Abstract
Round-shaped and uniformly distributed eyes are important quality features for several Swiss semi-hard cheese varieties such as Tilsit. Recently, the growth of histamine-producing strains of Lactobacillus parabuchneri has been associated with cheese defects, such as crack formation and burning taste. In this paper, the influence of pH on the metabolic activity of added strains of Lactobacillus buchneri and L. parabuchneri, which possess various CO2-producing activities, was studied in Tilsit-type model cheeses. Two different pH values were obtained by modifying the cheese-making process (curd washing and type of ripening). Lactate, free amino acids, free short-chain fatty acids and 1,2-propanediol were determined in the ripened cheeses. In the acidic cheeses (average pH = 5.40), significantly more 1,2-propanediol was produced, presumably from lactate. Lactobacillus parabuchneri FAM 21731, a histamine-producing strain, produced small amounts of 1,2-propanediol (0.2 mmol.kg−1) and high amounts of histamine (3.3 mmol.kg−1). Ornithine was produced by all the studied strains, with the highest amount of 9.0 mmol.kg−1 produced by L. parabuchneri FAM 21835 in the acidic cheeses. Standard cheese making representing the high pH group (curd washed and smear ripened, average pH = 5.70), and the addition of a glutamate decarboxylase-positive L. buchneri, resulted in higher amounts of γ-aminobutyric acid (GABA) (8.1 compared to 3.3 mmol.kg−1 in the control standard cheeses). Irrespective of the strain, the GABA level was much higher in all the acidic cheeses than in the standard cheeses (14.7 compared to 4.6 mmol.kg−1, respectively). The study clearly demonstrates the importance of the cheese pH in the metabolic activity of the added strains during cheese ripening.
Similar content being viewed by others
1 Introduction
The number, size, roundness and uniform distribution of eyes are important quality features for several Swiss semi-hard cheese varieties, such as Appenzeller and Tilsit. If eye formation is unsatisfactory, the cheese is downgraded. The production of CO2 by non-starter lactic acid bacteria (NSLAB) is an important contributor to eye formation. The NSLAB in cheese mainly consist of mesophilic lactobacilli. Based on their carbohydrate catabolism, they are subdivided into obligate homofermenters, facultative heterofermenters and obligate heterofermenters. Typically, facultatively heterofermentative lactobacilli predominate. Obligately heterofermentative lactobacilli (OHL) may also be present, especially during the later stages of cheese ripening. According to Coton et al. (2008), Lactobacillus brevis, Lactobacillus fermentum and Lactobacillus parabuchneri are the major OHL present in cheese. Park and Oh (2006) isolated various strains of Lactobacillus buchneri, another OHL, from naturally aged cheese.
Lactobacillus parabuchneri and L. buchneri are of interest because they possess various catabolic activities producing CO2. First, hexoses are degraded by the phosphoketolase pathway, yielding lactate, ethanol/acetate and CO2. Second, both species possess the arginine deiminase (ADI, EC 3.5.3.6) pathway by which arginine is converted into ornithine, with the concomitant release of CO2 and ammonia (Manca de Nadra et al. 1988). Histidine decarboxylase (HDC, EC 4.1.1.22) and glutamate decarboxylase (GAD, EC 4.1.1.15) activity was detected in some strains of L. parabuchneri and L. buchneri, respectively (Fröhlich-Wyder et al. 2013; Cho et al. 2007; Sumner et al. 1985). HDC removes the carboxyl group from histidine to yield histamine, and GAD removes the carboxyl group from glutamate to yield γ-aminobutyric acid (GABA). Finally, both species produce 1,2-propanediol and acetic acid from lactate, which is probably linked to the formation of CO2 (Oude Elferink et al. 2001).
