Abstract
Purpose of Review
NAD+ is a vital molecule that takes part as a redox cofactor in several metabolic reactions besides being used as a substrate in important cellular signaling in regulation pathways for energetic, genotoxic, and infectious stress. In stress conditions, NAD+ biosynthesis and levels decrease as well as the activity of consuming enzymes rises. Dietary precursors can promote NAD+ biosynthesis and increase intracellular levels, being a potential strategy for reversing physiological decline and preventing diseases. In this review, we will show the biochemistry and metabolism of NAD+ precursors NR (nicotinamide riboside) and NMN (nicotinamide mononucleotide), the latest findings on their beneficial physiological effects, their interplay with gut microbiota, and the future perspectives for research in nutrition and food science fields.
Recent Findings
NMN and NR demonstrated protect against diabetes, Alzheimer disease, endothelial dysfunction, and inflammation. They also reverse gut dysbiosis and promote beneficial effects at intestinal and extraintestinal levels. NR and NMN have been found in vegetables, meat, and milk, and microorganisms in fermented beverages can also produce them.
Summary
NMN and NR can be obtained through the diet either in their free form or as metabolites derivate from the digestion of NAD+. The prospection of NR and NMN to find potential food sources and their dietary contribution in increasing NAD+ levels are still an unexplored field of research. Moreover, it could enable the development of new functional foods and processing strategies to maintain and enhance their physiological benefits, besides the studies of new raw materials for extraction and biotechnological development.
Similar content being viewed by others
Introduction
NAD+ is the oxidized form of nicotinamide adenine dinucleotide, a molecule essential for living organisms for maintaining cellular health. It has been shown to promote several health benefits, including enhancing energy metabolism, cardio and neuroprotection, DNA repair, and anti-inflammatory and anti-aging effects [1••, 2,3,4,5,6].
In addition to its key role in energy metabolism as a coenzyme that accepts electrons for catabolic reactions, NAD+ also participates as a co-substrate in signaling pathways of intracellular calcium mobilization and post-translational protein modification [7]. The regulation of these important cellular processes mediated by NAD+ confers protection of mitochondrial function, redox homeostasis control, anti-inflammatory action, attenuation of age-related dysfunctions, cell differentiation, genomic stability, and epigenetic modulation among others [1••, 8,9,10].
NAD+ is constantly synthesized, catabolized, and recycled in the cell to sustain stable levels. Disturbances such as aging [11] and overnutrition by high-fat and -protein intake [12, 13] affect NAD+ synthesis and are associated with reduced levels of this important molecule. Low NAD+ levels is one of the hallmarks of physiological decline and the onset of age-associated diseases, such as neurodegenerative, metabolic, and ocular [14, 15]. Likewise, NAD+ depletion was related to complications and the worsening of coronavirus disease (Covid-19) [16, 17]. In summary, NAD+ decline can result from reduced NAD-synthesizing enzymes, increased NAD-consuming enzymes, or a combination of both.
Boosting NAD+ levels throught biosynthesis precursors has the potential to prevent or alleviate a wide range of diseases such as metabolic and age-related disorders. Based on their ability to elevate NAD+ levels, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) have been shown to mitigate the physiological decline, diabetes and diabetic neuropathy [1••, 13, 18,19,20], protect against hepatic steatosis [18, 21], decrease various pathological features of Alzheimer’s disease [22,23,24], protect neuronal cells from oxidative stress [25], and preserve cognition [26, 27]. In addition, they have demonstrated beneficial pharmacological activities in acute renal failure [28], anti-inflammatory [29,30,31], cardio and vasoprotective actions [32,33,34,35], telomere lengthening [36, 37•], extend lifespan and promote health in various organisms, from yeasts to mammals [10, 14, 15, 38].
The NMN and NR supplements have been the subject of clinical trials to assess their safety and applicability in humans [34, 38,39,40]. However, these NAD+ precursors are also present in foods and may have a potential dietary therapeutic role, similar to that of phenolic groups and other bioactive food compounds. Evidence from NMN and NR presence in vegetables, meat, and milk reinforce their natural occurrence [1••, 41, 42], although there is still limited data on NMN and NR content in foods. While vegetal sources, such as edamame, avocate, and broccoli, have shown slightly higher content until now; research is essential to identify the best food sources and determine whether a normal diet provides a sufficient amount of these precursors to increase NAD+ levels. The distribution of these novel NAD+ precursors in food sources presents an exciting new research field, particularly regarding their potential contribution to health promotion through diet.
Additionally, the potential impact of link between gut microbiota and NAD+ metabolites on overall host health is noteworthy. While microorganisms that inhabit the gut play a role in the metabolism of NAD+ and its metabolites [43, 44], NMN and NR also affect the composition of the gut microbiota, reversing dysbiose and promoting beneficial effects at both intestinal and extraintestinal levels [45,46,47•].
Hence, this review will cover the biochemistry and metabolism of NAD+ precursors NMN and NR, as well as their latest beneficial physiological effects, interplay with gut microbiota, and future research prospects in the fields of nutrition and food science.
NAD+, NMN and NR Metabolism
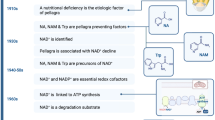
In eukaryotes, NAD+ performs two important functions: energy transduction and cell signaling. NAD+ was first established as a vital redox cofactor for ATP (adenosine triphosphate) synthesis. Subsequently, NAD+ degradation processes were unveiled, using it as a substrate by CD38/CD157/SARM1, ADP-ribosyl transferases (ARTs), poly-ADP polymerases (PARPs), and sirtuins [7, 48,49,50] (Fig. 1). These NAD-dependent enzymes mediate essential cellular processes for organismal homeostases, such as DNA repair [51], apoptosis [52], cell survival [53], lifespan regulation [10, 49], metabolic adjustments [49], inflammation, and infection [54].
NAD+ is a pyridine nucleotide made up of two nucleosides that are joined by a pyrophosphate group. Each nucleoside contains a ribose ring, with one nucleoside containing adenine attached to the first carbon atom (adenosine diphosphate ribose) and the other containing nicotinamide at the same position (NMN) (Fig. 1).
NAD+ synthesis can occur from tryptophan and vitamin B3 (niacin and nicotinamide). Later, the intermediates NR and NMN have also been identified to promote NAD+ synthesis [13, 55]. NR is a pyridine nucleoside consisting of an amine (nicotinamide) with a beta-N-glycosidic bond to a ribose. NMN is a pyridine nucleotide which contains a NR molecule bounded to a phosphate group. NMN and NR are involved in the degradation and re-synthesis of NAD+ (Fig. 1), an this metabolic cycle of NAD+ varies between species and kingdoms [56,57,58].
In mammals, NAD can be produced in different ways: by de novo generation from the amino acid tryptophan, by the nicotinamide (NAM) in the salvage pathway which is released from these consuming reactions as a NAD recycling system, or by niacin/nicotinic acid (NA) in the Preiss-Handler pathway (Fig. 2A). The precursors amidated nicotinamide (NAM), NMN and NR are the main substrates in the salvage pathway. Furthermore, more recently the reduced forms of NR and NMN, namely dihydronicotinamide riboside (NRH) [59, 60] and dihydronicotinamide mononucleotide (NMNH) [61], respectively, have also been shown to promote synthesis (Fig. 2A).
NAD biosynthesis pathways in humans (A) and plants (B). NMN could be uptake through CD73-mediated dephosphorylating to NR, or via a transporter encoded by gene SLC12A8. Arrows dotted indicate a putative pathway. Precursors/Metabolites: KYN: N-formylkynurenine; NMN (nicotinamide mononucleotide); NMNH (dihydronicotinamide mononucleotide); NR (nicotinamide riboside); NRH (dihydronicotinamide riboside); NAR (nicotinic acid riboside); NAM (nicotinamide); NA (nicotinic acid); NAMN (nicotinic acid mononucleotide); NAAD (nicotinic acid adenine dinucleotide), QA (quinolinic acid); TRYP (tryptophan). Enzymes: AK, adenosine kinase; CD73/CD38/CD157, ectoenzimas; IDO, indoleamine 2,3-dioxygenase; NADPPase (NAD pyrophosphatase); NADS (NAD synthase); NAMPT, nicotinamide phosphoribosyltransferase; NAPT (nicotinate phosphoribosyltransferase); NARK (nicotinic acid ribose kinase); NIC (nicotinamidase); NMNAT (nicotinamide/nicotinic acid mononucleotide adenylyltransferase); NRase (nicotinamide riboside nucleosidase); NRD (nicotinamide riboside deaminase); NRK1/2: nicotinamide riboside kinases; NUDase (5′- nucleotidase); PARPs, poli-ADPR polymerases; PNP, purine nucleoside phosphorylase; QPRTase: QA-phosphoribosyltransferase; SIRTs, sirtuins, TDO, tryptophan 2,3-dioxygenase. Image created with https://www.biorender.com/
Moreover, it is worth mentioning the role of the microbiota in NAD+ synthesis. Unlike mammals, the bacteria present in the gut possess enzymes that convert NMN to nicotinic acid mononucleotide (NAMN) through a process of deamidation [62]. After that, NAMN follows via the Preiss-Handler pathway to NAD+ synthesis. The isotope-labeled NMN orally administered in rats was partially deamidated into NAMN by the intestinal microbiota before its absorption. Despite to a noticeably lesser extent than the amidated metabolites NMN and NAD+, nicotinic acid adenine dinucleotide (NAAD) levels in the liver increased [43], as was observed for NR supplementation, which resulted in increased NAAD [63]. Shats et al. (2020) also reported that NAD+ enhancement by NAM and NR was mostly through microbiota-dependent deamidated pathway [44].
