Abstract
We examined the chemical constitution of the red alga Laurencia saitoi Perestenko, collected from Katsuura, Boso Peninsula, Chiba Prefecture, Japan. This specimen produced a new polyhalogenated acetogenin, named katsuurallene (1), which structure was determined by the spectral methods, along with known diterpene, deoxyparguerol (2) and triterpene, thyrsiferol (3). In this paper we describe the structural elucidation of katsuurallene together with some biological activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Red algae of the genus Laurencia (Rhodomelaceae, Ceramiales) are the most intensively studied of all algal genera. More than 1000 secondary metabolites with intriguing skeletal structures, including about 800 halogenated compounds, were reported from this unique genus [1,2,3,4]. New halogenated metabolites are being discovered from Laurencia [5, 6], which seem to be an endless source of novel compounds.
The Laurencia species, from which halogenated metabolites have been isolated, possess “corps en cerise” in both superficial cortical cells and trichoblast cells. “Corps en cerise”, an unusually swollen refractile inclusion, is recognized as the site of synthesis and/or storage of halogenated compounds [7]. On the other hand, some species without “corps en cerise” produce no halogenated compound. To date, the chemical constitution of 22 species of Japanese Laurencia with “corps en cerise”, including 7 taxonomically undescribed species, have been investigated [8,9,10,11].
As part of our additional studies of the chemical diversity in the Japanese Laurencia species, we examined the chemical composition of Laurencia spp. from Katsuura, Boso Peninsula, Chiba Prefecture. Boso Peninsula is situated in the southeast side of Kanto area on Honshu, the largest island of Japan. Its coastline faces Tokyo Bay to the west and Pacific Ocean to the east and south.
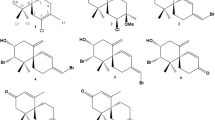
The coasts of Katsuura are influenced by both the Kuroshio Current (warm current) and the Oyashio Current (cold current), and its marine flora contains more subtropical elements than subarctic elements. In the intertidal coast of Yoshio, Katsuura, four Laurencia spp., L. saitoi, L. intricata, L. okamurae, and L. japonensis, grow sympatrically from April and July [12]. Among them L. intricata contained zagashimallene (4), cyclocolorenone (5), and intricatetraol (6) [13]. L. japonensis contained two new brominated acetogenins katsuurenyne A (7) (Fig. 1) and katsuurenyne B (8) along with known 2,10-dibromo-3-chloro-α-chamigrene (9) and aplysiadiol (10) [14]. Furthermore, in the coast of Yoshio, L. okamurae unusually grow sympatrically in morphological variation, a clumpy type and a non-clumpy type. The extracts of both specimens showed almost identical patterns on TLC and contained laurinterol (11) as the major metabolite (unpublished result).
In this paper we describe the chemical composition of Laurencia saitoi and structure elucidation of a new polyhalogenated acetogenin, designated as katsuurallene (1), together with the biological activities of the isolated compounds.
2 Results and discussion
2.1 Chemical composition of L. saitoi
The EtOAc-soluble fraction was subjected to a combination of column and preparative thin-layer chromatography to yield a halogenated acetogenin, named katsuurallene (1), along with two known terpenoids, deoxyparguerol (2) and thyrsiferol (3).
Katsuurallene (1), [α] 28D + 73.5 (c 0.15; CHCl3), was analyzed for C15H20Br2Cl2O2 by HRESI-MS. The presence of a terminal bromoallenic side chain was proven by typical signals in the 1H- and 13C-NMR spectra (Table 1) [δH 6.09 (1H, dd, J = 6.0, 1.8 Hz) and 5.43 (1H, dd, J = 6.0, 6.0 Hz); δC 201.68 (C), 101.37 (CH), and 74.39 (CH)] [10]. Since the IR spectrum revealed no hydroxy and carbonyl absorptions, the two oxygen atoms in 1 were assumed to be involved in ether linkages.
