to prevent obesity [21••].
Precision Nutrition for Obesity
Low-calorie diets with different macronutrient compositions, such as low-fat, low-carbohydrate, or high protein, has been investigated for years to find optimal diet strategy for weight loss. Across all dietary interventions, significant variability in weight loss is observed [54]. Lately, obesity studies’ focus has shifted from modeling the metabolic effects of macro- and micronutrients to meal frequency and timing (intermittent fasting), eating window (early or late time-restricted eating), sleep quantity and quality, postprandial responses through circadian rhythms, and genetic variants to overcome the interindividual differences in weight loss [55, 56••, 57•, 58]. If more objective and precise data is collected from individuals, the basis of interindividual differences can be understood, and thereby tailored recommendations can be offered for weight loss optimization, weight regain prevention, and long-term weight loss maintenance (Fig. 2). However, it's essential to ensure that these recommendations align with the individual food preferences, economic and social circumstances, food accessibility, culinary expertise, and other relevant considerations [54, 55, 56••].
Interindividual variability in response to same dietary pattern, and lifestyle habits. The figure was modified with text and after adaptation of images from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com)
Personalized Data Collection and Analysis
Precision nutrition requires an in-depth quantitative level of information from genetics and digital health profiles using technology-based assessment methods for dietary intake, physical activity (e.g. Fitbit, Garmin, Polar), flash (e.g. Abbott FreeStyle Libre), or real-time blood glucose levels (e.g. Nintamed Dexon G6, Medtronic Guardian Connect, Senseonics Eversense), blood pressure (e.g. Qardio Arm, Omron RS8, HeartGuide, CNAP2GO), heart health (e.g. Qardio Chest, iHeart) and sleep (e.g. ActiGraph, Fitbit, Garmin, Fatigue Science) [59, 60, 61•, 62,63,64]. Many physiological markers can now be monitored for patients outside of clinics thanks to advancements in wearable and non-invasive sensors. The Novel Coronavirus Disease (COVID-19) epidemic's rules requiring social distance and home isolation have once again highlighted the significance of telehealth programs that provide home screening, diagnosis, and monitoring. Improving patient monitoring with these methods can help alleviate the overcrowding of hospitals and the increasing workload of healthcare professionals, which are among the biggest issues of the healthcare system.
Dietary Assessment
Dietary history, food record, 24-h dietary recall, and food frequency questionnaire (FFQ), subjective dietary assessment methods, are widely used in human nutritional studies [65]. However, these methods are all to some extent subjective and in general are biased due to misreporting or underreporting of dietary intake and is affected by individual characteristics such as age, gender, and BMI [66, 67]. While adults with overweight or obesity tend to under-report, adults with underweight tend to over-report [66]. Additionally, the reliability of information obtained from the elderly may decrease with increasing age of the respondent due to cognitive impairments (memory loss, decreased cognitive function, common neurological diseases such as dementia), reductions in functional capacity (problems with vision, hearing, and writing), reduced or absent participation in food-related processes [67]. Also, accurate dietary assessment is challenging in children due to the lack or limited literacy and writing skills, limited food recognition and portion size estimation skills, memory limitations, and short focusing time [68]. Therefore, both in clinical and non-clinical settings, more objective dietary measurements are needed for the implementation of precision nutrition across all age groups [69]. Web-based, image-assisted, and image-based dietary assessment methods may be more useful than traditional dietary assessment methods for patient monitoring outside clinical settings, as they offer remote access. Furthermore, image-assisted and image-based methods may prevent misreporting [70].
Web-based Dietary Assessment
The increase in internet use in recent years has popularized web-based dietary methods for both research and individual purposes. While most of the web-based dietary assessment tools developed are based on 24-h dietary recall and FFQ, some are based on food records or diaries. These methods can be supplemented with images to improve the estimation of portion size. They provide a cost-effective collection of data in large-sample studies [71]. It has been shown that web-based tools such as FIRRSt (Food Intake Recording Software System), and INTAKE24, for measuring dietary intake in children are often more engaging than traditional, paper-based methods and keep the child focused. Therefore, it may be beneficial to prefer web-based methods for assessing nutritional status, especially in children [72]. Although childhood and adolescence are important periods for the development of obesity, few studies have focused on these periods [73].
Image-assisted Dietary Assessment
Image-assisted dietary assessment refers to any method that uses images or videos of mealtimes to improve the reliability of traditional methods. It is a new technology that uses food recognition, segmentation, classification, volume estimation, and deep learning techniques via mobile phones, depth cameras, RGB (red-green-blue) cameras, wearable sensors, or smart glasses [74]. Images can be captured in two different approaches, active and passive [70]. The active approach requires individuals to take images with a handheld device such as a digital camera or smartphone. Images are usually taken before and after meals to distinguish the leftovers, holding the device at an angle of about 45°. A reference marker of known dimensions and color is placed on each image. Images are often supplemented with additional text or audio recordings describing the foods [75•]. The passive approach does not depend on users capturing images of meals. It requires wearable cameras to automatically capture images of everyday events, including mealtimes. However, the captured images are not only for mealtimes, and this may not be accepted by everyone due to privacy concerns [70].
Image-based Dietary Assessment
While image-assisted methods are used to assist traditional dietary assessment methods or to remember the foods consumed, image-based methods are the primary recording method of dietary assessment. Images can be actively captured by the individual using a camera or smartphone, or passively recorded by a wearable device, as in image-assisted methods [70].
Dietary Monitoring Techniques
Motivating people to alter their lifestyle, including eating habits, is the largest barrier in improving any behavior. By continuously monitoring individuals outside of the clinics, these monitoring techniques can boost motivation and compliance (Fig. 3). There is a growing interest in smartphone-based self-monitoring apps such as My Meal Mate and My Fitness Pal. Although these apps sometimes lack evidence-based content, potentially limiting their efficacy, they can also provide rapid and personalized awareness through direct feedback [76]. The biggest advantage of monitoring via smartphone is that all the computing power required for data processing, hardware such as a camera, display for test results, a power supply, and most of the required auxiliary support is already available in an owned smartphone [77].
Precision nutrition can be considered to occur at three levels: (1) conventional diet based on individuals’ age, gender, and other determinants, (2) phenotypic information such as anthropometry, biochemical and metabolic markers, (3) genotypic information based on omics technologies. The figure was modified with text and after adaptation of images from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com)
As dietary assessment methods evolve, so does the demand for comprehensive and precise techniques to monitor dietary intake. Although some are only in the project phase for now, smart plate [78], smart dining tray [79], wearable sensors such as wrist-worn devices (e. g. smartwatch-based eating detection) for monitoring energy intake [80], smart fork for monitoring eating rate [81], or smart water bottle for monitoring water intake are other novel monitoring techniques [82]. Moreover, with technological advances, ingestible sensors are being developed that can measure parameters such as pH, temperature, pressure, gas, microbial population, and digestion and fermentation metabolites [83].
Biomarkers of Food Intakes
Metabolomics aims to determine the metabolome, considered a chemical representation of a biological phenotype. Blood (plasma or serum), saliva, urine, faces, tissue, exhaled breath, hair, and nail are frequently used biological samples in metabolomics studies [84, 85]. However, there are differences between using urine and blood (plasma or serum) as biological samples. Urine collection is less expensive and more practical than blood collection, enables quick collection of large quantities, and is less invasive for the subject. More than 4000 metabolites have been detected in the urine, although not all of them are related to dietary intake [86]. Urine is rich in non-nutrient bioactive compounds such as phytochemicals and their metabolites, and most of these metabolites are excreted rapidly. Blood, on the other hand, has a higher concentration of metabolically active chemicals, and fat-soluble metabolites are only found in plasma. Moreover, urinary biomarkers are considered short-term biomarkers of food intake (BFIs) [87]. Urinary naringenin and hesperetin are examples of short-term biomarkers for citrus fruits especially, orange juice intake [88]. As an exception, Saenger et al. showed that urinary proline betaine is traceable for at least 72 h after orange juice intake, and it is identified as a relatively long-term biomarker [89].
Table 2 summarizes some of the potential biomarkers of food intake from human studies. According to the scoring system developed by Rafiq et al. 69 metabolites were identified from 11 food-specific categories or dietary patterns that scored ≥ 5 points pointing to good evidence as a potential BFI. Through BFIs, information can be obtained not only on specific foods but also on dietary patterns. For example, it has been shown that 24-h urinary N-(4-methyl-5-oxo-1-imidazolin-2-yl)sarcosine (MG-HCr) excretion differs in individuals with different dietary habits such as omnivores (0.39 − 9.67 μmol/day), vegetarians (0.18 − 0.97 μmol/day), and vegans (0.10 − 0.27 μmol/day) [90]. Treibmann et al. suggested that urinary MG-HCr could be used as a short-term biomarker for heat-treated animal food intake [91].