During cheese making, lactose is rapidly metabolised by the starter culture to lactate (McSweeney 2011), so carbohydrate catabolism by OHL does not essentially contribute to CO2 formation in cheese; other substrates must be available. During cheese ripening, free amino acids accumulate due to the proteolysis of caseins. Both lactate and amino acids are potential substrates for gas formation in L. parabuchneri and L. buchneri. In a previous study, when used as adjuncts for cheese making, we observed that both species metabolised arginine to ornithine and produced 1,2-propanediol (Fröhlich-Wyder et al. 2013). Furthermore, HDC-positive L. parabuchneri and GAD-positive L. buchneri produced histamine and GABA, respectively, in cheese. That study clearly showed that these CO2-producing pathways from L. parabuchneri and L. buchneri were active in a cheese environment.
Both the ADI pathway and the formation of 1,2-propanediol were reported to be regulated by environmental factors. Manca de Nadra et al. (1986) demonstrated that arginine stimulated the enzymes of the ADI pathway in L. buchneri NCDO 110. Other studies confirmed that low pH activated the production of 1,2-propanediol (Oude Elferink et al. 2001) and that the formation of biogenic amines, such as histamine and GABA, depended on external factors, including the pH, salt concentration and temperature (Linares et al. 2011).
In this work, we studied the influence of pH on gas production by a thermophilic starter culture and an adjunct culture (L. parabuchneri and L. buchneri) in Tilsit-type model cheeses. Furthermore, we investigated the metabolic pathways involved in the formation of CO2 in L. buchneri and L. parabuchneri during cheese ripening.
2 Materials and methods
2.1 Adjunct cultures and physiological testing
The adjuncts used in this study are listed in Table 1. They were cultured in MRS broth at 30 °C (de Man et al. 1960). To assess the formation of GABA, the adjunct cultures were grown in MRS medium, supplemented with 1 % monosodium L-glutamate (pH 6.2). For the assessment of the formation of histamine, the adjunct cultures were grown in MRS broth, supplemented with 0.2 % L-histidine (pH 6.2). To detect the formation of ornithine from L-arginine, the adjunct cultures were grown in MAM medium (pH 5.8). To assess the formation of 1,2-propanediol, the adjunct cultures were cultivated in MRS-MOD medium (modified MRS medium, pH 3.8), as described by Oude Elferink et al. (2001). The formation of GABA, histamine, ornithine and 1,2-propanediol was verified by high-performance thin-layer chromatography on cellulose plates (Merck, Darmstadt, Germany) by comparing their migration distances to those of standard compounds. The methods used have been previously described in detail (Fröhlich-Wyder et al. 2013).
2.2 Cheese making
To produce different pH conditions, half the cheeses were produced and ripened according to the standard protocol, including curd washing and smear ripening (standard cheeses). For the other half, the curd washing step was omitted, and the cheeses were ripened in a plastic film (acidic cheeses). The Tilsit-type model cheeses of 30 cm in diameter were manufactured in a pilot plant of the Agroscope Research Station at Liebefeld, comprising eight vats. Four standard and four acidic cheeses were produced each from 70 L of the same batch of pasteurised full-fat (37 g.kg−1) cow’s milk. To each vat containing the 70 L of milk, 5 L of water and 0.2 % starter culture MK 401 (Agroscope, Liebefeld, Switzerland) containing various strains of Lactobacillus delbrueckii subsp. lactis, Streptococcus thermophilus and Lactococcus lactis subsp. lactis and 0.01 % adjunct culture (strain L. buchneri FAM 22050, L. parabuchneri FAM 21731 or L. parabuchneri FAM 21835 pre-cultured in MRS medium) were added. The milk was then pre-ripened at 31–32 °C for 15 min. The standard and acidic control cheeses were made without the addition of any adjunct culture. For coagulation, 10 mL of rennet (Winkler GR orange, Winkler AG, Konolfingen, Switzerland) was diluted