In addition, NR can increase NAD+ levels in two different ways. Through direct absorption following the salvage pathway, or be hydrolyzed to nicotinamide (NAM) by the glycohydrolase bone marrow stromal cell antigen 1 (BST1), and further metabolized by the gut microbiota to nicotinic acid, following through the Preiss–Handler pathway. Furthermore, was reported BST1 has a base-exchange activity against both NR and niacin riboside (NAR) to generate NAR and NR, respectively, connecting the amidated and deamidated pathways [64].
Thus, several pathways allow alternative precursors to be used to maintain intracellular NAD levels. Whether this redundancy is vital or has tissue-specific importance remains unexplored.
Sources of NMN and NR
The biosynthesis NAD+ precursors can be obtained trough the diet or by supplementation. The precursors tryptophan, NAM, NA, NMN, and NR are obtained through the diet either in their free form or as metabolites derivatives from the digestion of NAD+ .
NR has been named as the third NAD+ vitamin precursor, alongside NAM and NA [65]. One of the official methods for measuring NA content in foods is by microbiological assay. In the assessment, the food matrix is submitted to an acid or alkaline treatment at a high temperature (AOAC Method 944.13). It is known that under these conditions, NAM, NAD, and NADP are converted into NA, and the alkaline treatment releases NA eventually bound to polysaccharides, peptides, or glycopeptides. Ummarino et al. (2017) investigated whether NMN and NR would also produce NA when exposed to hot acid or alkaline extraction procedures. And indeed, NR and NMN molecules were hydrolyzed to NA and could account for the total niacin content in the milk samples analyzed [42]. Hence, this indicates that the niacin quantified by microbiological assay includes NR and NMN eventually present in the food matrix.
Although NMN and NR can be found in foods of animal origin and mushrooms, this section will focus on their content in plants as a potentially good dietary source, as there are ongoing experiments (results unpublished) supporting this notion.
NAD+ is essential for plants’ adaptation to environmental stresses such as UV irradiation, salinity, heat shock, and drought stress [66, 67]. Additionally, NAD+ is involved in several key factors in plant biology including growth and development, metabolism (energy, reactive oxygen and nitrogen species, harvest), signaling, gene expression, immunity, and biosynthesis [58, 68]. Gene transcription studies have revealed different expression patterns during fruit development and growth. Among the specific genes related to NAD metabolism, de novo pathway biosynthetic genes were transcriptionally induced in young tomatoes. Later stages of fruit growth showed an accumulation of genes involved in the salvage pathway, which coincided with increased NAD levels, most likely to sustain the high metabolic activity during the ripening process [69].
In plants, NAD is synthesized by the de novo pathway from the amino acid aspartate and by the salvage pathway [66]; however, nicotinic acid (NA) is the key metabolite of the pyridine nucleotide cycle [70] (Fig. 2B). Previously, the pathways in NAD+ biosynthesis consisting of seven metabolites were the most known; however, a cycle that includes nicotinamide riboside deaminase was found in potato tubers [71]. The new cycle bypasses nicotinamide and nicotinic acid pathways (Fig. 2B) indicating that it could occur in some plant species [58].
NMN is generated by breaking the diphosphate link of the NAD+ molecule via the action of NAD diphosphatases (EC 3.6.1.22), such as the nudix hydrolases [66], also named NAD pyrophosphatase [58]. Although the salvage pathway deamidated of 6 or 8 steps is most prevalent, NMN can also be converted to NAD in plants by a single step catalyzed by nicotinamide mononucleotide adenyltransferase (NMNAT) [58, 66]. The NMNAT activity specific in the mitochondrial fraction was demonstrated in Jerusalem artichoke (Helianthus tuberosus L.) and plays an important role in NAD metabolism [72]. In lentil and prickly pear (opuntia), the enzyme nucleotide pyrophosphatase (EC 3.6.1.9) was identified and exhibited hydrolytic activities on the pyrophosphate bonds of the reduced and oxidized forms of NAD(P), producing NMN and AMP [73, 74]. In plants such as tea, potatoes, gymnosperms, and Fabaceae, there is the conversion of NMN to NR by activity 5′nucleotidase (EC 3.1.3.5) [58, 66]. These findings characterizes NMN and NR as metabolites of the plant NAD+ pool [67, 69], and vegetal foodstuffs as sources of these compounds.
Accordingly, mammals are exposed to these precursors through the digestive breakdown of dietary NAD+ [39]. Bioavailability studies showed that ingested NAD+ was mainly hydrolyzed in the small intestine by pyrophosphatases present in brush border cells or intestinal secretions [75]. The expression of a transporter in the gut [76] reinforces that NMN could be made bioavailable through oral delivery.
To date, only a few studies have measured NMN and NR in foods, and as a result, the best sources have not been clearly identified. Mills et al. (2016) published the first evidence of NMN in foods such as edamame beans, avocado, broccoli, cucumber, cabbage, and tomato. Among the investigated sources, the highest amount was found in edamame ranging at 0.47–1.88 (mg/100 g) and avocado at 0.36–1.60 (mg/100 g), and at smaller amounts in seafood and raw meats (0.06–0.42 mg/100 g) [1••]. Additionally, NR and NMN were also determined in different species of milk and beer [42, 77•]. Since NR was first discovered in milk [55] it had only been measured in milk so far, with bovine milk having the highest NR content (0.5–3.6 μM,) and human milk as the richest NMN source (2.1–9.8 μM) [42]. As molarity is usually expressed in units of moles of solute per liter of solution, it is assumed that the concentration is for the volume of 1 L of milk, since this information is not stated in the referenced article.
Recently, yeast-mediated NR and NMN production in craft beers was demonstrated, and hop indicated a role in the enhancement of NR levels during fermentation [77•]; thus, opens new perspectives for enriched sources of NAD+ precursors through fermented foods and beverages. This would bring new possibilities alongside the potential biotechnological use of microbial species (e.g., fructophilic lactic acid bacteria) to produce NMN and NR [78] for functional probiotic products and supplements.
Last, NMN, and NR are marketed at high prices in capsule or powder form, with dosages ranging from 100 to 1000 mg, which are doses utilized per day in the studies. However, it is important to note that the optimal dose of NMN and NR supplements for humans is not yet fully understood and further research is needed to determine the most effective and safe dosage. Obtaining NAD+ precursors through the diet may be a more accessible and natural alternative, despite their lower content in food sources compared to supplements. Further research is needed to clarify these issues.
Safety and Pharmacokinetics
Several studies are ongoing to evaluate the physiological effects of NMN and NR supplementation, including safety and pharmacokinetics in humans (NCT04228640, NCT04910061), on aging (NCT04823260, NCT04685096), on hypertension (NCT04903210), on heart failure (NCT03565328, NCT04528004), on COVID-19 (NCT04407390, NCT05175768, NCT04818216), on mitochondrial function (NCT03789175, NCT03951285), metabolic and cardiovascular functions (NCT04571008), skeletal muscle and bone metabolism functions (NCT03818802, NCT04691986), on muscle physiology and physical capacity (NCT04691986, NCT04664361), and among others (listed in the database ClinicalTrials.Gov). Although the results of pre-clinical and clinical trials with NR and NMN supplementation are promising, it remains a need to determine if long-term supplementation and high doses may show side effects.
For example, NAD synthesis inhibition due to NAMPT inhibition decreases cell growth and increases susceptibility to oxidative stress emerging as a therapeutic concept for cancer treatment [79]. While the NAD+ precursors, NMN and NR, reversed cell death induced by the NAMPT inhibitor, the CD38 favored cell death, and CD73 allowed cell viability by degrading NAD and NMN into NAM, and NMN into NR, respectively, thus sustaining NAD+ synthesis [80]. In an oncogenic mouse model, the supplementation with NMN (500 mg/kg body weight daily for 13 days) significantly increased the secretory phenotype associated with pro-inflammatory senescence, promoting pancreatic ductal adenocarcinoma progression. This raises the hypothesis that dietary supplements can increase NAD+ and may be tumorigenic in vivo under stress conditions, such as premalignant senescent lesions induced by activated oncogenes [81].
Therefore, it is worth considering the extent of complex interactions and regulation between NAD+-dependent processes.
A toxicity study in rats demonstrated that repeated oral administration of synthetic NMN (NMN-C®) at doses up to 1500 mg/kg/day over a sub-chronic (90-day) treatment period appears to be safe and did not promote toxic effects as seen from body weight change, food and water consumption, feed conversion efficiency, biochemical, and blood parameters [82], although differences in several physiological and biochemical parameters were found in animals from mid and high-treatment doses (750 mg/kg/day and 1500 mg/kg/day, respectively). There was an increase in the kidney, liver, and adrenal gland weights relative to control, correlating with histopathology findings in the kidney and liver. Correspondingly, elevated levels of hepatic enzymes alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase were found. Thus, the established upper intake level of NMN for a 60 kg individual was 900 mg per day [82].
The first clinical trial reported that a single oral dose of NMN (from 100 to 500 mg) was safely and effectively metabolized in healthy subjects. It showed a significant increase of metabolites in plasma in a dose-dependent manner, without causing adverse effects or any significant clinical symptoms or changes in heart rate, blood pressure, oxygen saturation, and body temperature [40]. However, NMN was not detected in plasma, most likely because the sample freezing before extraction may have caused NMN degradation [40]. It has been reported that NMN degraded very rapidly in blood at − 80 °C [83].
In healthy subjects, NMN (250 mg/day for 12 weeks) elevated NAD+ levels in whole blood and no obvious adverse effects, no abnormalities in physiological and clinical laboratory tests were observed [84]. Another study involving healthy subjects aged between 40 and 65 years found that oral NMN supplementation (300 mg) for 60 days was also well-tolerated, with no observed deleterious effects. Although the difference between the NMN and placebo groups was not statistically significant, NAD+ /NADH levels in the serum increased by 38% (from 6.57 pmol/ml baseline to 9.07 pmol/ml at the end of the study) [85].