Most food-specific metabolites are detected in human blood and urine for 5 to 10 h, with others detected for up to 48 h. It is recommended to use a model of at least 24–48 h in which several biological samples are obtained to assess variations in metabolite concentration over time or to produce an average value representative of the genuine concentration [90]. In general, to assess potential BFIs, two different metabolomic techniques are used: (a) mass spectrometry (MS) combined with gas (GC-MS, gas spectrum-MS) or liquid phase chromatography (UPLC-qTOF-MS, ultra-high-performance liquid chromatography-quadrupole time-of-flight-MS), and (b) proton nuclear magnetic resonance (1H-NMR) spectroscopy [88]. Although 1H-NMR-based techniques are non-destructive and offer greater reproducibility, and less inter-laboratory variance, these techniques have a lower sensitivity compared to MS-based approaches [113]. Thereby, NMR is best at detecting high amounts of metabolites, while GC–MS and UPLC-qTOF-MS are best at low amounts [114•]. Otherwise, the efficacy of BFIs may vary depending on the diurnal variance, the day of the week, or the season of sample collection. Sample collection procedures, transportation, processing, storage settings, and storage period of acquired biological samples are also effective aspects in determining the efficacy of possible BFIs [115].
Cheung et al. showed urinary and plasma trimethylamine N-oxide (TMAO) as a potential biomarker for fish intake in a controlled dietary intervention study [92]. Yin et al. reported that although urinary TMAO showed a strong dose-response relationship with fish consumption (r = 0.148, p < 0.01), the use of urinary TMAO alone to determine fish intake in a free-living population was insufficient [116]. Combined use of BFIs should also be considered, as the combinations of BFIs may be more effective in predicting food intake rather than using one biomarker alone [117]. Databases of dietary phytochemicals and their metabolites in humans such as Phenol-Explorer, PhytoHub, and FooDB, and a novel inventory of BFIs in blood, urine, or other tissues/biofluids called Food Biomarker Alliance (FoodBAll) have been developed. Since, precision nutrition cannot deliver on its promises unless there is an accurate dietary assessment, databases containing many foods and their metabolites in humans should be developed in different populations to expand the use of metabolomics to identify BFIs in dietary assessment [114•].
Precision nutrition approaches require a thorough comprehension of how genetic-metabotype-diet interactions influence dietary biomarker levels. Genetic variations, such as SNPs, can significantly impact metabolic differences and influence dietary requirements and responses to various diets. Considering these interactions, BFIs may potentially play an important role in precision nutrition interventions by providing objective and accurate measurement of dietary habits and nutrient intake. With the identification of specific BFIs, individual responses to dietary interventions can be predicted, thereby enhancing the effectiveness of precision nutrition interventions, and leading to improved health outcomes [118]. Nonetheless, the limited utilization of BFIs into precision nutrition studies indicates a gap that requires further exploration and integration into future research endeavors, emphasizing the challenges associated with their incorporation [119•]. While there is ongoing debate regarding whether omics technology is superior to traditional biochemical markers in guiding precision nutritional assessment, the most critical challenge lies in determining which genetic variants or potential BFIs to prioritize [120]. Although BFIs provide more objective results than traditional nutritional assessment methods, the metabolomics approach has some limitations such as being more expensive and invasive, not representing the usual long-term intake, may not be sensitive or specific to the relevant food or nutrient intakes, may not be reproducible, variations may exist between ethnic groups and the most importantly, there are still no valid biomarkers for every food and nutrients [87, 121]. Therefore, future metabolomics research should concentrate on discovering novel biomarkers of specific food intake and validating these candidate biomarkers against eight validity criteria: dose-response, time-response, plausibility, reproducibility, reliability, robustness, analytical performance, and stability, and overcoming existing barriers to their application in precision nutrition studies [122].
Microbiome-based Analysis
The ratio of human to bacterial cells within the human body is approximately 1:1, these microbial members can encode 150 times more genes than the human genome. Microbial DNA can be passed from parent to child, thus making the microbiome our "second genome" [123]. Diet is a significant modifiable factor that influences the composition of the human gut microbiota, and its modulation with prebiotics, probiotics, and postbiotics, are well-known strategies for improving health. Since, obesity results from persistent positive energy balance due to increased energy intake and/or decreased energy expenditure, traditional weight-loss therapies have relied on both hypocaloric diets and increased physical activity. Yet, the genetic and/or epigenetic structure of each individual and the uniqueness of energy metabolism controlled by mechanisms related to the microbiota are mostly ignored. Findings suggest that gut microbiota profile might contribute to explaining some of these interindividual differences to the same diet for weight loss [124]. Adults with overweight or obesity and prediabetes participating in the large multicenter clinical research, baseline gut microbiota characteristics could explain roughly 25% of the change in total body fat, after following 8 weeks of a low-energy diet providing 800 kcal to 1200 kcal per day for weight loss [125•].
Gut dysbiosis, which particularly causes short-chain fatty acid (SCFA)-producing bacteria depletion, is associated with the etiology of various diseases by inducing inflammation and disrupting gut integrity and function [126•]. In addition, Canfora et al. found that rectal SCFA mixtures including acetate, propionate, and butyrate administration decreased lipolysis and increased fatty acid oxidation, energy expenditure, and plasma peptide YY concentration in normoglycemic participants with overweight and obesity [127]. O'Grady and Shanan, highlighted that more attention needs to be paid to the details of food-microbe interactions for precision nutrition to deliver on its promise [128].
The week-long continuous monitoring of glucose levels in an 800-person cohort, along with measurement of responses to nearly 47,000 meals, revealed significant variability in response to identical meals, suggesting that the effectiveness of general dietary recommendations may be limited. Furthermore, a machine-learning algorithm was developed that accurately predicted the postprandial glycemic responses, integrating blood parameters, dietary habits, anthropometrics, physical activity, and gut microbiota from this cohort [129••]. Similarly, great interindividual variability was observed in the postprandial responses of blood glucose, triglycerides, and insulin following the same meals in the Personalised REsponses to DIetary Composition Trial (PREDICT) 1, which included over 1,000 healthy adults in the UK. Importantly, individual factors, such as the deep metagenomic sequencing of gut microbiomes, have been shown to have a greater impact on metabolic responses than meal macronutrients for postprandial lipemia, while for glucose, meal macronutrient composition explained the greatest proportion of variance in response. Using multi-omics data with the PREDICT 1, a machine-learning model was developed that predicts both postprandial glycemic and triglyceride responses. Also, Berry et al. reported that the gut microbiome deep metagenomic sequencing can be used to categorize generalizable health levels in individuals even without clinically evident disease [130].
The Diet Intervention Examining the Factors Interacting with Treatment Success (DIETFITS) trial, which included 609 adults with overweight or obesity in the U.S. and randomly assigned participants to either a low-carbohydrate or low-fat diet for 12 months, showed that the weight change between groups did not differ significantly. No associations were found between genotype patterns or baseline insulin secretion and the dietary effects on weight loss. Moreover, among the 263 proteins examined at baseline, only FGF-21 (Fibroblast growth factor-21) was identified as a predictor of weight loss, with none contributing to personalized dietary recommendations [131, 132]. While the PREDICT 1 and DIETFITS investigate the relationship between dietary response and genetic differences, the NOW (Nutrigenomics, Overweight/Obesity and Weight Management), POUNDS LOST (Prevention of Obesity Using Novel Dietary Strategies), and Food4Me (Proof of Principle) trials show the relationship between diet adherence and genetic differences [132,133,134,135].
Chen et al. reported that diet and gut microbiome play a more dominant role than genetics in explaining interindividual variability in metabolism by assessing 1,183 plasma metabolites. They emphasized that there is a significant correlation between diet quality predicted by using a machine learning-based prediction model through an individual's plasma metabolome, and the diet quality predicted by the FFQ [136•]. With omics technology, the gut microbiome can be used as a predictive indicator of interindividual variability in response to the same dietary pattern. As a result, identifying the microbiomics involved in altering human gut homeostasis, with a new sequencing approach revealing possible microbial genes, might open the way to the development of tailored treatments [137].