in 1 L of water and added to the milk, which was then incubated at 32 °C for 35 min. According to the manufacturer’s instructions, one part of rennet Winkler GR orange clots 9000 parts of non-heated full-fat cow’s milk (pH 6.65 at 32 °C) within 30 min, which is equivalent to 194 IMCU.mL−1. The coagulum was cut into cubes of about 10 mm using knives with vertical wires. Thereafter, for the standard cheeses, 20 L of water was added to the curd–whey mixture. This step was omitted in the acidic cheeses. The curd–whey mixture was then cooked at 44 °C for 20 min, followed by a final stirring (43 °C, 20 min). To remove the whey, the curds were transferred into perforated moulds and pressed for 7.5 h. The cheeses were then immersed in brine solution for 16 h at 11–13 °C. The four standard cheeses were finally smear ripened at 14–15 °C and 90–96% relative humidity for 90 days. During the first 10 days of ripening, the cheeses were smeared daily with the brine solution, which had previously been inoculated with a mixture of Brevibacterium linens, Arthrobacter ssp. and Debaryomyces hansenii (OMK 702, Agroscope, Liebefeld, Switzerland). Thereafter, the brine solution was applied twice a week. The four acidic cheeses were wrapped under vacuum (−980 mbar) in a PA/PE 20/70 plastic film (CO2 permeability at 23 °C and 75% humidity 146 mL.(m2.day.bar)−1; Inauen Maschinen AG, Herisau, Switzerland) and ripened, together with the standard cheeses, at a temperature of 14–15 °C. The experiment was replicated on the second day (N = 2 runs), yielding a total of 16 cheeses. The design is shown in Table 2.
2.3 Cheese sampling
Cheese samples were taken aseptically from cheeses after 1 and 90 days of ripening with a cheese trier. The rind (thickness, 5 mm) was discarded, and the remaining cheese sample was ground and mixed.
2.4 Microbiological analyses
To enumerate OHL, the OH medium described by Isolini et al. (1990) and the method described by Fröhlich-Wyder et al. (2013) were used.
2.5 Chemical and biochemical analyses
The pH value of the cheeses was determined with a Metrohm pH-metre 605 equipped with a spear-tip pH electrode (Metrohm AG, Herisau, Switzerland). Free amino acids and histamine were determined with a high-performance liquid chromatograph (HPLC). A gas chromatograph was used to analyse the free short-chain fatty acids and the amount of 1,2-propanediol. These methods were described in detail by Fröhlich-Wyder et al. (2013).
L-, D-lactate and citrate were quantified enzymatically according to the instruction protocol on the kit (R-Biopharm, Darmstadt, Germany).
2.6 Sensory analysis
The sensory analysis was performed by a panel of seven experts according to the Agroscope standard protocol. The procedure was described by Guggisberg et al. (2013).
2.7 Statistical analysis
A factorial design with two factors (adjunct and pH level; treated as categorical variables) on four levels (one control and three adjuncts from Table 1) and two levels (standard pH and acidic pH) was applied (Table 2). The experiment was replicated on the second day (N = 2 runs, treated as random categorical variable). Statistical analysis of the instrumental data was carried out using the method of analysis of variance with the general linear model (GLM). The software used was SYSTAT 13 (Systat Software, Inc., Chicago IL, USA). Significant differences between the various levels within a factor were accepted at P ≤ 0.05.
The analysis of covariance was performed with GLM, and the results are given as squared multiple R (coefficient of determination). It explains how well the model fits the data.
3 Results and discussion
3.1 Biochemical properties of the adjunct cultures
The results of the physiological testing of the three adjunct cultures used in this study are listed in Table 1. All the selected strains showed the capacity to produce 1,2-propanediol and ornithine. One of the two strains of L. parabuchneri additionally produced histamine (FAM 21731), whereas the selected strain of L. buchneri (FAM 22050) isolated from Lalsil fresh LB produced GABA.