Detailed analysis of the 1H- and 13C-NMR spectra, as well as HMQC and 1H-1H COSY spectra, led to the partial structure 1a (Fig. 2) for katsuurallene (1). In 1a, the oxygen atoms at C-4, C-7, C-9, and C-13 were verified based upon the chemical shift values of the pertinent carbons at 73.79 (C-4), 76.05 (C-9), 77.94 (C-7), and 83.79 (C-13), respectively. Moreover, the substituent at C-6 and C-10 were proven to be chlorine atom by the chemical shifts at 62.83 (C-6) and 61.87 (C-10). This was also confirmed by observation of the halogen-induced 13C isotope shifts [15] in the 13C-NMR spectrum. Therefore, the remaining bromine atom is attached to C-12.
The 13C-NMR spectrum showed that there were no other double bonds apart from those of the bromoallene moiety, and therefore katsuurallene (1), having four degrees of unsaturation, must be composed of two oxide rings. In the HMBC spectra of 1, the long-range correlations between H-4/C-7, H-9/C-13 and H-13/C-9 (Table 1) were observed. This meant that two ether rings must be formed between C-4 and C-7 and between C-9 and C-13, leading to a planar structure 1 for katsuurallene.
The relative stereochemistry was partly determined as follows. In the NOESY spectrum of 1, the nuclear Overhauser effect was observed between H-9 and H-13, thus indicating that both H-9 and H-13 have axial configurations on a tetrahydropyran ring with a chair-like conformation (Fig. 3). Furthermore, in the NMR spectrum (in C6D6) of 1 (Table 1, footnote), the H-12 showed the coupling constants of J12,13 = 10.9 Hz, J11a,12 = 11.9 Hz and J11b,12 = 4.6 Hz, which are typical axial/axial, axial/axial, and axial/equatorial coupling constants, respectively, indicating that the H-12 has axial configuration (equatorial bromine atom) on a tetrahydropyran ring. On the other hand, the H-9 showed the coupling constant of J9,10 = 2.3 Hz, which is a typical equatorial/axial coupling constant, indicating that the H-10 has an equatorial configuration (axial chlorine atom) on a tetrahydropyran ring.
The relative configuration on the oxolane ring was also determined by the NOESY spectrum. The cis-relationship of the substituents at C-6 and C-7 was shown by a NOE correlation between H-6 and H-7. H-6 was further correlated to Ha-5. On the other hand, Hb-5 was correlated to H-4, thus indicating the trans-relationship between H-4 and H-7 (Fig. 3).
In view of the positive sign of the optical rotation of 1, the absolute configuration of the bromoallene moiety was suggested S-configuration, according to Lowe’s rule [16, 17], though a few exceptions to Lowe’s rule were reported in the case of microcladallenes [18] and also (E)- and (Z)-9-epi-omaezallene [10]. Consequently, the structure of katsuurallene would be represented by formula 1.
As shown in Fig. 4, the related acetogenins have been found; 12 from L. obtusa (Canary Island) [19], 13 from L. paniculata (Turkey) [20] and bisezakyne-B (14), which may be a plausible shunt product of katsuurallene (1) biosynthetic pathway, from Japanese Laurencia sp. (Okinawa Prefecture) [15]. Furthermore, sargonenyne (15) and its related bromoallene (16) have been isolated from L. obtusa collected in Corsica [21, 22]. We are currently attempting to prepare a crystal suitable for X-ray crystallographic analysis in order to confirm the structure and establish the absolute stereochemistry for katsuurallene (1).
2.2 Biological activity
Katsuurallene (1) was evaluated for insect repellent assay, Arabidopsis growth inhibition assay, antioxidant assay, brine shrimp assay and antimicrobial assay. Furthermore, zagashimallene (4), cyclocolorenone (5), and laurinterol (11) were evaluated brine shrimp assay, antioxidant assay and antimicrobial assay. 1 had weak toxicity for brine shrimp (LC50 = 855 μg/mL) and weak antimicrobial activity (no inhibition zone at 10 μg/disc, 11.0 mm inhibition ring at 30 μg/disc). 1 did not have insect repellent activity at 104 μg/cm2 (1.0 mg/disc), Arabidopsis growth inhibition activity at 100 μg/mL, and antioxidant activity at 100 μg/mL. Compounds 4, 5 and 11 had toxicity for brine shrimp. LC50 of 4, 5 and 11 were 3 μg/mL, 6 μg/mL and 37 μg/mL, respectively. Compounds 4, 5 and 11 did not have antioxidant activity at 100 μg/mL and antimicrobial activity at 30 μg/disc.