On the contrary, inaccurate results can be achieved if the wrong variants are selected [120, 132]. The likelihood of getting false positives is enhanced by considerable interindividual variation in microbiota composition and population-wide variances in human lifestyle [138]. Although these clinical trials have offered some clues for future studies, available data in the microbiomics field remain limited. Regarding the type of microbiome data employed, most studies have relied on 16S rRNA gene sequencing data for microbial profiling. Despite the growing accessibility of metagenomic data from whole-genome sequencing, its application in precision nutrition trials is not yet widespread [137, 139]. Biesiekierski et al. suggested that there is still insufficient evidence to support baseline microbiota as a reliable predictor of body weight and weight loss [140]. A major challenge is a limited concordance between research studying the role of the microbiota in obesity pathophysiology, limiting the ability to determine causal links between microorganisms and body weight. Since finding or selecting the right variants is subtle, variant calling algorithms with large-sample cohorts are necessary to match obesity gene mapping.
Tailored Interventions for Weight Loss
By objective and real-time (or continuous) monitoring of individuals’ dietary intake, physical activity, physiological parameters (heart rate, blood glucose levels, sleep), or gut microbiota composition, precision nutrition has immense potential to improve the effectiveness of obesity management [141]. Previous studies assessing personalized interventions or monitoring through wearable sensors in motivating people to change their diet or lifestyle behaviors have been inconsistent. The result of the Food4Me study, which included 1,279 adults across seven European countries, indicates that personalized nutrition advice increased diet quality. Although receiving personalized nutrition advice resulted in a decrease in red meat, saturated fat, and salt consumption and an increase in folate intake, knowledge of MTHFR genotype did not significantly improve dietary folate intakes [133, 142, 143]. Hietaranta-Luoma et al. reported that genotype-based recommendations resulted in greater improvement in dietary fat quality in the ApoE Ɛ4+ group than in the ApoE Ɛ4- group or controls [144].
de Hoogh et al. reported that dietary intake of energy, carbohydrates, sugar, total fat, saturated fat, and polyunsaturated fatty acids decreased after personalized nutrition intervention. Additionally, participants’ BMI, body fat, and hip circumference; total cholesterol and low-density lipoprotein(LDL)-cholesterol levels decreased [145]. A meta-analysis of randomized controlled trials found that consumer wearable activity trackers-based interventions significantly increased physical activity while significantly decreasing waist circumference, LDL-cholesterol level, and systolic blood pressure in individuals with chronic diseases [146]. By contrast, a meta-analysis of randomized controlled trials, including vignette studies, revealed that genetic risk communication had no appreciable impact on the motivation to lose weight or on dietary or physical activity changes [147]. Similarly, another meta-analysis evaluating the effect of genotype-based dietary or physical activity advice in changing behavior also showed no significant difference from traditional advice [148]. Moreover, among European adults from Food4Me study, no significant physical activity changes were found during the 6-month intervention after disclosing FTO risk [149].
A meta-analysis evaluating nutrigenetic studies has shown that the Mediterranean diet can have a protective role against obesity, type 2 diabetes, and metabolic syndrome [150]. The long-term adherence to the Mediterranean diet may potentially lead to the mitigation of specific genes' obesogenic effects, although this assertion remains speculative. Analytical methods in nutrigenetics and nutrigenomics have used genetic risk score algorithms to capture the effects of genes and nutrients or dietary patterns on disease susceptibility. Examples of this modeling approach have been shown to have a higher risk of developing obesity among individuals at high genetic risk scores (having ≥ 7 SNPs) following a Western diet. In addition, it has been shown that dietary fiber and vegetable protein intake have protective effects, especially in low-risk individuals (< 7 SNPs) [151]. Thanks to recent advances in genomics, metabolomics, and the gut microbiome, as well as wearable technologies, precision nutrition holds out solutions to obesity [152]. Through converting genomics data into molecular phenotypes with multi-omics technology, long-term adjustments can be achieved. Further along the road, it might even be possible to establish recommended daily intake or safe upper limits for subgroups based on the individual’s genomic risk profile, as well as design appropriate screening and follow-up tools, to assess and monitor nutritional status and interindividual response to dietary intervention [6, 39].
The randomized controlled trials on weight loss with precision nutrition approaches in overweight and obese individuals are provided in Table 3. Among the five identified studies, each trial employed varying levels of personalization, including dietary recommendations based on body weight, phenotype, and genotype. In total, analyses of 5, 35, 95, and 10 SNPs were conducted as part of the genotypic information aspect by Celis-Morales et al. [153], Aldubayan et al. [155••], Cuevas-Sierra et al. [156•], and Höchsmann et al. [157], respectively. Some of these obesity-associated SNPs (IL-6 rs1800795, MTHFR rs1801133, PPARG rs1801282, FABP2 rs1799883, ADRB3 rs4994, UCP2 rs659366, FTO rs9939609, and MC4R rs17782313) had previously been presented in Table 1. Notably, the study by Celis-Morales et al. demonstrated a significant reduction in body weight among FTO risk carriers in the genetic group compared to the control group, emphasizing the potential efficacy of genotype-based interventions [153]. However, other trials did not report significant differences in weight loss percentage or other anthropometric measurements across different levels of personalization [154•, 155••, 156•, 157]. These diverse findings underscore the complexity of precision nutrition interventions for weight loss and highlight the need for further randomized controlled trials to elucidate optimal strategies for tailored dietary recommendations.
Challenges for Clinical Use and Future Prospects
While precision medicine can currently be applied for predictive genomics in specific monogenic disorders such as cystic fibrosis, the integration of precision nutrition into healthcare systems is still in its early stages and has not yet been implemented [158]. It raises economic, legal, ethical, and social issues, including the participation of children, and the collection and storage of biological samples [159]. In a study aiming at assessing the perceptions of nutritional genomics by hospital-based registered dietitians or nutritionists, cost and ethics were identified as two major aspects that must be addressed before integrating gene-based recommendations into clinical practice [160•]. We believe that registered dietitians or nutritionists should be equipped to provide nutritional advice based on genetics with continuous learning.
The prevalence of analyzed SNPs varies considerably among different ethnic backgrounds, and the relationship with the identified phenotype cannot always be confirmed in populations with different ethnicities, limiting the application of nutrigenetics [19]. To strengthen the scientific aspect of precision nutrition, risk mapping, reliable polymorphism banks, clinically applicable epigenetic assessment, and the development of new technologies (bioinformatics) for the identification and analysis of valid, specific, and sensitive biomarkers including BFIs are required [6•]. Also, companies must meet certain standards before genetic testing can be commercialized. Data from large population-based epidemiological studies are needed to establish reliable gene banks for different genders, age groups, and ethnicities, and to better understand the obesity-gene relationship [158]. A notable example of this is the UK Biobank, a prospective cohort study involving more than 450,000 participants [161].
Conclusion
Although different nutritional recommendations and dietary guidelines were developed for different nations, different age groups (infants, older adults), or special groups (athletes, vegetarians, or those with chronic diseases), these recommendations do not consider the wide interindividual differences. While precision nutrition offers a highly personalized perspective, the number of studies conducted so far is quite limited, and there are studies indicating no superiority over traditional dietary recommendations. Despite recent technological advancements enabling the detection of obesity-related genetic polymorphisms and the prediction of postprandial metabolic responses, the integration of precision nutrition into routine clinical practice requires further validation and refinement. It appears imperative to consider exploring the integration of various 'omics' technologies, such as genomics, metabolomics, and microbiomics, along with objective dietary assessment data, for the potential enhancement of future predictive models of individual responses to diet. Moreover, precision nutrition practices might exacerbate health disparities, as only a small percentage of the population will have access to them. Therefore, as healthcare professionals, we must not lose sight of improving the overall public health and not ignore the impact of population-based effective policies and regulations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• WHO European Regional Obesity Report 2022. Copenhagen: World Health Organization Regional Office for Europe. 2022. This report provides comprehensive data on obesity trends in the European region, highlighting the critical need for policy interventions and public health strategies to address the rising obesity rates.
•• Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the american college of cardiology/american heart association task force on practice guidelines and the obesity society. Circulation. 2013;129(25_suppl_2):S102-S38. https://doi.org/10.1161/01.cir.0000437739.71477.ee. This guideline serves as a comprehensive reference point for healthcare professionals, offering evidence-based recommendations to guide the effective treatment strategies for overweight and obesity in adult populations.
Bush CL, Blumberg JB, El-Sohemy A, Minich DM, Ordovás JM, Reed DG, et al. Toward the definition of personalized nutrition: a proposal by the American Nutrition Association. J Am Coll Nutr. 2020;39(1):5–15. https://doi.org/10.1080/07315724.2019.1685332.
Chaudhary N, Kumar V, Sangwan P, Pant NC, Saxena A, Joshi S, et al. Personalized nutrition and-omics. Comprehensive Foodomics. 2021:495. https://doi.org/10.1016/B978-0-08-100596-5.22880-1.