Orally administered NMN was rapidly synthesized into NAD+ in mouse tissues. After oral gavage (300 mg/kg body weight), NMN was quickly absorbed from the intestine into the bloodstream and eliminated from circulation within 15 min, with a consistent increase in hepatic NAD+ levels at 15 to 30 min [1••]. Suggesting plasma NMN levels may also be kept low and constant due to balanced NMN distribution from plasma to tissues.
Oral NR increased NAD+ concentrations > twofold (NR 47.75 μM versus placebo 20.90 μM), NAAD by 4.5-fold, and NMN by 1.4-fold, without causing NAM increases in blood. NAM removal pathways were highly active after NR, with an excess of MeNAM, Me-2py, and Me-4py, and about a 20-fold increase of NAR in urine [29].
Martens et al. (2018) identified around a fivefold increase in NAAD levels in the blood of healthy middle-aged men and women supplemented with NR (1 g/day) for 6 weeks [34]. In another study, NAAD was not detected in human blood prior to NR supplementation, while there was a ∼2,900% increase in NAAD baseline in healthy individuals taking single doses of up to 1 g NR (100, 300, and 1000 mg) [63]. Thus, confirming that NAAD is a product of NR utilization in humans and a biomarker of increased NAD+ metabolism, which corroborates with findings in mice that the metabolic NR deamidated Preiss–Handler pathway is predominant [64].
NMN exerted a pronounced effect on skeletal muscle biology, with a significant increase in differentially-expressed genes of platelet-derived growth factor binding pathway compared placebo group [38]. Although muscle NAD+ content did not change after 10 weeks, NMN treatment increased muscle metabolites N1-methylnicotinamide (MeNAM), N1-methyl-2-pyridone-5-carboxamide (Me-2py), and N1-methyl-4-pyridone-5-carboxamide (Me-4py). Irie et al. [40] also observed the raising of these metabolites after oral NMN supplementation, suggesting increased muscle NAD+ turnover by NMN [40].
Preventive and Therapeutic Effects of NMN and NR
Historically, the NAD biosynthesis precursors tryptophan, NA, and NAM have been used to prevent and treat pellagra, a disease characterized by darkly pigmented skin eruptions causing the so-called three D’s (dermatitis, diarrheal, and dementia). Tryptophan alone is not sufficient at sustaining cellular levels of NAD+ [86] as it takes part in other metabolic pathways, such as serotonin, melatonin, and picolinic acid synthesis. NA at pharmacological doses reduced serum cholesterol levels in humans; however, its use is limited due to the side effect causing skin flushing, accompanied by an unpleasant warmth sensation, and often itching [87].
Although NAM has been shown to be effective to increase NAD+ in tissues, it also exerts an inhibitory effect on sirtuin [88] and PARPs activity [89]. At high doses, NAM results in increased methylation to generate N1-methylnicotinamide (MeNAM) via nicotinamide N-methyltransferase (NNMT) for elimination in urine, which can lower the cellular methyl pool over time [89]. This could lead to a reduction in DNA and protein methylation, and as consequence, alter gene expression patterns and protein activity, once that DNA methylation in gene promoter regions is typically associated with transcriptional repression.
Furthermore, blood and urine pharmacokinetic data performed in rats suggested that NMN administered intraperitoneally appears to be retained in the body longer than NAM [90]. It is still unclear why NR administration increases NAD+ levels more than NAM and NA treatment [63], while NMN and NAM administration showed no difference in NAD levels in blood and liver [90]. Further studies with direct comparisons of all precursors at the same dose are important to elucidate the superiority purpose of each one.
Proposed as a nutraceutical to prevent age-related physiological decline [1••, 91], NMN has shown to improve diabetes [13, 20, 38] and Alzheimer’s disease [22, 23], enhance aerobic capacity [92], and exhibit cardio- and vasoprotective actions. It inhibits inflammation and decreases oxidative stress, preventing arterial and endothelial dysfunction [32, 93], and protecting against heart failure [94], ischemia, and reperfusion [33].
NR demonstrated protective effects on cognitive function, synaptic plasticity, learning, memory, and motor function in Alzheimer’s disease models [24, 27]. It reduced the beta-amyloid levels, increasing the chance of recognizing a novel object by 20% in NR-treated Tg2576 mice [27]. Futhermore, NR significantly reduced neuroinflammation, apoptosis of hippocampal neurons, phosphorylated Tau and DNA damage, while also increasing neurogenesis by almost 20% [24].
Additionally, NR has been shown to prevent and improve metabolic disorders such as high-fat diet- induced obesity and non-alcoholic fatty liver disease (NAFLD). It promoted metabolic flexibility by enhancing energy expenditure, leading to a significant attenuation of high-fat diet-induced body weight gain and a decrease in fat mass, particularly in the liver, where triglyceride levels decreased by 40% [95]. NR increased hepatic β-oxidation and mitochondrial content reversing glucose intolerance, insulin resistance, liver lipid accumulation, and hepatic fibrosis [21].
Although it may have other as yet unraveled mechanisms, the beneficial effects of NMN and NR have been attributed mainly to increase NAD+ levels enabling the sirtuins activities and their underlying targets.
Anti-Aging Effect
The classification of aging as a disease or a natural and universal process is seen as controversial; however, it is well known the fact that a decline in cellular functions is part of the aging process.
It is recognized the decrease of NAD+ levels in various tissues during aging [11, 13, 96, 97], playing a critical role in the pathophysiology of several diseases, including age-associated metabolic disorders, neurodegenerative diseases, and mental disorders [14]. A significant cause for this age-associated NAD+ decline is the decrease in NAMPT-mediated biosynthesis. The NAMPT (nicotinamide phosphoribosyltransferase) protein and NAD+ levels decreased significantly in organs, such as white adipose tissue and skeletal muscle in old mice [13] affecting the activity of NAD+ -dependent enzymes such as sirtuins [15, 98]. As consequence, affect the balance of redox reactions in the cell, leading to functional decline [14].
Another responsible for the low NAD+ levels is the ectoenzyme CD38. The levels and activity of CD38 increase with aging and are necessary for NAD decline and mitochondrial dysfunction [99]. It was also found that CD38 degraded NMN altering the pharmacokinetics of both NMN and NR [99]. Thus, CD38 regulates the levels of NAD+, NMN, and additionally to SIRT1 activity, playing a critical role in the onset of age-related diseases. And indeed, CD38 is a candidate molecule for regulating neurodegeneration. CD38 knockdown in mice suppressed axon degeneration, demyelination, and immune cell infiltration after facial nerve axotomy compared to wild-type mice. Correspondingly, intraperitoneal injection of NR (400 mg/kg) once daily for 1 week prior to facial nerve axotomy, delayed axon degeneration, and demyelination, increasing significantly facial nucleus and facial nerve NAD+ levels in wild-type mice [100].
During the aging process, DNA damage accumulates in the cell nucleus causing PARPs activation and reducing both NAD+ levels and SIRT1 activity [15]. SIRT1 is one of the sirtuins (SIRT1-7) that use NAD+ as a substrate catalyzing the reaction of deacetylation or mono-ADP-ribosylation of proteins. In addition, accumulated DNA damage in chromosomal regions leads to a progressive and cumulative loss of protective telomere sequences at the chromosome end [101]. Short telomeres showed decreased expression and activity of sirtuins and marked mitochondrial dysfunction.
NMN promoted telomere lengthening in aged mice [36], pre-aging mice and humans [37•]. Treatment with NMN (dissolved in 5-mM drinking water for 8 weeks) promoted telomere lengthening in generation 4 (G4) mice, dampened the DNA damage response and p53, functionally rescued liver fibrosis, and improved the mitochondrial biogenesis and function in a partially SIRT1-dependent manner [36]. The increased NAD+ levels in the liver tissue of NMN-treated G4 mice reversed the hyperacetylation of several sirtuin targets indicating the reinforcement of sirtuin activity [36]. SIRT1 increases mitochondrial activity and biogenesis by deacetylating and activating PGC1α (peroxisome proliferator-activated receptor-γ co-activator 1α) [102]. PGC1α is a transcriptional co-regulator of mitochondrial genes and detoxifies enzymes that eliminate ROS (reactive oxygen species), leading to metabolism improvement and antioxidant protection [103].
Mitochondria is a vital organelle for the body which from food produces most of the energy used by cells. Almost all metabolic processes depend on NAD+, consequently, the maintenance of mitochondrial NAD+ levels is crucial for cell survival [104]. As we age, mitochondria become increasingly dysfunctional, with defective mitophagy and bioenergetic capabilities which end up producing excess free radicals inside the cell, damaging the global cellular environment [101]. A long-term NMN administration (given in drinking water ad libitum at either 100 or 300 mg/kg/day for 12 months) increased mitochondrial respiratory ability in skeletal muscle and reversed age-associated gene expression changes in a tissue-specific manner [1••]. In addition, the orally administered NMN was quickly absorbed, transported into blood circulation, and immediately converted to NAD+ in major metabolic tissues such as the liver and soleus muscle, improving energy expenditure, oxygen consumption, insulin sensitivity, lipid plasma profile, eye function, bone density, and myeloid-lymphoid composition in aged mice [1••].
Other animal and human studies of the NR and NMN supplementation, including anti-aging effects, are summarized in Table 1.
Anti-COVID-19 Effect
Age is also a risk factor for patients with COVID-19, increasing the chances of severe disease and death [105]. The reason is that the aging process leads to several changes, especially immunosenescence and inflammaging that compromise the immune response. The shortening of telomeres and decrease in naïve lymphocytes leading to impaired immune function and a rise in circulating pro-inflammatory cytokines are factors contributing to the worsening of COVID-19 cases [105, 106].
Evidence points to the potential relevance of NAD+ in modulating the COVID-19 disease outcomes, the pandemic public health outbreak. During the SARS-CoV-2 infection, the set of genes related to NAD+ synthesis and use is dysregulated [16], potentially due to the increased demand the NAD+ metabolic pathways.