2.3 Conclusion
As described above, the specimen of Laurencia saitoi from Katsuura contained a new halogenated acetogenin, katsuurallene (1), as a characteristic major metabolite, along with deoxyparguerol (2) and thyrsiferol (3). On the other hand, the specimens from Teuri Island [23,24,25,26,27] and Suttsu [28] in Hokkaido contained diterpenes and triterpenes as characteristic metabolites. Since L. saitoi Perestenko has passed under the name L. obtusa (Hudson) Lamouroux in Japan [29], the former specimen from Teuri Island was first reported as L. obtusa.
Three Chinese specimens of L. saitoi have also been examined. The specimen collected from the coast of Yantai, Shandong Province, produced several parguerane-diterpenes and two triterpenes thyrsiferol and thyrsiferyl 23-acetate [30], which are very similar to the metabolites of the specimens from Hokkaido. However, the specimen collected from the coast of Rongcheng, northern Shandong Province, produced halogenated chamigrane-, bisabolane- and laurane-sesquiterpenes [31]. And the specimen collected from Hainan coastlines produced halogenated snyderane-sesquiterpenes [32]. The difference in chemical composition of the specimens of L. saitoi strongly requires doing taxonomical reexamination.
3 Experimental
3.1 General experimental procedures
IR spectra were recorded on a PerkinElmer FT-IR Spectrum Two spectrophotometer. 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were measured in CDCl3 or C6D6 by using JEOL-JNM-ECS-400 spectrometer. ESI-MS were obtained on a Hitachi High-Technologies Corporation NanoFrontier eLD spectrometer. Optical rotations were measured on a HORIBA SEPA-500 polarimeter. UV-Vis spectra were recorded on a JASCO V-650 spectrophotometer. Silica gel (Merck, Kieselgel 60, 70-230 mesh) were used for column chromatography (CC). Silica gel plate (Merck, Kieselgel 60 F254S) was used for preparative thin-layer chromatography (TLC).
3.2 Plant material
Laurencia saitoi Prestenko was collected from the coast of Yoshio (35°8′N, 140°17′E), Katsuura, Boso Peninsula, Chiba Prefecture, on 18 May 2018. The voucher specimen has been deposited in the Herbarium of the Coastal Branch of Natural History Museum and Institute, Chiba (CMNH).
3.3 Extraction and isolation of L. saitoi
The algal sample (58.0 g dry weight) was extracted twice with MeOH. The resulting MeOH solution was concentrated in vacuo and partitioned between EtOAc and H2O. The EtOAc layer was washed with water, dried over dry Na2SO4 and evaporated to leave an oily substance. The EtOAc-soluble extract (545 mg) was then fractionated by Si gel column chromatography with a step gradient (hexane and EtOAc). A portion (120 mg) of the fraction (158 mg) eluted with hexane–EtOAc (4:1) was further subjected to prep. TLC with toluene to yield a crude substance which was purified by prep. TLC with hexane-EtOAc (9:1) to give katsuurallene (1) (19.6 mg). A portion (85 mg) of the fraction (99 mg) eluted with hexane-EtOAc (1:1) was subjected to prep. TLC with hexane-EtOAc (1.5:1) to yield a crude substance which was further subjected to prep. TLC with toluene-EtOAc (2:1) to yield two fractions. The less polar fraction (25 mg) was then purified by prep. TLC with CHCl3-MeOH (95:5) to give deoxyparguerol (2) (6.3 mg). The polar fraction (30 mg) was purified by prep. TLC with CHCl3-MeOH (95:5) to give thyrsiferol (3) (7.7 mg).
Katsuurallene (1): Colorless solid; [α] 28D + 73.5 (c 0.15; CHCl3); IR νmax (film) cm−1; 3057, 1960, 1372, 1309, 1196, 1090, 1026, 994, 819; 1H- and 13C-NMR spectra, Table 1 (Additional file 1: Figs. S1–S7); HR-ESIMS m/z; 460.9280. Calc. for C15H2079Br235Cl2O2, 460.9285 [M + H]+.