3.2 pH values in the cheeses
As expected, the different cheese-making steps in the group of standard cheeses (curd washing and smear ripening) and group of acidic cheeses (no curd washing and ripening in a plastic film) led to significant differences in the pH values and lactate content (Table 3). The lack of a curd washing step had a considerable effect on the amount of lactate in the fresh cheese. As the lactose was not diluted, the lactate fermentation was much more intensive. The ripening in the plastic film and, thus, the absence of lactate-degrading surface flora maintained the pH of the acidic cheeses at a low level during ripening. These cheeses contained about twice as much lactate as that of the standard cheeses at 90 days of ripening. Accordingly, the mean pH value of the acidic cheeses was significantly lower than that of the standard cheeses throughout the whole ripening period (5.40 and 5.70, respectively, on day 90) (Table 3).
There was a noticeable difference in the lactate content between the acidic cheeses on day 1 and day 90. In contrast to expectations, the acidic cheeses contained more lactate on day 90 than on day 1. This finding was likely due to the loss of water during the brine salting and ripening (Table 3). Additionally, as the lactose was not diluted in the vat, the LAB of the starter probably could not degrade all the galactose before brining and thus continued to produce lactic acid during the first days of ripening.
3.3 Fermentation of lactate to 1,2-propanediol
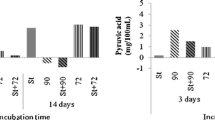
The amount of 1,2-propanediol measured in the standard cheeses was very low (0.29 mmol.kg−1) and comparable to the concentration previously found in other cheeses made with the same adjuncts (Fröhlich-Wyder et al. 2013). However, in the acidic cheeses, the degradation of lactate to 1,2-propanediol was significantly higher (3.12 mmol.kg−1; P ≤ 0.001). Remarkable differences were observed between the three adjuncts (Table 4): Although L. buchneri FAM 22050 and, particularly, L. parabuchneri FAM 21835 produced high amounts of 1,2-propanediol in the acidic cheeses, L. parabuchneri FAM 21731 did not (Fig. 1). Given the low amount of 1,2-propanediol produced by L. parabuchneri FAM 21731 and assuming that the fermentation of lactate by L. buchneri and L. parabuchneri to 1,2-propanediol contributed to pH homeostasis, it appears that L. parabuchneri FAM 21731 used an alternative pathway to control acidic stress.
Content of 1,2-propanediol in the 90-day aged Tilsit-type model standard cheeses (black circle) and acidic cheeses (black star). Both groups of cheeses included cheeses without adjuncts (control) and with adjuncts of Lactobacillus parabuchneri FAM 21731 (Lpbu FAM 21731), L. parabuchneri FAM 21835 (Lpbu FAM 21835) or L. buchneri FAM 22050 (Lbu FAM 22050)
Acetic acid is an end product of various fermentative pathways, such as the fermentation of citrate by NSLAB or the fermentation of lactate by propionibacteria or clostridia. Moreover, acetic acid is formed during the degradation of lactate to 1,2-propanediol by L. buchneri or L. parabuchneri. According to Oude Elferink et al. (2001), 1,2-propanediol and acetic acid are produced in equimolar amounts. Figure 2 shows that there was a high correlation in the acidic cheeses between the content of acetic acid and 1,2-propanediol (r = 0.994, with P < 0.001). Therefore, most of the acetic acid likely originated from the degradation of lactate by L. buchneri or L. parabuchneri. However, it can be concluded from Fig. 2 that approximately 4–5 mmol of the acetic acid in the acidic cheeses seem to originate from metabolic pathways other than lactate degradation. Based on the analysis of the volatile fatty acids, the fermentation of citrate by NSLAB or lactate by propionibacteria or clostridia was not responsible for the additional acetic acid (data not shown). In the standard cheeses, the correlation between the acetic acid and 1,2-propanediol content was weak but still significant (r = 0.793, with P = 0.019; Fig. 2). In these cheeses, up to 14 mmol of the acetic acid could not be attributed to the formation of 1,2-propanediol by NSLAB nor to the metabolic activity of propionibacteria and clostridia (data not shown). Possible explanations for the excess acetic acid could be the degradation of selected amino acids (Yvon et al. 2011; Skeie et al. 2008).