PARPs are known to play a critical antiviral role in inhibiting the translation of transcripts through ADP-Ribosylation in the viral genome, with NAD+ requirement. However, several viral families, including SARS-CoV, encode a macrodomain protein that hydrolyses the ADPR units of proteins and nucleic acids, inhibiting PARPs protective effect and then facilitating replication and virulence. Consequently, excessive activation of PARPs occurs to compensate for ADPR hydrolysis, accompanied by NAD+ consumption, suggesting that increasing NAD+ levels may restore the antiviral functions of PARPs to support immunity to SARS-CoV-2 [16, 106].
In addition to the role of PARPS on immune responses, sirtuins can also coordinate the intensity of inflammatory responses, preventing the effects of cytokine storms. SIRT1, SIRT2, and SIRT3 all suppress the activity of NF-κB (factor nuclear kappa B) and the NLRP3 (Nod-like receptor family protein 3) inflammasome via multiple mechanisms [107]. SIRT6 attenuates NF-κB signaling via histone H3 lysine 9 (H3K9) deacetylation at chromatin of NF-κB target gene promoters, which are related to apoptosis, and cellular senescence [108].
In a case study of 10 critically-ill patients over 50 years, the NMN cocktail (83 mL of NMN mixed with 400 mL of water, consumed before breakfast and dinner) was strongly associated with the COVID-19 symptom resolution. Before treatment, patients had low-oxygen saturation, pulmonary infiltrates, and inflammation. Post-treatment, there was rapid improvement in bilateral pulmonary infiltrates, fever resolution, and lower inflammation biomarkers. However, one patient stopped the treatment after 3 days due to miscommunication and experienced a relapse with fever and pulmonary infiltrates 8 days later [17]. The rapid and effective clinical improvement suggests NMN may play a role in reversing the potentially fatal cytokine storm triggered by SARS-CoV-2 infection.
A study showed that combined metabolic activators (CMAs), consisting of NR (1 g), l-carnitine tartrate (3.73 g), N-acetylcysteine (2.55 g), and serine (12.35 g), improved the COVID-19 outcomes. Patients took the mixture orally one dose in the morning after breakfast and one dose in the evening after dinner for 14 days. In both a placebo-controlled, open-label phase 2 study and double-blinded phase 3 clinical trials, patients given CMAs had significantly faster complete recovery times compared to the placebo group (6.6 vs 9.3 days and 5.7 vs 9.2 days, respectively). CMAs were found to reinforce immune response and regulate amino acid and lipid metabolism. Moreover, patients treated with CMAs showed significant improvements in plasma levels of several inflammation and antioxidant metabolism-related biomarkers, such as alanine aminotransferase (ALT), lactate dehydrogenase (LDH), creatinine, glucose, and proteins [109].
It was found that alterations in the purinergic metabolism contribute to immune dysregulation during COVID-19, possibly contributing to disease severity. Unvaccinated severe COVID-19 patients presented higher ATP levels and lower levels of adenosine in plasma when compared to healthy controls. Ectonucleotidases CD39 and CD73 responsible for ATP cleavage to adenosine had reduced expression in severe COVID-19 patients’ blood. Besides the impaired generation from ATP, the adenosine receptors were also less expressed, in a disease severity-dependent manner [110]. Since adenosine and its receptors act on neutrophil and monocyte/macrophage suppressing cytokine production [111], an unbalanced ratio ATP/adenosine disturbs this anti-inflammatory regulation. In addition, in vitro administration of exogenous adenosine prevented inflammatory responses in the leukocytes of patients [110], whether this alteration in the metabolism of ATP is a cause or effect of the exacerbated inflammatory response to SARS-CoV-2 still needs to be explored. Given the function of NAD+ in ATP generation and the role of both extracellular NAD+ and ATP in the regulation of inflammation and immune response [112], it is worth investigating the possible involvement of NAD+ in this altered ATP metabolism during infection by SARS-CoV-2.
Anti-Inflammatory Effect
Boosting NAD+ can reduce inflammation. Consistent with NR’s known role in increasing NAD+ levels and consequently SIRT3 activation, the 24-h administration of NR (amount not described) to PBMCs from healthy subjects replicated the fasting effect of blunting NLRP3 inflammasome activation and enhancing mitochondrial quality control through SIRT3 [30]. NR reduced acetylation of SOD2 and isocitrate dehydrogenase 2, while increasing mitochondrial SOD2 activity, thereby reducing mitochondrial ROS levels. Additionally, NR decreased Interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) secretion in monocytes and macrophages from healthy volunteers, with the most significant reductions in monocytes of over 50% for IL-1β (from approximately 22,000 to 10,000 pg/mL) and over 75% for TNF-α (from above 2000 to less than 500 pg/mL). In macrophages, the most pronounced reduction was around 20% for IL-1β (from above 300 to less than 250 pg/mL) and approximately 45% in TNF-α levels (from around 1800 to 1000 pg/mL) [30].
In older men, supplementing with 1 g of oral NR per day for 3 weeks increased NAD+ metabolome levels in whole blood and skeletal muscle and significantly reduced circulating levels of the inflammatory cytokines IL-2, IL-5, IL-6, and TNF-α compared to baseline (from approximately 20 to 5 pg/ml for interleukins and from around 250 to 200 pg/ml for TNF-α) [29].
In addition to increasing the cellular NAD+ level decreased, treatment with NMN (500 μM) decreased pro-inflammatory cytokines production in LPS-activated mouse and human macrophage cell lines (THP-1, RAW264.7). NMN efficiently alleviated LPS-induced inflammation and oxidative stress via the COX-2-PGE2 axis and inhibition of inflammation-related pathways. Proteomics analysis identified that NMN downregulated the expression of cyclooxygenase-2 (COX-2) and markedly decreased mRNA expressions and extracellular secretion of IL-6 and IL-1β, and the cellular levels of prostaglandin E2 (PGE2). Proteins related to inflammatory response such as RELL1, PTGS2, FGA, FGB, and igkv12-44 were all decreased in LPS/NMN co-treated cells as compared with LPS-treated cells. NMN treatment also suppressed other inflammation-associated pathways such as prostanoid biosynthesis, LPS/IL-1 mediated inhibition of RXR function, IL-6 signaling, and NF-κB signaling [31].
Regulation of Energy Metabolism
NAD+ functions as a coenzyme in various redox reactions in the major energy production pathways, such as glycolysis, tricarboxylic acid (TCA) cycle, and fatty acid oxidation [113]. Declining NAD+ levels is a signature of an imbalance in energy homeostasis [114], which makes it an energy-sensing metabolite. Energy-sensing pathways are important for maintaining an adequate balance between energy production and expenditure. Disturbance of these pathways results in various metabolic disorders, such as insulin resistance and fatty liver [113].
Under disturbed nutrient conditions, such as high-fat and -protein intake, NAD+ levels decreases [12, 13]. When an excess calorie is consumed, a low AMP/ATP ratio can cause a decrease in NAD+ or NAD+ /NADH ratio. The adenosine monophosphate-activated protein kinase (AMPK) is a cellular energy regulator which senses changes in the intracellular AMP/ATP ratio. During energy limitation, low levels of ATP activate AMPK, which acts to maintain cellular energy stores. AMPK switches on catabolic pathways that produce ATP, mostly by enhancing oxidative metabolism and mitochondrial biogenesis, while switching off anabolic pathways that consume ATP, altering the NAD+ /NADH ratio. Thus, AMPK enhances the SIRT1 activity by increasing cellular NAD+ levels, resulting in the deacetylation and modulation of the activity of energy metabolism-related downstream SIRT1 targets, which include the PGC1α, the forkhead box O1 (FOXO1) and O3 (FOXO3a) transcription factors [102].
Correspondingly, NR or NMN administration can prevent the reduction in NAD levels. NR (450 mg/kg body weight for 45 days) stabilized myocardial NAD+ levels, increased glycolysis, and citrate and acetyl-coenzyme A metabolism, attenuating the development of heart failure in a mouse model with dilated cardiomyopathy [35]. By increasing NAD+ levels in mice’s liver and muscle, NR supplementation (400 mg/kg/day) stimulated SIRT1 and SIRT3 activity, enhancing the mitochondrial function, oxidative metabolism, energy expenditure, and endurance performance [95]. In addition, NR protected against the detrimental metabolic effects of a high-fat diet, including hyperinsulinemia, elevated levels of total and LDL cholesterol, and weight gain, although it had no effect on body weight when given with a normal chow diet [95].
NMN also demonstrated no benefic effect on glucose metabolism in mice consuming a chow diet. Male offspring of both lean and obese progenitress mice, fed either chow or high-fat diet (HFD) for 30 weeks post-weaning were intraperitoneally injected with NMN (500 mg/kg body weight) for 21 days. These mice had reduced adiposity, and hepatic and plasma triglyceride levels. Additionally, there was a reduction of hepatic genes involved in fat synthesis, transport, and uptake, while the genes involved in fatty acid oxidation were increased by NMN in offspring consuming HFD. However, in relation to glucose tolerance, NMN was beneficial only for the most metabolically compromised mice group (feed HFD and from obese progenitress). In addition, NMN impaired glucose tolerance in mice from lean progenitress consuming chow and had no additional impact in offspring consuming HFD [115]. In another study, NMN supplementation (400 mg/kg for 8 weeks) reduced the exercise-induced benefits in obese mice such as hepatic triglyceride accumulation reduction, glucose-stimulated insulin secretion from islets, and glucose tolerance. Although, NMN on its own significantly down-regulated TNF-α and Tlr4 expression and up-regulated expression of PGC-1α in islets supporting its antioxidative effects, the association of NMN and exercise enhanced the ratio of antioxidants to prooxidants, deregulating redox homeostasis [116].
These results suggested that supplementation with NAD+ precursors may be less effective in conditions where the NAD+ levels are balanced.