Tebani A, Bekri S. Paving the way to precision nutrition through metabolomics. Front Nutr. 2019;6:41. https://doi.org/10.3389/fnut.2019.00041.
• Ferguson LR, De Caterina R, Görman U, Allayee H, Kohlmeier M, Prasad C, et al. Guide and position of the international society of nutrigenetics/nutrigenomics on personalised nutrition: part 1-fields of precision nutrition. Lifestyle Genom. 2016;9(1):12–27. https://doi.org/10.1159/000445350. This review highlightes the endeavors, achievements, progress, and challenges of precision nutrition.
Bouchard C, Ordovas JM. Fundamentals of nutrigenetics and nutrigenomics. Prog Mol Biol Transl Sci. 2012;108:1–15. https://doi.org/10.1016/B978-0-12-398397-8.00001-0.
Pavlidis C, Nebel J-C, Katsila T, Patrinos GP. Nutrigenomics 2.0: The need for ongoing and independent evaluation and synthesis of commercial nutrigenomics tests’ scientific knowledge base for responsible innovation. Omics J Integr Biol. 2016;20(2):65–8. https://doi.org/10.1089/omi.2015.0170.
Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19(5):299–310. https://doi.org/10.1038/nrg.2018.4.
Di Renzo L, Gualtieri P, Romano L, Marrone G, Noce A, Pujia A, et al. Role of personalized nutrition in chronic-degenerative diseases. Nutrients. 2019;11(8):1707. https://doi.org/10.3390/nu11081707.
Palmnäs M, Brunius C, Shi L, Rostgaard-Hansen A, Torres NE, González-Domínguez R, et al. Perspective: metabotyping—a potential personalized nutrition strategy for precision prevention of cardiometabolic disease. Adv Nutr. 2020;11(3):524–32. https://doi.org/10.1093/advances/nmz121.
• Pigsborg K, Magkos F. Metabotyping for precision nutrition and weight management: hype or hope? Curr Nutr Rep. 2022;11(2):117–23. https://doi.org/10.1007/s13668-021-00392-y. This review provides up-to-date information in identifying metabotypes that predict weight loss outcomes in response to diet.
•• Hillesheim E, Yin X, Sundaramoorthy GP, Brennan L. Using a metabotype framework to deliver personalised nutrition improves dietary quality and metabolic health parameters: A 12‐week randomised controlled trial. Mol Nutr Food Res. 2023;2200620. https://doi.org/10.1002/mnfr.202200620. This study reported that personalized nutrition is significantly associated with higher diet quality and metabolic health parameters.
Rodgers GP, Collins FS. Precision nutrition—the answer to “what to eat to stay healthy”. JAMA. 2020;324(8):735–6. https://doi.org/10.1001/jama.2020.13601.
Reddy VS, Palika R, Ismail A, Pullakhandam R, Reddy GB. Nutrigenomics: Opportunities & challenges for public health nutrition. Indian J Med Res. 2018;148(5):632. https://doi.org/10.4103/ijmr.IJMR_1738_18.
Spanakis E, Day IN. The molecular basis of genetic variation: mutation detection methodologies and limitations. Genetics of Common Diseases: Garland Science. 2020;33–74. https://doi.org/10.1201/9781003076810-3.
Karki R, Pandya D, Elston RC, Ferlini C. Defining, “mutation” and “polymorphism” in the era of personal genomics. BMC Med Genomics. 2015;8(1):1–7. https://doi.org/10.1186/s12920-015-0115-z.
Mullins VA, Bresette W, Johnstone L, Hallmark B, Chilton FH. Genomics in personalized nutrition: Can you “eat for your genes?". Nutrients. 2020;12(10):3118. https://doi.org/10.3390/nu12103118.
Barrea L, Annunziata G, Bordoni L, Muscogiuri G, Colao A, Savastano S. Nutrigenetics—personalized nutrition in obesity and cardiovascular diseases. Int J Obes Suppl. 2020;10(1):1–13. https://doi.org/10.1038/s41367-020-0014-4.
•• Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587–96. e9. https://doi.org/10.1016/j.cell.2019.03.028. This study provided evidence for the significance of polygenic scores on weight, which appear early in life and increase as people age.
•• Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23(2):120–33. https://doi.org/10.1038/s41576-021-00414-z. This paper provides a comprehensive summary of monogenic and polygenic obesity, and GWAS results.
Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–84. https://doi.org/10.1038/s41576-019-0127-1.
da Silva TER, Andrade NL, de Oliveira CD, Leão-Cordeiro JAB, Vilanova-Costa CAST, Silva AMTC. The FTO rs9939609 polymorphism and obesity risk in teens: Evidence-based meta-analysis. Obes Res Clin Pract. 2018;12(5):432–7. https://doi.org/10.1016/j.orcp.2018.08.001.
Doaei S, Jarrahi SM, Moghadam AS, Akbari M, Kooshesh SJ, Badeli M, et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis. Biomol Concepts. 2019;10(1):237–42. https://doi.org/10.1515/bmc-2019-0025.
• Najd-Hassan-Bonab L, Safarpour M, Moazzam-Jazi M, Azizi F, Daneshpour MS. The role of FTO variant rs1421085 in the relationship with obesity: a systematic review and meta-analysis. Eat Weight Disord. 2022;27(8):3053–62. https://doi.org/10.1007/s40519-022-01509-0. This systematic review and meta-analysis investigates the association between theFTO gene variant rs1421085 and obesity, providing valuable insights into genetic factors contributing to obesity and potential targets for intervention.
Quan L, Wang H, Tian Y, Mu X, Zhang Y, Tao K. Association of fat-mass and obesity-associated gene FTO rs9939609 polymorphism with the risk of obesity among children and adolescents: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19(4):614–23.
Felix JF, Bradfield JP, Monnereau C, van der Valk RJ, Stergiakouli E, Chesi A, et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum Mol Genet. 2016;25(2):389–403. https://doi.org/10.1093/hmg/ddv472.
Yu K, Li L, Zhang L, Guo L, Wang C. Association between MC4R rs17782313 genotype and obesity: A meta-analysis. Gene. 2020;733:144372. https://doi.org/10.1016/j.gene.2020.144372.
Fu L, Li YN, Luo D, Deng S, Hu YQ. Plausible relationship between homocysteine and obesity risk via MTHFR gene: a meta-analysis of 38,317 individuals implementing Mendelian randomization. Diabetes Metab Syndr Obes. 2019;12:1201–12. https://doi.org/10.2147/DMSO.S205379.
Yu Z, Han S, Cao X, Zhu C, Wang X, Guo X. Genetic polymorphisms in adipokine genes and the risk of obesity: a systematic review and meta-analysis. Obesity (Silver Spring). 2012;20(2):396–406. https://doi.org/10.1038/oby.2011.148.
Akbarian SA, Salehi-Abargouei A, Pourmasoumi M, Kelishadi R, Nikpour P, Heidari-Beni M. Association of Brain-derived neurotrophic factor gene polymorphisms with body mass index: A systematic review and meta-analysis. Adv Med Sci. 2018;63(1):43–56. https://doi.org/10.1016/j.advms.2017.07.002.
Zhang H, Wu J, Yu L. Association of Gln27Glu and Arg16Gly polymorphisms in Beta2-adrenergic receptor gene with obesity susceptibility: a meta-analysis. PLoS ONE. 2014;9(6):e100489. https://doi.org/10.1371/journal.pone.0100489.
Yao Y-S, Li J, Jin Y-L, Chen Y, He L-P. Association between PPAR-γ2 Pro12Ala polymorphism and obesity: a meta-analysis. Mol Biol Rep. 2015;42(6):1029–38. https://doi.org/10.1007/s11033-014-3838-6.
Stijnen P, Tuand K, Varga TV, Franks PW, Aertgeerts B, Creemers JW. The association of common variants in PCSK1 with obesity: a HuGE review and meta-analysis. Am J Epidemiol. 2014;180(11):1051–65. https://doi.org/10.1093/aje/kwu237.
Chen X, Wang W, Wang Y, Han X, Gao L. Vitamin D Receptor Polymorphisms Associated with Susceptibility to Obesity: A Meta-Analysis. Med Sci Monit. 2019;25:8297–305. https://doi.org/10.12659/MSM.915678
Pei YF, Zhang L, Liu Y, Li J, Shen H, Liu YZ, et al. Meta-analysis of genome-wide association data identifies novel susceptibility loci for obesity. Hum Mol Genet. 2014;23(3):820–30. https://doi.org/10.1093/hmg/ddt464.