Deoxyparguerol (2): Colorless oil; The 1H-NMR data (Additional file 1: Fig. S8) were found to be identical to those previously reported [33].
Thyrsiferol (3): Colorless solid; The 1H-NMR data (Additional file 1: Fig. S9) were found to be identical to those previously reported [34].
3.4 Biological activity
3.4.1 Insect repellent assay
The repellent activities of some isolated compounds against the maize weevils Sitophilus zeamais were evaluated using the filter paper impregnation method as previously described [35]. The numbers of adult beetles present in each Petri dish were recorded after 24 h of exposure. Each treatment was repeated three times. Pyrethrin standard was used as a positive control.
3.4.2 Growth inhibition assay
The wild-type Arabidopsis seeds (Col-0) were immersed in 70% ethanol for 5 min and then 1.5% NaClO with Tween 80 for 5 min. Seeds were subsequently rinsed with distilled water, and then soaked in 0.1% agar solution for several hours at 4 °C. Surface sterilized seeds were grown in half-strength Murashige and Skoog (MS) medium supplemented with 0.8% agar and 1% sucrose for 1 week at 22 °C. For the plant growth assay, well-grown seedlings were selected and transferred to 12-well plate containing 1/2 MS medium with 1% sucrose. Test samples were dissolved in DMSO and prepared to a final concentration of 1 mg/mL (less than 1% DMSO). After the treatment of samples, the plates were incubated on a rotary shaker for 4 days at 22 °C under light–dark cycle conditions (12L:12D). The growth of the seedlings was estimated by the individual weights. All the experiments were performed three times.
3.4.3 Antioxidant assay
An aliquot of antioxidant Trolox or compounds diluted with ethanol (20 μL) was mixed with the 80 μM Tris–HCl buffer (pH 7.4) and then added to 100 μL of 200 μM 2,2-diphenyil-picrylhydrazyl (DPPH) (Alfa Aesar) in ethanol. The mixture was shaken vigorously and left to stand for 30 min at room temperature in the dark. The absorbance at 515 nm by DPPH was measured by UV–Vis spectrophotometer.
3.4.4 Brine shrimp assay
A bioassay of toxicity toward brine shrimp was performed as described in the literature [36]. Briefly, the compounds dissolved in ethanol were made up to 5, 10 and 50 μg/mL in artificial seawater. Serial dilution was made in the wells of 24-well microplates (Iwaki, Asahi Techno Glass Co., Tokyo, Japan) in triplicate in artificial seawater (2 mL). Brine shrimp eggs obtained locally (Japan Pet Design Co., Ltd., Tokyo, Japan) were hatched in artificial seawater at 25 °C. After 48 h, a suspension of nauplii containing 10–20 organisms (100 µL) was added to each well and incubated at 25 °C for 24 h and the numbers of non-motile and total nauplii in each well were counted in turn.
3.4.5 Antimicrobial assay
Antibacterial bioassays were carried out using Escherichia coli NBRC-3972 strain. Organism was precultured in LB medium for 2 days. The turbidity of the culture was adjusted to 107 cells/mL using hemocytometer. Then 0.2 mL of the precultured bacterial suspension was used to seed LB agar plate. Paper discs (6 mm; ADVANTEC Toyo, Tokyo, Japan) impregnated with various amounts of the respective pure compounds were placed on the seeded agar plates and the diameters of the inhibitory zones were measured after the plates were incubated at 37 °C for 2 days.
References
Wang BG, Gloer JB, Ji NY, Zhao JC. Halogenated organic molecules of rhodomelaceae origin: chemistry and biology. Chem Rev. 2013;113:3632–85.
Ji NY, Wang BG. Nonhalogenated organic molecules from Laurencia algae. Phytochem Rev. 2014;13:653–70.
Harizani M, Ioannou E, Roussis V. The Laurencia paradox: an endless source of chemodiversity. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, editors. Progress in the chemistry of organic natural products 102. Wien: Springer International Publishing; 2016. p. 91–252.