Anti-Diabetic Effect
The hallmark hyperglycemia of diabetes mellitus causes a redox imbalance between NAD+ and NADH ratio, which can lead to oxidative stress and a variety of metabolic syndromes. For cells whose glucose uptake is not dependent on insulin, glucose oversupply can lead to NADH overproduction by both the conventional glucose combustion pathways (glycolysis and Krebs cycle) and the polyol pathway. This NAD+ /NADH ratio imbalance, also known as pseudohypoxia, initially causes reductive stress, mitochondrial overload, and dysfunction, which lead to oxidative stress and oxidative damage to macromolecules, including DNA, lipids, and proteins. On the other hand, NAD+ can be diminished or depleted by the overactivation of PARPs that uses NAD+ as their substrate for DNA repair. Accordingly, redox imbalance might be a major factor contributing to the development of diabetic complications [117].
In addition, this redox change characterized by pseudohypoxia causes activation of two pathways: diacylglycerol (DAG)–protein kinase C (PKC) and (DAG)–protein kinase D (PKD) cascade, which impairs AMPK activity decreasing NAD+ synthesis. Another suggestion could be the insufficient phosphoribosyl pyrophosphate (PPRP) synthesis from the pentose phosphate pathway which is compromised by a decrease in plasma thiamine and protein kinase B activity in diabetes. Protein kinase B is responsible for phosphorylating and activating transketolase to PPRP production, which will be used in NAD+ pathway synthesis [114].
Decreased NAD+ content and imbalanced mitochondrial dynamics occur in the development of diabetic cardiomyopathy. Daily oral gavage of NR 400 mg/kg/day for 4 weeks alleviated diabetes-induced cardiomyopathy improving cardiac function in diabetic mice. NR elevated myocardium NAD+ content and promoted mitofusin 2-mediated mitochondrial fusion, suppressing oxidative stress and cell apoptosis via the SIRT1-PGC1α-PPARα pathway [19].
Chronic fructose feeding is known to cause suppression of eNAMPT secretion and pro-inflammatory cytokine-mediated islet dysfunction leading to impaired beta cell function and elevated blood glucose. In this case, NMN administration (100 μmol/l) restored insulin secretion in islets cultured with pro-inflammatory cytokines IL1β and TNFα, in a partially SIRT1-dependent manner. In addition, it corrected the inflammation-induced islet dysfunction, reversing the changes in the expression of genes encoding islet markers essential for glucose sensing and beta cell differentiation. Also by improving the glucose-stimulated insulin secretion in mice fed with a fructose-rich diet, NMN (500 mg/kg body weight administrated intraperitoneally 16 h prior to tissue sampling) may indicate an ability to protect against beta cell failure through an anti-inflammatory mechanism [20].
In another study, NMN demonstrated to be an effective intervention to treat the pathophysiology of type 2 diabetes. In both diet- and age-induced diabetic models, the administration of NMN via intraperitoneal (500 mg/kg body weight/day for 11 days) ameliorated impaired glucose tolerance substantially and enhanced hepatic insulin sensitivity. The indicated reversing changes in gene expression related to oxidative stress, inflammatory response, and circadian rhythm was in part a result of SIRT1 activation [13]. BESTO (beta cell-specific SIRT1-overexpressing) transgenic mice with advanced age had lost their phenotypes of glucose-stimulated insulin secretion and improved glucose tolerance as when they were young, despite maintaining SIRT1 overexpression in pancreatic β-cells. The NMN treatment (500 mg/kg body weight 14 h prior to performing the assay), besides increasing NAD+ levels that had decreased with age, was able to restore the phenotype in aged BESTO mice and also improved glucose-stimulated insulin secretion in aged wild-type mice [98].
Given the multifactorial nature of metabolic diseases, including diabetes, the mechanisms by which NAD+ precursors contribute to improved insulin sensitivity and glycemic control can be attributed to the replacement and/or increase in NAD+ levels and reversal of oxidative stress and inflammation.
In postmenopausal women with overweight or obesity and prediabetes, oral NMN supplementation (250 mg/day for 10 weeks) increased NAD+ content in peripheral blood mononuclear cells (PBMCs), and improved insulin signaling and sensitivity in skeletal muscle [38].
Interplay Between Gut Microbiota and NAD+ in Host Health
We reviewed the background and the latest findings about the central function of NAD+ in maintaining cellular homeostasis. It is also already known the important physiological role of gut microbiota in the metabolic homeostasis, hormones secretion, and brain function of the host [118,119,120,121]. In the same way, link between intestinal microorganisms and NAD+ metabolites suggests an important axis for beneficial physiological effects.
Some gut microbial can produce metabolites that exert a protective effect against various diseases [122,123,124]. NMN derived from the gut microbiota ameliorated acute pancreatitis (AP) injury by increasing NAD+ levels and activating the SIRT3-PRDX5 pathway in AP mouse models [125]. AP is exacerbated by gut microbiota dysbiosis, which is characterized by an imbalance between beneficial and pathogenic microbes that increases gut permeability, bacterial translocation, and systemic inflammatory responses. Besides reverting gut microbiota dysbiosis, the normobiotic fecal microbiota transplantation (FMT) induced higher levels of NMN in the serum, enhancing NAD+ biosynthesis in the pancreas. Similarly to FMT, pretreatment with NMN (500 mg/kg body weight/day intraperitoneally for 28 consecutive days) increased pancreatic NAD+ levels, mitigating AP-mediated mitochondrial dysfunction, oxidative damage, and inflammation in a partially SIRT3-dependent manner. These findings suggest that NMN is an important mediator of the protective effects of FMT in AP and reinforce the potential role of gut microbiota on NAD+ metabolism and pathological disorders outcomes of the host.
In addition to gut microbiota activity on B vitamins [118, 126] and NAD+ biosynthesis [44], recent evidence demonstrates the role of NAD+ and its precursors NR and NMN on the modulation of gut microbiota, which could be one of the underlying mechanism of beneficial effects observed. NAD+ precursors can modulate gut microbiota by providing an energy source for beneficial bacteria, activating sirtuins, and improving gut barrier function.
NMN and NR can selectively stimulate the growth of beneficial bacteria such as Bifidobacterium and Lactobacillus [46, 47•]. These bacteria have been associated with improved gut health, including prevention of diarrhea, improved gut barrier function, reduced inflammation, and immune system support [127]. Additionally, the activation of sirtuins by NAD+ precursors may also contribute to the beneficial effects on gut microbiota and intestinal health. Sirtuins are involved in regulating the gut microbiota [128, 129] and in protecting intestinal endothelial cells against oxidative injury [130]. Finally, NMN and NR can also improve intestinal barrier function [46, 130], which is important for preventing the translocation of harmful bacteria and toxins from the gut into the bloodstream.
Besides changing the composition and functional profiles, the modulation of gut microbiota by NR was demonstrated to be protective against high-fat diet (HFD)-induced weight gain in mice [131]. Mice fed with 60% HFD supplemented with 0.4% NR (for 168 days) had almost a 16% reduction in weight gain and a reduction in fasting blood glucose levels compared to the control supplemented with vehicle (water). Interestingly, the fecal transplant (FMT) from NR-treated donors to mice fed a HFD also attenuated the weight gain by decreasing energy efficiency. Both dietary NR supplementation and FMT-NR supplementation caused alteration of the intestinal microorganisms composition with a unique functional metabolic profile enriched of butyrate-producing Firmicutes. Even though some Firmicutes species have been associated with obesity [132], there are many species from the Firmicutes phylum that have probiotic and anti-obesity effects [133]. For instance, F. prausnitzii, Eubacterium rectale, and Roseburia produce short-chain fatty acids, including butyrate which has been shown to reduce adiposity by inhibiting intestinal cholesterol biosynthesis and promoting beneficial metabolic effects, such as insulin secretion, insulin sensitivity, and energy expenditure [133]. Butyrate can also increase satiety and gut mobility by upregulating the expression of peptide YY and Glucagon-like Peptide-1 (GLP-1) [133]. Additionally FMT-NR mice exhibited a significant increase in NADH levels in the cecal contents, indicating altered microbial metabolism in the cecum [131].
And indeed NAD+ can directly affect the metabolism of intestinal microorganisms. Because NAD+ is an important component in the energy metabolism of all living organisms, mainly to be a cofactor for many key enzymes involved in the production of cellular ATP, boosting NAD+ may promote microbial energy production [134]. Furthermore, NAD+ can indirectly affect gut microbes by regulating the movement of the colon through the key inhibitory neurotransmitter β-NAD and by affecting the synthesis of host bile acids [45], which can be antimicrobial when they destroy the bacterial cell membrane [134].
Dietary supplementation of mice with NR for 10 weeks (400 mg/kg daily) reduced both pro- and anti-inflammatory cytokine levels, inhibited microglial activation, and provided protection against alcohol-induced depression-like behaviors. This effect was attributed to NR’s ability to upregulate brain-derived neurotrophic factor (BDNF) by altering gut microbiota composition [135]. The mice model of alcohol-induced depression showed significant differences in intestinal microbiota diversity and composition compared to Control and NR groups. Notably, the model group was enriched with Akkermansia and Clostridium XVIII, while Barnesiella and Alloprevotella were dominant in the NR group. Brain inflammatory cytokines were positively correlated with model group-enriched bacteria, whereas brain BDNF levels were positively correlated with model group-deficient bacteria such as Barnesiella, Alloprevotella, Prevotella, Alistipes, Mucispirillum, and Odoribacter. Additionally, fecal microbiota transplantation (FMT) from alcohol-treated mice donors to germ-free recipient mice resulted in similar depression-like behaviors and alterations to microglial activation status, cytokines, and BDNF levels. Conversely, recipient mice of NR-treated donors had similar protective features to their donors [135].