Riestra P, Gebreab SY, Xu R, Khan RJ, Gaye A, Correa A, et al. Circadian CLOCK gene polymorphisms in relation to sleep patterns and obesity in African Americans: findings from the Jackson heart study. BMC Genet. 2017;18(1):58. https://doi.org/10.1186/s12863-017-0522-6.
• Rahati S, Qorbani M, Naghavi A, Nia MH, Pishva H. Association between CLOCK 3111 T/C polymorphism with ghrelin, GLP-1, food timing, sleep and chronotype in overweight and obese Iranian adults. BMC Endocr Disord. 2022;22(1):147. https://doi.org/10.1186/s12902-022-01063-x. This study explores the relationship between the CLOCK 3111 T/C polymorphism and various metabolic and behavioral factors, such as ghrelin, GLP-1 levels, food timing, sleep patterns, and chronotype, in overweight and obese Iranian adults, offering insights into how genetic variations can influence obesity-related traits.
Laddu D, Hauser M. Addressing the nutritional phenotype through personalized nutrition for chronic disease prevention and management. Prog Cardiovasc Dis. 2019;62(1):9–14. https://doi.org/10.1016/j.pcad.2018.12.004.
• Trevisano RG, Gregnani MF, de Azevedo BC, de Almeida SS. The Effect of Association between Fat Mass and Obesity-associated Gene Polymorphism (rs9939609) on the Body Composition of Older People: A Systematic Review. Curr Aging Sci. 2022;15(3):229–41. https://doi.org/10.2174/1874609815666220331090135. This systematic review examines the impact of the FTO gene polymorphism rs9939609 on body composition in older adults, highlighting how genetic factors contribute to variations in body composition in the aging population.
Wang H, Zhang D, Ling J, Lu W, Zhang S, Zhu Y, et al. Gender specific effect of LIPC C-514T polymorphism on obesity and relationship with plasma lipid levels in Chinese children. J Cell Mol Med. 2015;19(9):2296–306. https://doi.org/10.1111/jcmm.12663.
Eraslan G, Avsec Ž, Gagneur J, Theis FJ. Deep learning: new computational modelling techniques for genomics. Nat Rev Genet. 2019;20(7):389–403. https://doi.org/10.1038/s41576-019-0122-6.
Wu J, Liu Z, Meng K, Zhang L. Association of adiponectin gene (ADIPOQ) rs2241766 polymorphism with obesity in adults: a meta-analysis. PLoS ONE. 2014;9(4):e95270. https://doi.org/10.1371/journal.pone.0095270.
Xie C, Hua W, Zhao Y, Rui J, Feng J, Chen Y, et al. The ADRB3 rs4994 polymorphism increases risk of childhood and adolescent overweight/obesity for East Asia’s population: an evidence-based meta-analysis. Adipocyte. 2020;9(1):77–86. https://doi.org/10.1080/21623945.2020.1722549.
Wang R, Zhou D, Xi B, Ge X, Zhu P, Wang B, et al. ENPP1/PC-1 gene K121Q polymorphism is associated with obesity in European adult populations: evidence from a meta-analysis involving 24,324 subjects. Biomed Environ Sci. 2011;24(2):200–6.
Shabana HS. The fatty acid binding protein 2 (FABP2) polymorphism Ala54Thr and obesity in Pakistan: A population based study and a systematic meta-analysis. Gene. 2015;547(1):106–11. https://doi.org/10.1016/j.gene.2015.07.087.
Hu M, Yu Z, Luo D, Zhang H, Li J, Liang F, et al. Association between -174G>C polymorphism in the IL-6 promoter region and the risk of obesity: A meta-analysis. Medicine (Baltimore). 2018;97(33):e11773. https://doi.org/10.1097/MD.0000000000011773.
Wang D, Ma J, Zhang S, Hinney A, Hebebrand J, Wang Y, et al. Association of the MC4R V103I polymorphism with obesity: A Chinese case–control study and meta-analysis in 55,195 individuals. Obesity. 2010;18(3):573–9. https://doi.org/10.1038/oby.2009.268.
Xi B, Chandak GR, Shen Y, Wang Q, Zhou D. Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. 2012. https://doi.org/10.1371/journal.pone.0045731.
Zhu Z, Yang Q, Li C, Chen J, Xiang M, Chen M, et al. Association between the resistin gene-420 C> G polymorphism and obesity: an updated meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20(23):4922–9.
Abd El Daim HA, Elsaid AM, Mousa AA, El-Eshmawy MM, Lashin LS, Toraih EA, et al. Unleash the Association of Mitochondrial Uncoupling Protein (UCP2) Promoter Variant (G-866A; rs659366) with Obesity: Stepping from a Case-Control Study to a Meta-analysis. Biochem Genet. 2020;58(5):738–70. https://doi.org/10.1007/s10528-020-09973-y.
Liu L, Zhao X, Kang S, Zhang D. An association between− 866G/A polymorphism in the promoter of UCP2 and obesity: a meta-analysis. Gene. 2013;514(1):41–7. https://doi.org/10.1016/j.gene.2012.11.001.
Qian L, Xu K, Xu X, Gu R, Liu X, Shan S, et al. UCP2 -866G/A, Ala55Val and UCP3 -55C/T polymorphisms in association with obesity susceptibility - a meta-analysis study. PLoS ONE. 2013;8(4):e58939. https://doi.org/10.1371/journal.pone.0058939.
Kim JY. Optimal Diet Strategies for Weight Loss and Weight Loss Maintenance. J Obes Metab Syndr. 2021;30(1):20–31. https://doi.org/10.7570/jomes20065.
Greenhill C. Towards precision nutrition. Nat Rev Endocrinol. 2020;16(9):473. https://doi.org/10.1038/s41574-020-0385-1
• Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, et al. Calorie restriction with or without time-restricted eating in weight loss. N Eng J Med. 2022;386(16):1495–1504. https://doi.org/10.1056/NEJMoa2114833. This study provides valuable insights into the effectiveness of time-restricted eating compared to daily calorie restriction for weight loss and metabolic health among individuals with obesity.
• Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Pavlou V, Lin S, et al. The effect of 4-h versus 6-h time restricted feeding on sleep quality, duration, insomnia severity and obstructive sleep apnea in adults with obesity. Nutr Health. 2022;28(1):5–11. https://doi.org/10.1177/02601060211002347. This study investigates the impact of two different time-restricted feeding windows (4-hour vs. 6-hour) on sleep parameters in adults with obesity, providing important evidence on how meal timing can influence sleep quality, duration, and the severity of sleep disorders such as insomnia and obstructive sleep apnea.
St-Onge M-P, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–121. https://doi.org/10.1161/CIR.0000000000000476.
Moore JB. From personalised nutrition to precision medicine: the rise of consumer genomics and digital health. Proc Nutr Soc. 2020;79(3):300–10. https://doi.org/10.1017/S0029665120006977.
Spender A, Bullen C, Altmann-Richer L, Cripps J, Duffy R, Falkous C, et al. Wearables and the internet of things: Considerations for the life and health insurance industry. Br Actuar J. 2019;24. https://doi.org/10.1017/S1357321719000072.
• Hajj-Boutros G, Landry-Duval M-A, Comtois AS, Gouspillou G, Karelis AD. Wrist-worn devices for the measurement of heart rate and energy expenditure: A validation study for the Apple Watch 6, Polar Vantage V and Fitbit Sense. Eur J Sport Sci. 2022:1–13. https://doi.org/10.1080/17461391.2021.2023656. This validation study evaluates the accuracy of three popular wrist-worn devices in measuring heart rate and energy expenditure, providing valuable information on the reliability of these devices for health and fitness monitoring.
Fortin J, Rogge DE, Fellner C, Flotzinger D, Grond J, Lerche K, et al. A novel art of continuous noninvasive blood pressure measurement. Nat Commun. 2021;12(1):1387. https://doi.org/10.1038/s41467-021-21271-8.
Blum A. Freestyle libre glucose monitoring system. Clin Diabetes. 2018;36(2):203–4. https://doi.org/10.2337/cd17-0130.
Cappon G, Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G. Wearable continuous glucose monitoring sensors: a revolution in diabetes treatment. Electronics. 2017;6(3):65. https://doi.org/10.3390/electronics6030065.
Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36. https://doi.org/10.4178/epih/e2014009.
Lutomski JE, van den Broeck J, Harrington J, Shiely F, Perry IJ. Sociodemographic, lifestyle, mental health and dietary factors associated with direction of misreporting of energy intake. Public Health Nutr. 2011;14(3):532–41. https://doi.org/10.1017/S1368980010001801.