M. Suzuki, Database (2021), http://laurencia-database.jp. Accessed 29 Aug 2021
Koutsaviti A, Daskalaki MG, Agusti S, Kampranis SC, Tsatsanis C, Duarte CM, Roussis V, Ioannou E. Thuwalallenes A–E and Thuwalenynes A–C: new C15 acetogenins with anti-inflammatory activity from a Saudi Arabian Red Sea Laurencia sp. Mar Drugs. 2019;17:644.
Ghandourah M, Alarif W, Bawakid N. New bioactive C15 acetogenins from the red alga Laurencia obtusa. Pharmacogn Mag. 2019;15:199–203.
Young DN, Howard BM, Fenical W. Subcellular localization of brominated secondary metabolites in the red alga Laurencia snyderae. J Phycol. 1980;16:182–5.
Suzuki M, Vairappan CS. Halogenated secondary metabolites from Japanese species of the red algal genus Laurencia (Rhodomelaceae, Ceramiales). Cur Top Phytochemistry. 2005;7:1–34.
Suzuki M, Kawamoto T, Vairappan CS, Ishii T, Abe T, Masuda M. Halogenated metabolites from Japanese Laurencia spp. Phytochemistry. 2005;66:2787–93.
Umezawa T, Oguri Y, Matsuura H, Yamazaki S, Suzuki M, Yoshimura E, Furuta T, Nogata Y, Serisawa Y, Matsuyama-Serisawa K, Abe T, Matsuda F, Suzuki M, Okino T. Omaezallene from red alga Laurencia sp.: structure elucidation, total synthesis, and antifouling activity. Angew Chem Int Ed. 2014;53:3909–12.
Minamida Y, Matsuura H, Ishii T, Sato K, Kamada T, Kato A, Yamagishi Y, Abe T, Kikuchi N, Suzuki M. Chemical composition of Laurencia spp. collected from the Seto Inland Sea of Japan. Biochem Syst Ecol. 2021;96:104259.
Kikuchi N, Miyata M. Marine Flora at Katsuura-city, Southeastern Part of the Boso Peninsula, Japan (revised edition). J Nat Hist Mus Inst, Chiba, Special Issue. 2021;11:9–24.
Ishii T, Shinjo Y, Miyagi M, Matsuura H, Abe T, Kikuchi N, Suzuki M. Investigation of insect repellent activity of cyclocolorenone obtained from the red alga Laurencia intricata. Rec Nat Prod. 2019;13:81–4.
Ishii T, Miyagi M, Shinjo Y, Minamida Y, Matsuura H, Abe T, Kikuchi N, Suzuki M. Two new brominated C15-acetogenins from the red alga Laurencia japonensis. Nat Prod Res. 2020;34:2787–93.
Suzuki M, Nakano S, Takahashi Y, Abe T, Masuda M. Bisezakyne-A and -B, halogenated C15 acetogenins from a Japanese Laurencia species. Phytochemistry. 1999;51:657–62.
Lowe G. The absolute configuration of allenes. Chem Commun. 1965;411–3.
Elsevier CJ, Vermeer P, Gedanken A, Runge W. Synthesis and absolute configurations of halogenoallenes. J Org Chem. 1985;50:364–7.
Sohn TI, Kim D, Paton RS. Substrate-controlled asymmetric total syntheses of microcladallenes A, B, and C based on the proposed structures. Chem Eur J. 2015;21:15988–97.
Norte M, Fernandez JJ, Ruano JZ. Three new bromo ethers from the red alga Laurencia obtusa. Tetrahedron. 1989;45:5987–94.
Imre S, Aydogmus Z, Guner H, Lotter H, Wagner H. Polybrominated non-terpenoid C15 compounds from Laurencia paniculata and Laurencia obtusa. Z Naturforsch. 1995;50c:743–7.
Esselin H, Sutour S, Liberal J, Cruz MT, Salgueiro L, Siegler B, Freuze I, Castola V, Paoli M, Bighelli A, Tomi F. Chemical composition of Laurencia obtusa extract and isolation of a new C15-acetogenin. Molecules. 2017;22:779.