In a clinical trial, NR slightly modulated gut microbiota composition in twins. The long-term NR supplementation (250–1000 mg/day for 5 months) increased the abundance of Faecalibacterium prausnitzii [47•], which promotes metabolic health and anti-inflammatory responses [136, 137]. Patients with inflammatory bowel disease (IBD) have been associated with reduced bacterial diversity and a decline in butyrate-producing bacteria such as Faecalibacterium prausnitzii, indicating the importance of gut dysbiosis in IBD [138, 139]. A balanced ratio between the different commensal bacteria species is needed to maintaining the homeostasis intestinal and overall host health [132]. Association analysis suggests that the abundance of F. prausnitzii may contribute to the regulation of NAD+, inflammation, amino acid, and lipid metabolism [47•].
NMN also demonstrated protecting effect on IBD in an experimental mice model of colitis, reversing the intestinal dysbiosis [46]. NMN was administered via gavage at a dose of 1 mg/g of body weight either at the same time for 21 days or after colitis induction with dextran sodium sulfate for the last 14 days. In addition to increasing microbial abundance and diversity, NMN improved the mucus secretion and tight-junction proteins’ expression, which helped to attenuate intestinal mucosal permeability [46].
The long-term NMN treatment (0.1 to 0.6 mg/mL in drinking water for 12 weeks) modulated gut microbiota diversity and composition, exerting a protective effect on the intestinal tract of mice. Although NMN reduced the diversity of intestinal species, it increased the abundance of butyric acid-producing bacteria (Ruminococcae_UCG-014 and Prevotellaceae_NK3B31_group) and other probiotics such as Akkermansia muciniphila, while the abundance of several harmful bacteria (Bilophila and Oscillibacter) were decreased [45]. In addition, NMN increased the level of bile acid-related metabolites and beneficial metabolites, the number of goblet cells, mucus thickness, and expression of tight junction protein, maintaining the integrity of the intestinal epithelium and reducing mucosal permeability [45]. Niu et al. (2021) also found that NMN significantly reduced fecal bacterial diversity. The supplementation of NMN (500 mg/L (w/v) in drinking water for 40 days increased the abundance of Helicobacter, Mucispirillum, and Faecalibacterium, and lowered Proteobacteria and Akkermansia abundance in mice. This changed bacterial was correlated with the composition of serum metabolite constitutes, mainly involved in the purine, nicotinate/nicotinamide, and arginine/proline metabolism pathways [37•].
Although NAD+ precursors has a potential modulatory function on gut microbiota, further research is needed to clarify their role in intestinal microorganisms, underlying host physiological effects, and the related mechanisms. Finally, whether boosting host NAD+ levels may promote beneficial intestinal bacteria and inhibit harmful bacteria also remain to be more explored.
Conclusion and Future Perspectives
The natural occurrence of NR and NMN in food, along with their potential beneficial effects for animals and humans, is opening up an exciting new area in food science and nutrition. Investigating their content in dietary sources as well as bioaccessibility certainly justifies the research effort.
The search for potential food sources of NMN and NR, from commonly consumed items to waste by-products, could provide insights for improved diets and the development of functional foods. Additionally, this may lead to the identification of new raw materials for the extraction, isolation, and biotechnological production of these compounds. It is also important to evaluate food processing strategies that can preserve and enhance the physiological benefits of NR and NMN.
The metabolism and biodistribution of NAD+ precursors in various tissues and within cells are still little understood. Further research is necessary to investigate the bioavailability, quantitative metabolomics, and pharmacokinetics of dietary NMN and NR. These studies can help identify the optimal doses and contribution of each precursor in maintaining and boosting NAD+ levels, as well as their physiological effects through diet.
Finally, given the crucial role of gut microbiota in human health and the emerging evidence of intestinal microbes modulation by NMN and NR, there is a need to investigate the crosstalk between gut microbiota metabolism and NR and NMN. These studies can help identify the potential impact on health and offer insights into the underlying mechanisms.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
•• Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806. In addition to the anti-aging effect of NMN supplementation in mice preventing age associated gene expression changes and physiological decline, this article showed the first evidence of the natural presence of NMN in food such as fruits, vegetables, and meat.
Yamaguchi S, Franczyk MP, Chondronikola M, Qi N, Gunawardana SC, Stromsdorfer KL, et al. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc Natl Acad Sci. 2019;116(47):23822–8.
Abdellatif M, Sedej S, Kroemer G. NAD+ Metabolism in cardiac health, aging, and disease. circulation. 2021;144(22):1795–817.
Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 2019;30(4):630–55.
Huang Q, Sun M, Li M, Zhang D, Han F, Wu JC, et al. Combination of NAD+ and NADPH offers greater neuroprotection in ischemic stroke models by relieving metabolic stress. Mol Neurobiol. 2018;55(7):6063–75.
Saville KM, Clark J, Wilk A, Rogers GD, Andrews JF, Koczor CA, et al. NAD+-mediated regulation of mammalian base excision repair. DNA Repair (Amst). 2020;93:102930.
Berger F, Ramírez-Hernández MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci. 2004;29(3):111–8.
Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020;5(1).
Ummarino S, Hausman C, Gaggi G, Rinaldi L, Bassal MA, Zhang Y, et al. NAD modulates dna methylation and cell differentiation. Cells. 2021;10(11):2986.
Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (80-). 2016;352(6292):1436–43.
Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One [Internet]. 2012;7(7):e42357 Available from: https://doi.org/10.1371/journal.pone.0042357.
Seyedsadjadi N, Berg J, Bilgin AA, Braidy N, Salonikas C, Grant R. High protein intake is associated with low plasma NAD+ levels in a healthy human cohort. PLoS One. 2018;13(8).
Yoshino J, Mills KF, Yoon MJ, Imai SI. Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–36.
Johnson S, Imai S. NAD+ biosynthesis, aging, and disease [version 1; peer review: 2 approved]. F1000Research [Internet]. 2018;7(132). Available from: http://openr.es/a4e.
Imai S ichiro, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–71.
Heer CD, Sanderson DJ, Voth LS, Alhammad YMO, Schmidt MS, Trammell SAJ, et al. Coronavirus infection and PARP expression dysregulate the NAD metabolome: an actionable component of innate immunity. J Biol Chem. 2020;295(52):17986–96.
Huizenga R. Dramatic clinical improvement in nine consecutive acutely Ill elderly COVID-19 patients treated with a nicotinamide mononucleotide cocktail: a case series. SSRN Electron J [Internet]. 2020; Available from: https://ssrn.com/abstract=3677428.
Trammell SAJ, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016;6(1).
Hu L, Guo Y, Song L, Wen H, Sun N, Wang Y, et al. Nicotinamide riboside promotes Mfn2-mediated mitochondrial fusion in diabetic hearts through the SIRT1-PGC1α-PPARα pathway. Free Radic Biol Med. 2022;1(183):75–88.
Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia [Internet]. 2011;54(12):3083–92. Available from: https://doi.org/10.1007/s00125-011-2288-0.
Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology [Internet]. 2016;63(4):1190–204. Available from: https://doi.org/10.1002/hep.28245.
Wang X, Hu X, Yang Y, Takata T, Sakurai T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9.
Yao Z, Yang W, Gao Z, Jia P. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett. 2017;647:133–40.
Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci [Internet]. 2018;115(8):E1876 LP–E1885. Available from: http://www.pnas.org/content/115/8/E1876.abstract.
Ito S, Gotow T, Suetake I, Nagai K. Protective effects of nicotinamide mononucleotide against oxidative stress-induced PC12 cell death via mitochondrial enhancement. PharmaNutrition. 2020;1(11):100175.
Chandrasekaran K, Choi J, Arvas MI, Salimian M, Singh S, Xu S, et al. Nicotinamide mononucleotide administration prevents experimental diabetes-induced cognitive impairment and loss of hippocampal neurons. Int J Mol Sci. 2020;21(11).
Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34(6):1581–8.
Guan Y, Wang S-R, Huang X-Z, Xie Q, Xu Y-Y, Shang D, et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1–dependent manner. J Am Soc Nephrol [Internet]. 2017;28(8):2337 LP – 2352. Available from: http://jasn.asnjournals.org/content/28/8/2337.abstract.
Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717–1728.e6.
Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest. 2015;125(12):4592–600.
Liu J, Zong Z, Zhang W, Chen Y, Wang X, Shen J, et al. Nicotinamide mononucleotide alleviates lps-induced inflammation and oxidative stress via decreasing COX-2 expression in macrophages. Front Mol Biosci. 2021;6:8.
de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell [Internet]. 2016;15(3):522–30. Available from: https://doi.org/10.1111/acel.12461.
Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9(6).
Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun [Internet]. 2018;9(1):1286. Available from: https://doi.org/10.1038/s41467-018-03421-7.
Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation [Internet]. 2018;137(21):2256–73. Available from: https://doi.org/10.1161/CIRCULATIONAHA.116.026099.
Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab [Internet]. 2019;29(6):1274–1290.e9. Available from: https://doi.org/10.1016/j.cmet.2019.03.001.
• Niu K-M, Bao T, Gao L, Ru M, Li Y, Jiang L, et al. The impacts of short-term nmn supplementation on serum metabolism, fecal microbiota, and telomere length in pre-aging phase. Front Nutr. 2021;29:8. In addition to changes in the fecal microbiota and serum metabolite composition related to cofactors/vitamin metabolism in pre-aged mice, oral NMN supplementation for 40 days also resulted in an elongation of telomere length in peripheral blood mononuclear cells (PBMC) in both pre-aging mice and humans.
Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science (80-). 2021;372(6547):1224–9.
Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep [Internet]. 2019;9(1):9772. Available from: https://doi.org/10.1038/s41598-019-46120-z.
Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020;67(2):153–60.
Trammell SAJ, Y. L, Redpath P, Migaud ME, Brenner C. Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J Nutr. 2016;146(5):957–63.
Ummarino S, Mozzon M, Zamporlini F, Amici A, Mazzola F, Orsomando G, et al. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chem. 2017;15(221):161–8.