Shahar DR, Yu B, Houston DK, Kritchevsky SB, Newman AB, Sellmeyer DE, et al. Misreporting of energy intake in the elderly using doubly labeled water to measure total energy expenditure and weight change. J Am Coll Nutr. 2010;29(1):14–24. https://doi.org/10.1080/07315724.2010.10719812.
Livingstone MBE, Robson P, Wallace J. Issues in dietary intake assessment of children and adolescents. Br J Nutr. 2004;92(S2):S213–22. https://doi.org/10.1079/BJN20041169.
Picó C, Serra F, Rodríguez AM, Keijer J, Palou A. Biomarkers of nutrition and health: new tools for new approaches. Nutrients. 2019;11(5):1092. https://doi.org/10.3390/nu11051092.
Boushey C, Spoden M, Zhu F, Delp E, Kerr D. New mobile methods for dietary assessment: review of image-assisted and image-based dietary assessment methods. Proc Nutr Soc. 2017;76(3):283–94. https://doi.org/10.1017/S0029665116002913.
Kirkpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE, Kahle LL, et al. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr. 2014;100(1):233–40. https://doi.org/10.3945/ajcn.114.083238.
Foster E, Adamson A. Challenges involved in measuring intake in early life: focus on methods. Proc Nutr Soc. 2014;73(2):201–9. https://doi.org/10.1017/S0029665114000020.
Wu Y, Perng W, Peterson KE. Precision nutrition and childhood obesity: a scoping review. Metabolites. 2020;10(6):235. https://doi.org/10.3390/metabo10060235.
Eldridge AL, Piernas C, Illner A-K, Gibney MJ, Gurinović MA, De Vries JH, et al. Evaluation of new technology-based tools for dietary intake assessment—an ILSI Europe dietary intake and exposure task force evaluation. Nutrients. 2018;11(1):55. https://doi.org/10.3390/nu11010055.
• Wang W, Min W, Li T, Dong X, Li H, Jiang S. A review on vision-based analysis for automatic dietary assessment. Trends Food Sci Technol. 2022. https://doi.org/10.1016/j.tifs.2022.02.017. This review explores the use of vision-based technologies for automatic dietary assessment, highlighting recent advancements and potential applications in improving dietary tracking accuracy and facilitating better nutritional monitoring and management.
Cade JE. Measuring diet in the 21st century: use of new technologies. Proc Nutr Soc. 2017;76(3):276–82. https://doi.org/10.1017/S0029665116002883.
Srinivasan B, Lee S, Erickson D, Mehta S. Precision nutrition—Review of methods for point-of-care assessment of nutritional status. Curr Opin Biotechnol. 2017;44:103–8. https://doi.org/10.1016/j.copbio.2016.12.001.
Namgung K, Kim T-H, Hong Y-S. Menu recommendation system using smart plates for well-balanced diet habits of young children. Wireless Communications and Mobile Computing. 2019;2019. https://doi.org/10.1155/2019/7971381.
Zhou B, Cheng J, Lukowicz P, Reiss A, Amft O. Monitoring dietary behavior with a smart dining tray. IEEE Pervasive Comput. 2015;14(4):46–56. https://doi.org/10.1109/MPRV.2015.79.
Stankoski S, Jordan M, Gjoreski H, Luštrek M. Smartwatch-based eating detection: Data selection for machine learning from imbalanced data with imperfect labels. Sensors. 2021;21(5):1902. https://doi.org/10.3390/s21051902.
Hermsen S, Hermans RC, editors. Take It Slow: can feedback from a smart fork reduce eating speed. Front Pub Health Conference Proceed. 2016.
Borofsky MS, Dauw CA, York N, Terry C, Lingeman JE. Accuracy of daily fluid intake measurements using a “smart” water bottle. Urolithiasis. 2018;46(4):343–8. https://doi.org/10.1007/s00240-017-1006-x.
Kalantar-Zadeh K, Ha N, Ou JZ, Berean KJ. Ingestible sensors. ACS Sensors. 2017;2(4):468–83. https://doi.org/10.1021/acssensors.7b00045.
Bujak R, Struck-Lewicka W, Markuszewski MJ, Kaliszan R. Metabolomics for laboratory diagnostics. J Pharm Biomed Anal. 2015;113:108–20. https://doi.org/10.1016/j.jpba.2014.12.017.
Rollo ME, Williams RL, Burrows T, Kirkpatrick SI, Bucher T, Collins CE. What Are They Really Eating? A Review on New Approaches to Dietary Intake Assessment and Validation. Curr Nutr Rep. 2016;5(4):307–14. https://doi.org/10.1007/s13668-016-0182-6.
Clarke ED, Rollo ME, Pezdirc K, Collins CE, Haslam RL. Urinary biomarkers of dietary intake: a review. Nutr Rev. 2020;78(5):364–81. https://doi.org/10.1093/nutrit/nuz048.
Guasch-Ferré M, Bhupathiraju SN, Hu FB. Use of metabolomics in improving assessment of dietary intake. Clin Chem. 2018;64(1):82–98. https://doi.org/10.1373/clinchem.2017.272344.
Gibbons H, Michielsen CJ, Rundle M, Frost G, McNulty BA, Nugent AP, et al. Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol Nutr Food Res. 2017;61(10):1700037. https://doi.org/10.1002/mnfr.201700037.
Saenger T, Hübner F, Lindemann V, Ganswind K, Humpf HU. Urinary biomarkers for orange juice consumption. Mol Nutr Food Res. 2021;65(2):2000781. https://doi.org/10.1002/mnfr.202000781.
Rafiq T, Azab SM, Teo KK, Thabane L, Anand SS, Morrison KM, et al. Nutritional metabolomics and the classification of dietary biomarker candidates: a critical review. Adv Nutr. 2021;12(6):2333–57. https://doi.org/10.1093/advances/nmab054.
Treibmann S, Händler S, Hofmann T, Henle T. MG-HCr, the methylglyoxal-derived hydroimidazolone of creatine, a biomarker for the dietary intake of animal source food. J Agric Food Chem. 2020;68(17):4966–72. https://doi.org/10.1021/acs.jafc.0c00907.
Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, et al. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105(3):600–8. https://doi.org/10.3945/ajcn.116.146639.
Yin X, Gibbons H, Rundle M, Frost G, McNulty BA, Nugent AP, et al. Estimation of chicken intake by adults using metabolomics-derived markers. J Nutr. 2017;147(10):1850–7. https://doi.org/10.3945/jn.117.252197.
Giesbertz P, Brandl B, Lee YM, Hauner H, Daniel H, Skurk T. Specificity, dose dependency, and kinetics of markers of chicken and beef intake using targeted quantitative lc-ms/mS: A human intervention trial. Mol Nutr Food Res. 2020;64(5):1900921. https://doi.org/10.1002/mnfr.201900921.
Mitry P, Wawro N, Rohrmann S, Giesbertz P, Daniel H, Linseisen J. Plasma concentrations of anserine, carnosine and pi-methylhistidine as biomarkers of habitual meat consumption. Eur J Clin Nutr. 2019;73(5):692–702. https://doi.org/10.1038/s41430-018-0248-1.
Solvik BS, Øyen J, Kvestad I, Markhus MW, Ueland PM, McCann A, et al. Biomarkers and fatty fish intake: a randomized controlled trial in Norwegian Preschool Children. J Nutr. 2021;151(8):2134–41. https://doi.org/10.1093/jn/nxab112.
Cross AJ, Major JM, Sinha R. Urinary biomarkers of meat consumptionurinary biomarkers of meat consumption. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1107–11. https://doi.org/10.1158/1055-9965.EPI-11-0048.
Lloyd AJ, Fave G, Beckmann M, Lin W, Tailliart K, Xie L, et al. Use of mass spectrometry fingerprinting to identify urinary metabolites after consumption of specific foods. Am J Clin Nutr. 2011;94(4):981–91. https://doi.org/10.3945/ajcn.111.017921.
Fuchsmann P, Tena Stern M, Münger LH, Pimentel GG, Burton KJ, Vionnet N, et al. Nutrivolatilomics of urinary and plasma samples to identify candidate biomarkers after cheese, milk, and soy-based drink intake in healthy humans. J Proteome Res. 2020;19(10):4019–33. https://doi.org/10.1021/acs.jproteome.0c00324.
Ampatzoglou A, Atwal KK, Maidens CM, Williams CL, Ross AB, Thielecke F, et al. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J Nutr. 2015;145(2):215–21. https://doi.org/10.3945/jn.114.202176.
Wierzbicka R, Zamaratskaia G, Kamal-Eldin A, Landberg R. Novel urinary alkylresorcinol metabolites as biomarkers of whole grain intake in free-living Swedish adults. Mol Nutr Food Res. 2017;61(7):1700015. https://doi.org/10.1002/mnfr.201700015.