Relationship between the content of 1,2-propanediol and acetic acid in 90-day aged Tilsit-type model standard cheeses (black circle) and acidic cheeses (black star). Both groups of cheeses included cheeses without adjuncts (control) and with adjuncts of Lactobacillus parabuchneri FAM 21731, L. parabuchneri FAM 21835 or L. buchneri FAM 22050
1,2-Propanediol has a slightly sweet taste. The measured amounts of 1,2-propandiol were all well below the taste threshold of 13.9 mmol.L−1, indicated by Hufnagel and Hofmann (2008) for wine. All the same, there was a significant, negative correlation between the sensorial attributes ‘bitterness’ and ‘sweetness’ in the model Tilsit-type cheeses (r = −0.809; P < 0.001; Table 5).
3.4 Fermentation of arginine to ornithine
As previously shown by Fröhlich-Wyder et al. (2013), all the investigated strains had strong ADI activity. Independently of the pH value, almost all the available arginine was metabolised to ornithine, an indicator of an active ADI pathway (Table 6). In addition, in the control cheeses, elevated concentrations of ornithine (4.01 mmol.kg−1, on average) were observed. This was probably due to the lactobacilli present in the starter culture using the same pathway.
The enzymes involved in the ADI pathway appear to be inherently acid tolerant, displaying activity at pH 3.1, and even lower in some species (Cotter and Hill 2003). In acidic environments, arginine consumption leads to an increase in pH because ammonia is produced by the ADI pathway (Araque et al. 2013). In the present study, the low pH conditions of the acidic cheeses seemed to have only a slight influence on the formation of ornithine. However, there was one exception (Fig. 3): L. parabuchneri FAM 21835 produced significantly more ornithine in the acidic cheeses as compared to the standard cheeses (P = 0.046). During lactate fermentation under acid stress, it also produced considerably more 1,2-propanediol than the other two adjuncts (Fig. 1). The aforementioned may be due to L. parabuchneri FAM 21835 neutralising the cell environment only by the metabolism of lactate and arginine. In contrast, the two other adjuncts possess additionally amino acid decarboxylation activities, enabling them to respond to acid conditions by the formation of biogenic amines and by proton uptake (De Angelis and Gobbetti 2004; Molenaar et al. 1993). Linares et al. (2012) suggested that the formation of biogenic amines by the decarboxylation of amino acids might be a system for neutralisation of low extracellular pH, thereby increasing survival under acidic stress conditions.
Determination of ornithine in 90-day aged Tilsit-type model standard cheeses (grey bar) and acidic cheeses (black bar). Both groups of cheeses included cheeses without adjuncts (control) and with adjuncts of Lactobacillus parabuchneri FAM 21731 (Lpbu FAM 21731), L. parabuchneri FAM 21835 (Lpbu FAM 21835) or L. buchneri FAM 22050 (Lbu FAM 22050). The error bars indicate the standard error (N = 2). *P ≤ 0.05
3.5 Formation of GABA
Lactobacillus buchneri FAM 22050 showed GAD activity during laboratory characterisation, showing that it has the ability to decarboxylase glutamic acid to GABA (Table 1). As expected, in the standard pH group, the cheeses with this adjunct contained more GABA than the cheeses with the other adjuncts and the control. In the acidic cheeses, the control and the cheeses made with the adjuncts had comparable amounts of GABA. The optimum pH for decarboxylases is around 5.0. As shown in Fig. 4, there was a very significant interaction (P < 0.001) between the strain-specific capability of GABA formation and the pH level of the cheeses, revealing that the starter culture also possessed GAD activity. A previous study demonstrated that the same starter culture produced considerable amounts of GABA under similar acidic conditions (Bisig et al. 2014). Various species of LAB possess GAD activity, as demonstrated by several studies (e.g. Dhakal et al. 2012). It seems that the GAD activity of the applied starter was even more sensitive than L. buchneri FAM 22050 to acidic stress. A possible explanation could be that L. buchneri FAM 22050 is able to ferment lactate and arginine, both of which are also involved in its pH homeostasis (Cotter and Hill 2003). In contrast, the LAB present in the starter culture fermented only arginine, to some extent, but not lactate. Thus, the decarboxylation of glutamic acid was probably the main type of metabolism used by the starter culture to achieve pH homeostasis, whereas L. buchneri FAM 22050 combined several metabolic activities to do so (De Angelis and Gobbetti 2004).