Esselin H, Tomi F, Bighelli A, Sutour S. New metabolites isolated from a Laurencia obtusa population collected in Corsica. Molecules. 2018;23:720.
Suzuki T, Suzuki M, Furusaki A, Matsumoto T, Kato A, Imanaka Y, Kurosawa E. Teurilene and thyrsiferyl 23-acetate, meso and remarkably cytotoxic compounds from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Tetrahedron Lett. 1985;26:1329–32.
Suzuki T, Takeda S, Suzuki M, Kurosawa E, Kato A, Imanaka Y. Cytotoxic squalene-derived polyethers from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem Lett. 1987;16:361–4.
Suzuki T, Takeda S, Hayama N, Tanaka I, Komiyama K. The structure of brominated diterpene from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem Lett. 1989;18:969–70.
Takeda S, Matsumoto T, Komiyama K, Kurosawa E, Suzuki T. A new cytotoxic diterpene from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem Lett. 1990;19:277–80.
Takeda S, Kurosawa E, Komiyama K, Suzuki T. The structures of cytotoxic diterpenes containing bromine from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Bull Chem Soc Jpn. 1990;63:3066–72.
Kurata K, Taniguchi K, Agatsuma Y, Suzuki M. Diterpenoid feeding-deterrents from Laurencia saitoi. Phytochemistry. 1998;47:363–9.
Masuda M, Abe T. The occurrence of Laurencia saitoi Perestenko (L. obtusa auct. japon.) (Ceramiales, Rhodophyta) in Japan. Jpn J Phycol. 1993;41:7–18.
Ji NY, Li XM, Wang BG. Halogenated terpenes and a C15-acetogenin from the marine red alga Laurencia saitoi. Molecules. 2008;13:2894–9.
Ji NY, Li XM, Li K, Wang BG. Halogenated sesquiterpenes from the marine red alga Laurencia saitoi (Rhodomelaceae). Helv Chim Acta. 2009;92:1873–9.
Su H, Yuan ZH, Li J, Guo SJ, Deng LP, Han LJ, Zhu XB, Shi DY. Sesquiterpenes from the marine red alga Laurencia saitoi. Helv Chim Acta. 2009;92:1291–7.
Schmitz FJ, Michaud DP, Schmidt PG. Marine natural products: parguerol, deoxyparguerol, and isoparguerol. New brominated diterpenes with modified pimarane skeletons from the sea hare Aplysia dactylomela. J Am Chem Soc. 1982;104:6415–23.
Manzo E, Gavagnin M, Bifulco G, Cimino P, Micco SD, Ciavatta ML, Guo YW, Cimino G. Aplysiols A and B, squalene-derived polyethers from the mantle of the sea hare Aplysia dactylomela. Tetrahedron. 2007;63:9970–8.
Ishii T, Nagamine T, Nguyen BCQ, Tawata S. Insecticidal and repellent activities of laurinterol from the Okinawan red alga Laurencia nidifica. Rec Nat Prod. 2017;11:63–8.
Solis PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993;59:250–2.
Acknowledgements
The authors are grateful to Mr. H. Akutsu (Central Laboratory for Research and Education, Center for Advanced Research and Education, Asahikawa Medical University) for the measurement of high resolution mass spectra. This work was supported by JSPS KAKENHI Grant Numbers 18K05799 and 21K14904.
Author information
Authors and Affiliations
Contributions
Y. Minamida, HM, TI and MS conceived and designed the research; Y. Minamida, HM, TI, MM, YS, K. Sato, TK and MS carried out the experiment and wrote the manuscript; Y. Mihara, IT, K. Sugimoto carried out biological assays; TA and NK collected and identified the algal species; HM and MS supervised the whole study and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
No conflict of interest is declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
1H-NMR (1D), 13C-NMR, DEPT, COSY, HMQC, HMBC, NOESY.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minamida, Y., Matsuura, H., Ishii, T. et al. New acetogenin katsuurallene from Laurencia saitoi collected from Katsuura, Japan. Nat. Prod. Bioprospect. 12, 10 (2022). https://doi.org/10.1007/s13659-022-00328-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-022-00328-1