Edmands WM, Ferrari P, Rothwell JA, Rinaldi S, Slimani N, Barupal DK, et al. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am J Clin Nutr. 2015;102(4):905–13. https://doi.org/10.3945/ajcn.114.101881.
Llorach R, Medina S, García-Viguera C, Zafrilla P, Abellán J, Jauregui O, et al. Discovery of human urinary biomarkers of aronia-citrus juice intake by HPLC-q-TOF-based metabolomic approach. Electrophoresis. 2014;35(11):1599–606. https://doi.org/10.1002/elps.201300565.
Andersen M-BS, Kristensen M, Manach C, Pujos-Guillot G, Poulsen SK, Larsen TM, et al. Discovery and validation of urinary exposure markers for different plant foods by untargeted metabolomics. Anal Bioanal Chem. 2014;406(7):1829–44. https://doi.org/10.1007/s00216-013-7498-5.
Tahiri I, Garro-Aguilar Y, Cayssials V, Achaintre D, Mancini FR, Mahamat-Saleh Y, et al. Urinary flavanone concentrations as biomarkers of dietary flavanone intakes in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2020;123(6):691–8. https://doi.org/10.1017/S0007114519003131.
Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5(3):184–95. https://doi.org/10.1016/S2213-8587(16)30419-3.
Edmands WM, Beckonert OP, Stella C, Campbell A, Lake BG, Lindon JC, et al. Identification of human urinary biomarkers of cruciferous vegetable consumption by metabonomic profiling. J Proteome Res. 2011;10(10):4513–21. https://doi.org/10.1021/pr200326k.
Lafrenière J, Couillard C, Lamarche B, Laramée C, Vohl M-C, Lemieux S. Associations between self-reported vegetable and fruit intake assessed with a new web-based 24-h dietary recall and serum carotenoids in free-living adults: a relative validation study. J Nutr Sci. 2019;8. https://doi.org/10.1017/jns.2019.23.
Schulz M, Hövelmann Y, Hübner F, Humpf H-U. Identification of Potential Urinary Biomarkers for Bell Pepper Intake by HPLC–HRMS-Based Metabolomics and Structure Elucidation by NMR. J Agric Food Chem. 2021;69(45):13644–56. https://doi.org/10.1021/acs.jafc.1c04210.
Hövelmann Y, Lewin L, Steinert K, Hübner F, Humpf HU. Mass Spectrometry-Based Analysis of Urinary Biomarkers for Dietary Tomato Intake. Mol Nutr Food Res. 2020;64(12):2000011. https://doi.org/10.1002/mnfr.202000011.
Rothwell JA, Keski-Rahkonen P, Robinot N, Assi N, Casagrande C, Jenab M, et al. A metabolomic study of biomarkers of habitual coffee intake in four European countries. Mol Nutr Food Res. 2019;63(22):1900659. https://doi.org/10.1002/mnfr.201900659.
Lin X, Racette SB, Ma L, Wallendorf M, Spearie CA, Ostlund RE Jr. Plasma biomarker of dietary phytosterol intake. PLoS ONE. 2015;10(2):e0116912. https://doi.org/10.1371/journal.pone.0116912.
Gibbons H, O’Gorman A, Brennan L. Metabolomics as a tool in nutritional research. Curr Opin Lipidol. 2015;26(1):30–4. https://doi.org/10.1097/MOL.0000000000000140.
• LeVatte M, Keshteli AH, Zarei P, Wishart DS. Applications of metabolomics to precision nutrition. Lifestyle Genomics, 2022, 15(1):1–9. https://doi.org/10.1159/000518489. This article discusses the application of metabolomics in precision nutrition, illustrating how metabolic profiling can be used to tailor dietary recommendations, thereby enhancing the effectiveness of nutritional interventions.
Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125(5):507–25. https://doi.org/10.1007/s00439-009-0662-5.
Yin X, Gibbons H, Rundle M, Frost G, McNulty BA, Nugent AP, et al. The Relationship between Fish Intake and Urinary Trimethylamine-N-Oxide. Mol Nutr Food Res. 2020;64(3):1900799. https://doi.org/10.1002/mnfr.201900799.
Cuparencu C, Rinnan Å, Dragsted LO. Combined markers to assess meat intake—human metabolomic studies of discovery and validation. Mol Nutr Food Res. 2019;63(17):1900106. https://doi.org/10.1002/mnfr.201900106.
Maruvada P, Lampe JW, Wishart DS, Barupal D, Chester DN, Dodd D, et al. Perspective: dietary biomarkers of intake and exposure—exploration with omics approaches. Adv Nutr. 2020;11(2):200–15. https://doi.org/10.1093/advances/nmz075.
• Livingstone KM, Ramos-Lopez O, Perusse L, Kato H, Ordovas JM, Martínez JA. Reprint of: Precision nutrition: A review of current approaches and future endeavors. Trends Food Sci Technol. 2022;130:51–62. https://doi.org/10.1016/j.tifs.2022.08.017. This review provides an overview of the current methodologies and future directions in precision nutrition, focusing on how personalized dietary strategies can be developed using genetic, phenotypic, and lifestyle data to improve individual health outcomes.
Garcia-Bailo B, El-Sohemy A. Recent advances and current controversies in genetic testing for personalized nutrition. Curr Opin Clin Nutr Metab Care. 2021;24(4):289–95. https://doi.org/10.1097/MCO.0000000000000763.
McNamara AE, Brennan L. Potential of food intake biomarkers in nutrition research. Proc Nutr Soc. 2020;79(4):487–97. https://doi.org/10.1017/S0029665120007053.
Dragsted LO, Gao Q, Scalbert A, Vergères G, Kolehmainen M, Manach C, et al. Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes Nutr. 2018;13(1):1–14. https://doi.org/10.1186/s12263-018-0603-9.
Shine EE, Crawford JM. Molecules from the microbiome. Annu Rev Biochem. 2021;90:789–815. https://doi.org/10.1146/annurev-biochem-080320-115307.
San-Cristobal R, Navas-Carretero S, Martínez-González MÁ, Ordovas JM, Martínez JA. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. 2020;16(6):305–20. https://doi.org/10.1038/s41574-020-0346-8.
• Jian C, Silvestre MP, Middleton D, Korpela K, Jalo E, Broderick D, et al. Gut microbiota predicts body fat change following a low-energy diet: a PREVIEW intervention study. Genome Med. 2022;14(1):1–18. https://doi.org/10.1186/s13073-022-01053-7. This study examines the role of gut microbiota in predicting body fat changes in response to a low-energy diet, offering insights into how individual variations in gut bacteria can influence the effectiveness of dietary interventions for weight loss.
• Lubomski M, Xu X, Holmes AJ, Muller S, Yang JY, Davis RL, et al. Nutritional intake and gut microbiome composition predict Parkinson’s disease. Front Aging Neurosci. 2022;10. https://doi.org/10.3389/fnagi.2022.881872. This study explores the relationship between nutritional intake, gut microbiome composition, and the prediction of Parkinson’s disease, highlighting the potential of dietary and microbial biomarkers in early detection and prevention strategies for the disease.
Canfora EE, van der Beek CM, Jocken JW, Goossens GH, Holst JJ, Olde Damink SW, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):1–12. https://doi.org/10.1038/s41598-017-02546-x.
O’Grady J, Shanahan F. Macronutrients, microbiome and precision nutrition. Curr Opin Gastroenterol. 2021;37(2):145–51. https://doi.org/10.1097/MOG.0000000000000705.
•• Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. https://doi.org/10.1016/j.cell.2015.11.001. This study highlights the potential of personalized dietary interventions, informed by machine-learning algorithms and comprehensive data analysis, to effectively manage elevated postprandial blood glucose levels, representing a promising approach for personalized nutrition and metabolic health management.
Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26(6):964–73. https://doi.org/10.1038/s41591-020-0934-0.
Figarska SM, Rigdon J, Ganna A, Elmståhl S, Lind L, Gardner CD, et al. Proteomic profiles before and during weight loss: Results from randomized trial of dietary intervention. Sci Rep. 2020;10(1):1–8. https://doi.org/10.1038/s41598-020-64636-7.
Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JP, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–79. https://doi.org/10.1001/jama.2018.0245.
Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O’Donovan CB, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4me European randomized controlled trial. Int J Epidemiol. 2017;46(2):578–88.
Horne JR, Gilliland JA, O’Connor CP, Seabrook JA, Madill J. Change in Weight, BMI, and Body Composition in a Population-Based Intervention Versus Genetic-Based Intervention: The NOW Trial. Obesity. 2020;28(8):1419–27. https://doi.org/10.1002/oby.22880.