Determination of γ-aminobutyric acid (GABA) in the standard and acidic Tilsit-type model cheeses. Both groups of cheeses included cheeses with adjuncts of Lactobacillus buchneri FAM 22050 (black star), L. parabuchneri FAM 21731 (black triangle), L. parabuchneri FAM 21835 (black square) and control cheeses without an adjunct (black circle). The error bars indicate the standard error (N = 2).
3.6 Formation of histamine
Lactobacillus parabuchneri FAM 21731 showed HDC activity in laboratory analyses (Table 1) and converted histidine in cheese to histamine (Table 6). The decarboxylation of histidine is considered to contribute to pH homeostasis and the control of acidic stress (Linares et al. 2012; Molenaar et al. 1993). Moreover, acidic pH conditions induce structural changes in HDC that are necessary for its activity (Coton et al. 1998; Linares et al. 2011); HDC is inactive at alkaline to neutral pH. As expected, only L. parabuchneri FAM 21731 produced histamine in the cheeses. Histamine formation tends to be higher in standard cheeses than in the acidic ones (Table 6). This finding can be explained by the different availability of histidine in the standard and acidic cheeses. In the standard cheeses, the pH throughout the whole ripening period was closer to the pH optimum of most bacterial proteinases and peptidases, the latter being responsible for the release of free amino acids (Upadhyay et al. 2004). As a result, more intensive proteolysis was observed in the standard cheeses than in the acidic cheeses. The latter is the most likely explanation for the higher soluble nitrogen and non-protein nitrogen and thus the histidine content in the standard cheeses (Table 4).
In the studies of Bisig et al. (2014) and Fröhlich-Wyder et al. (2013), L. parabuchneri FAM 21731 played an important role in CO2 production and in a strong burning taste detected in the cheeses. CO2 originates from the decarboxylation of histidine, and the burning taste is associated with the inflammation of mucosa in the mouth by histamine (Bachmann et al. 2011).
3.7 Influence on pH in cheese
As shown in the previous sections, the different pH values in the two groups of Tilsit-type model cheeses had a strong effect on the metabolism of the investigated L. buchneri and L. parabuchneri strains responding to acidic stress. Previous studies showed that these pathways—fermentation of lactate and of arginine, decarboxylation of amino acids—contributed to the neutralisation of the surrounding matrix and thus, to some extent, influenced the development of the pH during cheese ripening (Araque et al. 2013; Cotter and Hill 2003; De Angelis and Gobbetti 2004; Dhakal et al. 2012; Fröhlich-Wyder et al. 2013). In the present study, the increases in pH throughout the whole ripening time were dependent on the applied strains (Table 4 and Fig. 5). Compared to the control, the HDC-positive strain L. parabuchneri FAM 21731 had the most potent effect on pH, both in the acidic and standard cheeses, and was able to raise the pH by about 0.2 units. Although histamine production was less pronounced in the acidic cheeses (see section 3.6) in the present study, the results confirm the important role of histamine production in the neutralisation of the surrounding cheese body. The other two strains, L. parabuchneri FAM 21835 and L. buchneri FAM 22050, also had a significant alkalising effect, with FAM 22050 being the less potent. An analysis of covariance showed that the increase in pH could be explained by the degradation of lactate and the production of histamine and ornithine (R 2 = 0.966, with P < 0.001).