Kim L-J, Chalmers TJ, Smith GC, Das A, Poon EWK, Wang J, et al. Nicotinamide mononucleotide (NMN) deamidation by host-microbiome interactions. bioRxiv [Internet]. 2020;2020.09.10.289561. Available from: http://biorxiv.org/content/early/2020/09/11/2020.09.10.289561.abstract.
Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, et al. Bacteria boost mammalian host nad metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020;31(3):564–579.e7.
Huang P, Jiang A, Wang X, Zhou Y, Tang W, Ren C, et al. NMN maintains intestinal homeostasis by regulating the gut microbiota. Front Nutr. 2021;29:8.
Huang P, Wang X, Wang S, Wu Z, Zhou Z, Shao G, et al. Treatment of inflammatory bowel disease: potential effect of NMN on intestinal barrier and gut microbiota. Curr Res Food Sci. 2022;5:1403–11.
• Lapatto HAK, Kuusela M, Heikkinen A, Muniandy M, van der Kolk BW, Gopalakrishnan S, et al. Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci Adv. 2023;9(2). It was the first evidence of gut microbiota modulation in humans mediated by NR. This altered gut microbiota composition was associated with changes in the plasma metabolomic profile, which are related to mitochondrial energy metabolism, lipids, and amino acids. In addition, NR supplementation boosted mitochondrial biogenesis and influenced global DNA methylation in a tissue-specific manner.
Fabrizio G, Scarpa ES, Di Girolamo M. State of the art of protein mono-ADP-ribosylation: biological role and therapeutic potential. Front Biosci - Landmark. 2015;20(3):405–30.
Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol [Internet]. 2012;13(4):225–38. Available from: https://doi.org/10.1038/nrm3293.
Vyas S, Matic I, Uchima L, Rood J, Zaja R, Hay RT, et al. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat Commun. 2014;5(1).
Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res - Fundam Mol Mech Mutagen. 2001;477(1–2):97–110.
Pittelli M, Felici R, Pitozzi V, Giovannelli L, Bigagli E, Cialdai F, et al. Pharmacological effects of exogenous NAD on mitochondrial bioenergetics, DNA repair, and apoptosis. Mol Pharmacol. 2011;80(6):1136–46.
Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient forpoly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30(8):2967–78.
Fehr AR, Singh SA, Kerr CM, Mukai S, Higashi H, Aikawa M. The impact of PARPs and ADP-ribosylation on inflammation and host–pathogen interactions. Genes Dev. 2020;34(5–6):341–59.
Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495–502.
Gazzaniga F, Stebbins R, Chang SZ, McPeek MA, Brenner C. Microbial NAD metabolism: lessons from comparative genomics. Microbiol Mol Biol Rev. 2009;73(3):529–41.
Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. BioEssays [Internet]. 2003;25(7):683–90. Available from: https://doi.org/10.1002/bies.10297.
Zrenner R, Ashihara H. Nucleotide Metabolism. In: Plant Metabolism and Biotechnology. Chichester, UK: John Wiley & Sons, Ltd; 2011.
Giroud-Gerbetant J, Joffraud M, Giner MP, Cercillieux A, Bartova S, Makarov MV, et al. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol Metab. 2019;30:192–202.
Yang Y, Zhang N, Zhang G, Sauve AA. NRH salvage and conversion to NAD+ requires NRH kinase activity by adenosine kinase. Nat Metab. 2020;2(4):364–79.
Liu Y, Luo C, Li T, Zhang W, Zong Z, Liu X, et al. Reduced nicotinamide mononucleotide (NMNH) potently enhances NAD+ and suppresses glycolysis, the TCA cycle, and cell growth. J Proteome Res [Internet]. 2021;20(5):2596–606. Available from: https://doi.org/10.1021/acs.jproteome.0c01037.
Galeazzi L, Bocci P, Amici A, Brunetti L, Ruggieri S, Romine M, et al. Identification of nicotinamide mononucleotide deamidase of the bacterial pyridine nucleotide cycle reveals a novel broadly conserved amidohydrolase family. J Biol Chem. 2011;286(46):40365–75.
Trammell SAJ, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7(1).
Yaku K, Palikhe S, Izumi H, Yoshida T, Hikosaka K, Hayat F, et al. BST1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat Commun. 2021;12(1):6767.
Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr [Internet]. 2008;28(1):115–30. Available from: https://doi.org/10.1146/annurev.nutr.28.061807.155443.
Gakière B, Hao J, de Bont L, Pétriacq P, Nunes-Nesi A, Fernie AR. NAD+ biosynthesis and signaling in plants. CRC Crit Rev Plant Sci [Internet]. 2018;37(4):259–307. Available from: https://doi.org/10.1080/07352689.2018.1505591.
Hashida S, Takahashi H, Uchimiya H. The role of NAD biosynthesis in plant development and stress responses. Ann Bot [Internet]. 2009;103(6):819–24. Available from: https://doi.org/10.1093/aob/mcp019.
Smith EN, Schwarzländer M, Ratcliffe RG, Kruger NJ. Shining a light on NAD- and NADP-based metabolism in plants. Trends Plant Sci. 2021;26(10):1072–86.
Decros G, Beauvoit B, Colombié S, Cabasson C, Bernillon S, Arrivault S, et al. Regulation of pyridine nucleotide metabolism during tomato fruit development through transcript and protein profiling. Front Plant Sci [Internet]. 2019;10:1201. Available from: https://www.frontiersin.org/article/10.3389/fpls.2019.01201.
Ashihara H, Stasolla C, Yin Y, Loukanina N, Thorpe TA. De novo and salvage biosynthetic pathways of pyridine nucleotides and nicotinic acid conjugates in cultured plant cells. Plant Sci [Internet]. 2005;169(1):107–14. Available from: https://www.sciencedirect.com/science/article/pii/S0168945205000828.
Katahira R, Ashihara H. Profiles of the biosynthesis and metabolism of pyridine nucleotides in potatoes (Solanum tuberosum L.). Planta [Internet]. 2009;231(1):35–45. Available from: https://doi.org/10.1007/s00425-009-1023-2.
Di Martino C, Pallotta ML. Mitochondria-localized NAD biosynthesis by nicotinamide mononucleotide adenylyltransferase in Jerusalem artichoke (Helianthus tuberosus L.) heterotrophic tissues. Planta [Internet]. 2011;234(4):657. Available from: https://doi.org/10.1007/s00425-011-1428-6.
Medda R, Padiglia A, Lorrai A, Murgia B, Agrò AF, Castagnola M, et al. Purification and properties of a nucleotide pyrophosphatase from lentil seedlings. J Protein Chem [Internet]. 2000;19(3):209–14. Available from: https://doi.org/10.1023/A:1007007803996.
Spanò D, Pintus F, Pes R, Medda R, Floris G. Purification and characterisation of a soluble nucleotide pyrophosphatase/phosphodiesterase from prickly pear (Opuntia ficus indica) fruits. Food Res Int. 2011;44(7):2264–70.
Cantó C, Menzies KJ, Auwerx J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22(1):31–53.
Grozio A, Mills KF, Yoshino J, Bruzzone S, Sociali G, Tokizane K, et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab [Internet]. 2019;1(1):47–57. Available from: https://doi.org/10.1038/s42255-018-0009-4.
• Garofalo C, Sabbatini R, Zamporlini F, Minazzato G, Ferrocino I, Aquilanti L, et al. Exploratory study on the occurrence and dynamics of yeast-mediated nicotinamide riboside production in craft beers. LWT. 2021;1(147): 111605. In this article, the authors demonstrated the production of NR and NMN in craft beers using yeast-mediated fermentation, and the role of hop in enhancing NR levels during the process. This finding is of great interest to the fields of biotechnology and food science and technology, as it highlights the potential for utilizing fermentation processes to produce NAD+ precursors.
Sugiyama K, Iijima K, Yoshino M, Dohra H, Tokimoto Y, Nishikawa K, et al. Nicotinamide mononucleotide production by fructophilic lactic acid bacteria. Sci Rep [Internet]. 2021;11(1):7662. Available from: https://doi.org/10.1038/s41598-021-87361-1.
Xu R, Yuan Z, Lijuan Y, Li L, Li D, Lv C. Inhibition of NAMPT decreases cell growth and enhances susceptibility to oxidative stress. Oncol Rep. 2017;38(3):1767–73.
Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, et al. CD73 Protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated Tumor Cells. J Biol Chem. 2013;288(36).
Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol [Internet]. 2019;21(3):397–407. Available from: https://doi.org/10.1038/s41556-019-0287-4.
Cros C, Cannelle H, Laganier L, Grozio A, Canault M. Safety evaluation after acute and sub-chronic oral administration of high purity nicotinamide mononucleotide (NMN-C®) in Sprague-Dawley rats. Food Chem Toxicol. 2021;1(150):112060.
Liu L, Su X, Quinn WJ, Hui S, Krukenberg K, Frederick DW, et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018;27(5):1067–1080.e5.
Okabe K, Yaku K, Uchida Y, Fukamizu Y, Sato T, Sakurai T, et al. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front Nutr. 2022;11:9.
Huang H. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Front Aging. 2022;5:3.
Nikiforov A, Dölle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286(24):21767–78.
Capuzzi DM, Morgan JM, Brusco OA, Intenzo CM. Niacin dosing: relationship to benefits and adverse effects. Curr Atheroscler Rep [Internet]. 2000;2(1):64–71. Available from: https://doi.org/10.1007/s11883-000-0096-y.
Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast Sir2 and human SIRT1. J Biol Chem. 2002;277(47):45099–107.
Hwang ES, Song SB. Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules. 2020;10(5).
Kawamura T, Mori N, Shibata K. β-Nicotinamide mononucleotide, an anti-aging candidate compound, is retained in the body for longer than nicotinamide in rats. J Nutr Sci Vitaminol (Tokyo). 2016;62(4):272–6.