Liu X, Liang J, Geng H, Xu W, Teng F, Yang M. Association of the CDKAL1 polymorphism rs10946398 with type 2 diabetes mellitus in adults: a meta-analysis. Medicine. 2020;99(30). https://doi.org/10.1097/MD.0000000000021383.
• Chen L, Zhernakova DV, Kurilshikov A, Andreu-Sánchez S, Wang D, Augustijn HE, et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat Med. 2022;28(11):2333–43. https://doi.org/10.1038/s41591-022-02014-8. This study investigates how the microbiome, diet, and genetics contribute to individual differences in the human plasma metabolome, offering insights into the complex interactions that influence metabolic profiles and their implications for personalized medicine.
Mills S, Lane JA, Smith GJ, Grimaldi KA, Ross RP, Stanton C. Precision nutrition and the microbiome part II: potential opportunities and pathways to commercialisation. Nutrients. 2019;11(7):1468. https://doi.org/10.3390/nu11071468.
Vujkovic-Cvijin I, Sklar J, Jiang L, Natarajan L, Knight R, Belkaid Y. Host variables confound gut microbiota studies of human disease. Nature. 2020;587(7834):448–54. https://doi.org/10.1038/s41586-020-2881-9.
Ramos-Lopez O, Aranaz P, Riezu-Boj JI, Milagro FI. Application of gut bacterial profiling information in precision nutrition for obesity and weight loss management. Lifestyle Genom. 2024;17(1):22–30. https://doi.org/10.1159/000536156.
Biesiekierski JR, Jalanka J, Staudacher HM. Can Gut Microbiota Composition Predict Response to Dietary Treatments? Nutrients. 2019;11(5). https://doi.org/10.3390/nu11051134.
Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6(5):416–26. https://doi.org/10.1016/S2213-8587(18)30037-8.
Celis-Morales C, Lara J, Mathers JC. Personalising nutritional guidance for more effective behaviour change. Proc Nutr Soc. 2015;74(2):130–8. https://doi.org/10.1017/S0029665114001633.
O’Donovan CB, Walsh MC, Gibney MJ, Brennan L, Gibney ER. Knowing your genes: does this impact behaviour change? Proc Nutr Soc. 2017;76(3):182–91. https://doi.org/10.1017/S0029665116002949.
Hietaranta-Luoma H-L, Tahvonen R, Iso-Touru T, Puolijoki H, Hopia A. An intervention study of individual, apoE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics. 2015;7(3):161–74. https://doi.org/10.1159/000371743.
de Hoogh IM, Winters BL, Nieman KM, Bijlsma S, Krone T, van den Broek TJ, et al. A novel personalized systems nutrition program improves dietary patterns, lifestyle behaviors and health-related outcomes: results from the habit study. Nutrients. 2021;13(6):1763. https://doi.org/10.3390/nu13061763.
Franssen W, Franssen GH, Spaas J, Solmi F, Eijnde BO. Can consumer wearable activity tracker-based interventions improve physical activity and cardiometabolic health in patients with chronic diseases? A systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 2020;17(1):1–20. https://doi.org/10.1186/s12966-020-00955-2.
Li SX, Ye Z, Whelan K, Truby H. The effect of communicating the genetic risk of cardiometabolic disorders on motivation and actual engagement in preventative lifestyle modification and clinical outcome: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2016;116(5):924–34. https://doi.org/10.1017/S0007114516002488.
King A, Graham CA-M, Glaister M, Da Silva Anastacio V, Pilic L, Mavrommatis Y. The efficacy of genotype-based dietary or physical activity advice in changing behavior to reduce the risk of cardiovascular disease, type II diabetes mellitus or obesity: a systematic review and meta-analysis. Nutr Rev. 2023:nuad001. https://doi.org/10.1093/nutrit/nuad001.
Marsaux CF, Celis-Morales C, Livingstone KM, Fallaize R, Kolossa S, Hallmann J, et al. Changes in physical activity following a genetic-based internet-delivered personalized intervention: randomized controlled trial (Food4Me). J Med Internet Res. 2016;18(2): e30. https://doi.org/10.2196/jmir.5198.
Tuncay C, Ergoren MC. A systematic review of precision nutrition and Mediterranean Diet: A personalized nutrition approaches for prevention and management of obesity related disorders. Clin Nutr ESPEN. 2020;38:61–4. https://doi.org/10.1016/j.clnesp.2020.04.005.
Goni L, Cuervo M, Milagro FI, Martínez JA. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr. 2015;10(1):1–10. https://doi.org/10.1007/s12263-014-0445-z.
Özdemir V, Kolker E. Precision nutrition 4.0: A big data and ethics foresight analysis—Convergence of agrigenomics, nutrigenomics, nutriproteomics, and nutrimetabolomics. Omics J Integrative Biol. 2016;20(2):69–75. https://doi.org/10.1089/omi.2015.0193.
Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O’Donovan CB, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46(2):578–88.
• Popp CJ, Hu L, Kharmats AY, Curran M, Berube L, Wang C, et al. Effect of a Personalized Diet to Reduce Postprandial Glycemic Response vs a Low-fat Diet on Weight Loss in Adults With Abnormal Glucose Metabolism and Obesity: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(9):e2233760. https://doi.org/10.1001/jamanetworkopen.2022.33760. This randomized clinical trial highlights the significant benefits of personalized dietary approaches in managing weight and metabolic health.
•• Aldubayan MA, Pigsborg K, Gormsen SMO, Serra F, Palou M, Galmés S, et al. A double-blinded, randomized, parallel intervention to evaluate biomarker-based nutrition plans for weight loss: The PREVENTOMICS study. Clin Nutr. 2022;41(8):1834–44. https://doi.org/10.1016/j.clnu.2022.06.032. This study holds importance as it underscores the need for further exploration and refinement of precision nutrition approaches, highlighting the current limitations in achieving superior outcomes with personalized dietary plans in individuals with overweight or obesity.
• Cuevas-Sierra A, Milagro FI, Guruceaga E, Cuervo M, Goni L, García-Granero M, et al. A weight-loss model based on baseline microbiota and genetic scores for selection of dietary treatments in overweight and obese population. Clin Nutr. 2022;41(8):1712–23. https://doi.org/10.1016/j.clnu.2022.06.008. This study presents a weight-loss model that uses baseline microbiota and genetic scores to personalize dietary treatments for overweight and obese individuals, demonstrating the potential of integrating genetic and microbiome data to enhance the effectiveness of weight management strategies.
Höchsmann C, Yang S, Ordovás JM, Dorling JL, Champagne CM, Apolzan JW, et al. The personalized nutrition study (POINTS): evaluation of a genetically informed weight loss approach, a randomized clinical trial. Nat Commun. 2023;14(1):6321. https://doi.org/10.1038/s41467-023-41969-1.
Koromina M, Konstantinidou V, Georgaka M, Innocenti F, Patrinos GP. Nutrigenetics and nutrigenomics: ready for clinical use or still a way to go? Future Med. 2020. https://doi.org/10.2217/pme-2020-0007.
Kohlmeier M, De Caterina R, Ferguson LR, Görman U, Allayee H, Prasad C, et al. Guide and position of the International Society of Nutrigenetics/Nutrigenomics on personalized nutrition: part 2-ethics, challenges and endeavors of precision nutrition. Lifestyle Genom. 2016;9(1):28–46. https://doi.org/10.1159/000446347.
• Nacis JS, Galang MR, Labrador JPH, Gonzales MS, Dablo AMFD, Domalanta-Ronquillo DGA, et al. “Right diet for the right person”: a focus group study of nutritionist-dietitians’ perspectives on nutritional genomics and gene-based nutrition advice. J Community Genet. 2022;13(1):49–57. https://doi.org/10.1007/s12687-021-00560-1. This study explores nutritionist-dietitians' perspectives on nutritional genomics and gene-based nutrition advice, highlighting the challenges and opportunities of implementing personalized nutrition based on genetic information in clinical practice.
Backman JD, Li AH, Marcketta A, Sun D, Mbatchou J, Kessler MD, et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 2021;599(7886):628–34. https://doi.org/10.3389/fsufs.2018.00087.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.G.U.G. and N.R. prepared the original draft of the manuscript. N.R. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ulusoy-Gezer, H.G., Rakıcıoğlu, N. The Future of Obesity Management through Precision Nutrition: Putting the Individual at the Center. Curr Nutr Rep 13, 455–477 (2024). https://doi.org/10.1007/s13668-024-00550-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-024-00550-y