Imai S ichiro. A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res. 2010;62(1):42–7.
Liao B, Zhao Y, Wang D, Zhang X, Hao X, Hu M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. J Int Soc Sports Nutr [Internet]. 2021;18(1):54. Available from: https://doi.org/10.1186/s12970-021-00442-4.
Mateuszuk Ł, Campagna R, Kutryb-Zając B, Kuś K, Słominska EM, Smolenski RT, et al. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem Pharmacol. 2020;1(178):114019.
Zhang R, Shen Y, Zhou L, Sangwung P, Fujioka H, Zhang L, et al. Short-term administration of nicotinamide mononucleotide preserves cardiac mitochondrial homeostasis and prevents heart failure. J Mol Cell Cardiol. 2017;112:64–73.
Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–47.
McReynolds MR, Chellappa K, Chiles E, Jankowski C, Shen Y, Chen L, et al. NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Syst [Internet]. 2021;12(12):1160–1172.e4. Available from: https://www.sciencedirect.com/science/article/pii/S2405471221003380.
Zhu X-H, Lu M, Lee B-Y, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci. 2015;112(9):2876–81.
Ramsey KM, Mills KF, Satoh A, Imai SI. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7(1):78–88.
Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23(6):1127–39.
Takaso Y, Noda M, Hattori T, Roboon J, Hatano M, Sugimoto H, et al. Deletion of CD38 and supplementation of NAD+ attenuate axon degeneration in a mouse facial nerve axotomy model. Sci Rep. 2020;10(1).
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194.
Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature [Internet]. 2009;458(7241):1056–60. Available from: https://doi.org/10.1038/nature07813.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell [Internet]. 2006;127(2):397–408. Available from: https://www.sciencedirect.com/science/article/pii/S0092867406012281.
Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130(6):1095–107.
Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) [Internet]. 2020;12(10):9959–81. Available from: https://doi.org/10.18632/aging.103344.
Omran HM, Almaliki MS. Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: a hypothesis. J Infect Public Health. 2020;13(9):1196–201.
Zheng M, Schultz MB, Sinclair DA. NAD+ in COVID-19 and viral infections. Trends Immunol. 2022;43(4):283–95.
Kawahara TLA, Michishita E, Adler AS, Damian M, Berber E, Lin M, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74.
Altay O, Arif M, Li X, Yang H, Aydın M, Alkurt G, et al. Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19. Adv Sci. 2021;8(17):2101222.
Pietrobon AJ, Andrejew R, Custódio RWA, Oliveira L de M, Scholl JN, Teixeira FME, et al. Dysfunctional purinergic signaling correlates with disease severity in COVID-19 patients. Front Immunol. 2022;13.
Haskó G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4.
Haag F, Adriouch S, Braß A, Jung C, Möller S, Scheuplein F, et al. Extracellular NAD and ATP: partners in immune cell modulation. Purinergic Signal. 2007;3(1–2):71.
Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci [Internet]. 2019;26(1):34. Available from: https://doi.org/10.1186/s12929-019-0527-8.
Fan L, Cacicedo JM, Ido Y. Impaired nicotinamide adenine dinucleotide (NAD+) metabolism in diabetes and diabetic tissues: implications for nicotinamide-related compound treatment. J Diabetes Investig [Internet]. 2020;11(6):1403–19. Available from: https://doi.org/10.1111/jdi.13303.
Uddin GM, Youngson NA, Chowdhury SS, Hagan C, Sinclair DA, Morris MJ. Administration of nicotinamide mononucleotide (NMN) reduces metabolic impairment in male mouse offspring from obese mothers. Cells. 2020;9(4).
Yu J, Laybutt DR, Kim L-J, Quek L-E, Wu LE, Morris MJ, et al. Exercise-induced benefits on glucose handling in a model of diet-induced obesity are reduced by concurrent nicotinamide mononucleotide. Am J Physiol Metab [Internet]. 2021;321(1):E176–89. Available from: https://doi.org/10.1152/ajpendo.00446.2020.
Wu J, Jin Z, Zheng H, Yan L-J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab Syndr Obes [Internet]. 2016;9:145–53. Available from: https://pubmed.ncbi.nlm.nih.gov/27274295.
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature [Internet]. 2019;572(7770):474–80. Available from: https://doi.org/10.1038/s41586-019-1443-5.
Sun L-J, Li J-N, Nie Y-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin Med J (Engl). 2020;133(7):826–33.
Mishra AK, Dubey V, Ghosh AR. Obesity: an overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism. 2016;65(1):48–65.
Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71.
Yang C, Du Y, Ren D, Yang X, Zhao Y. Gut microbiota-dependent catabolites of tryptophan play a predominant role in the protective effects of turmeric polysaccharides against DSS-induced ulcerative colitis. Food Funct. 2021;12(20):9793–807.
Corral-Jara KF, Nuthikattu S, Rutledge J, Villablanca A, Fong R, Heiss C, et al. Structurally related (−)-epicatechin metabolites and gut microbiota derived metabolites exert genomic modifications via VEGF signaling pathways in brain microvascular endothelial cells under lipotoxic conditions: Integrated multi-omic study. J Proteomics. 2022;263:104603.
Marć MA, Jastrząb R, Mytych J. Does the gut microbial metabolome really matter? The connection between GUT metabolome and neurological disorders. Nutrients. 2022;14(19):3967.
Liu L, Xie Y, Li G, Zhang T, Sui Y, Zhao Z, et al. Gut microbiota‐derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br J Pharmacol. 2022.
Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;20:6.
Turroni F, Ventura M, Buttó LF, Duranti S, O’Toole PW, Motherway MO, et al. Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci. 2014;71(2):183–203.
Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. gastroenterology. 2017;153(3):772–86.
Zhang Y, Wang X, Zhou M, Kang C, Lang H, Chen M, et al. Crosstalk between gut microbiota and sirtuin-3 in colonic inflammation and tumorigenesis. Exp Mol Med. 2018;50(4):1–11.
Zhang X, Tian B, Deng Q, Cao J, Ding X, Liu Q, et al. Nicotinamide riboside relieves the severity of experimental necrotizing enterocolitis by regulating endothelial function via eNOS deacetylation. Free Radic Biol Med. 2022;184:218–29.
Lozada-Fernández VV, DeLeon O, Kellogg SL, Saravia FL, Hadiono MA, Atkinson SN, et al. Nicotinamide riboside-conditioned microbiota deflects high-fat diet-induced weight gain in mice. mSystems. 2022;7(1).
Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. microorganisms. 2020;8(11):1715.
Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr. 2019;59(sup1):S130–52.
Ren Z, Xu Y, Li T, Sun W, Tang Z, Wang Y, et al. NAD+ and its possible role in gut microbiota: Insights on the mechanisms by which gut microbes influence host metabolism. Anim Nutr. 2022;10:360–71.
Jiang Y, Liu Y, Gao M, Xue M, Wang Z, Liang H. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020;378–91.
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux J-J, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci. 2008;105(43):16731–6.
Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11(7):1667–79.
Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83.
Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, et al. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52(2):398–406.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. GeroScience [Internet]. 2019;41(5):619–30. Available from: https://doi.org/10.1007/s11357-019-00074-2.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;1(24):101192.
Bertoldo MJ, Listijono DR, Ho WHJ, Riepsamen AH, Goss DM, Richani D, et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020;30(6):1670–1681.e7.
Wu K, Li B, Lin Q, Xu W, Zuo W, Li J, et al. Nicotinamide mononucleotide attenuates isoproterenol-induced cardiac fibrosis by regulating oxidative stress and Smad3 acetylation. Life Sci. 2021;1(274):119299.
Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. GeroScience [Internet]. 2019;41(4):419–39. Available from: https://doi.org/10.1007/s11357-019-00095-x.
Kiss T, Nyúl-Tóth Á, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. GeroScience [Internet]. 2020;42(2):527–46. Available from: https://doi.org/10.1007/s11357-020-00165-5.
Li D-J, Sun S-J, Fu J-T, Ouyang S-X, Zhao Q-J, Su L, et al. NAD(+)-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin. Theranostics [Internet]. 2021;11(9):4381–402. Available from: https://pubmed.ncbi.nlm.nih.gov/33754067.
Wang Z, Bao X, Hu J, Shen S, Xu G, Wu Y. Nicotinamide riboside enhances endothelial precursor cell function to promote refractory wound healing through mediating the Sirt1/AMPK pathway [Internet]. Front Pharmacol. 2021;12:1081. Available from: https://www.frontiersin.org/article/10.3389/fphar.2021.671563.
Park JM, Han YM, Lee HJ, Park YJ, Hahm KB. Nicotinamide riboside vitamin B3 mitigated C26 adenocarcinoma-induced cancer cachexia. Front Pharmacol [Internet]. 2021;12:665493. Available from: https://pubmed.ncbi.nlm.nih.gov/34262449.
Seldeen KL, Shahini A, Thiyagarajan R, Redae Y, Leiker M, Rajabian N, et al. Short-term nicotinamide riboside treatment improves muscle quality and function in mice and increases cellular energetics and differentiating capacity of myogenic progenitors. Nutrition [Internet]. 2021;87–88:111189. Available from: https://www.sciencedirect.com/science/article/pii/S0899900721000514.
Funding
The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES) (grant number 88887.353067/2019–00) and São Paulo Research Foundation, FAPESP-Brazil (grant number 2020/08761–4) for their financial support.
Author information
Authors and Affiliations
Contributions
ALEGRE, GFS: designed research and wrote paper (conceptualization, writing, review, and editing); PASTORE, GM: designed research (conceptualization and supervision). All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any potential conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alegre, G.F.S., Pastore, G.M. NAD+ Precursors Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR): Potential Dietary Contribution to Health. Curr Nutr Rep 12, 445–464 (2023). https://doi.org/10.1007/s13668-023-00475-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00475-y