Determination of the pH value in 90-day aged Tilsit-type model standard cheeses (black circle) and acidic cheeses (black star). Both groups of cheeses included cheeses without adjuncts (control) and with adjuncts of Lactobacillus parabuchneri FAM 21731 (Lpbu FAM 21731), L. parabuchneri FAM 21835 (Lpbu FAM 21835) or L. buchneri FAM 22050 (Lbu FAM 22050)
3.8 CO2 formation in the cheese
As reported elsewhere, the ADI metabolism, degradation of lactate to 1,2-propanediol and decarboxylation of amino acids by L. buchneri and L. parabuchneri all contribute to the production of CO2 and thus influence eye formation and overall cheese quality (Fröhlich-Wyder et al. 2013; Bisig et al. 2014). In the present work, the lower pH value in the acidic cheeses promoted the degradation of lactate to 1,2-propanediol and of glutamic acid to GABA, both of which affected the production of CO2 and eye formation. The production of CO2 can be estimated by the quantity and types of metabolites generated in the abovementioned pathways. During the ADI metabolism, CO2 and ornithine are released in equimolar amounts (Manca de Nadra et al. 1986). Lactate degradation results in equimolar amounts of 1,2-propanediol, acetic acid and CO2 (Oude Elferink et al. 2001), and the decarboxylation of amino acids yields equimolar amounts of the corresponding amine and CO2 (e.g. Park and Oh 2006). The data presented in Fig. 6 reveal that the amount of CO2 produced in the acidic cheeses was twice as high as that produced in the standard cheeses.
Estimation of theoretically produced amounts of CO2 (mL.kg−1 under atmospheric pressure at room temperature) in the 90-day aged Tilsit-type model standard and acidic cheeses (N = 8 each). The calculation of estimated CO2 was made on the basis of the following metabolites: 1,2-propanediol, ornithine, γ-aminobutyric acid (GABA) and histamine
In terms of cheese quality, the pH of the cheese plays an important role not only in the development of the texture and flavour during ripening but also in the solubility of CO2. Figure 7a of the cross-sections of the standard cheeses shows that they exhibited mostly round-shaped eyes. It also shows that the Tilsit-type model cheeses with the addition of an adjunct culture tended to have more eyes than the control cheeses (nos. 2 and 13). In contrast to the standard cheeses, the acidic cheeses exhibited mainly splits and cracks and no round-shaped eyes (Fig. 7b). The pH of a cheese has an important influence on the texture of the cheese body and the solubility of CO2 (Upadhyay et al. 2004; Jakobsen and Jensen 2009). A cheese with a low pH is associated with the formation of a brittle texture and reduced solubility of CO2 in the cheese body. Therefore, it is not surprising that the eye formation in the acidic cheeses was defective. These observations reflect the results of the sensorial evaluation of the attributes eye quantity and quality (Table 5).
Sectional view of the 90-day aged Tilsit-type model standard cheeses (a) and acidic cheeses (b). Both groups of cheeses included cheeses with adjuncts of Lactobacillus parabuchneri FAM 21731 (cheese nos. 3, 4, 15 and 16), L. parabuchneri FAM 21835 (cheese nos. 5, 6, 9 and 10) or L. buchneri FAM 22050 (cheese nos. 7, 8, 11 and 12). The control cheeses (nos. 1, 2, 13 and 14) were made without an adjunct
The sensorial evaluation of the cheeses primarily revealed that L. parabuchneri FAM 21731 resulted in an unpleasant burning taste in the mouth (Bachmann et al. 2011). The burning taste was mirrored in the high scores for the attribute ‘bitter’ as the closing discussion within the panel revealed. As expected, the acidic cheeses did not meet the quality expectations of the panellists: Compared to the standard cheeses, they were sour, less aromatic, less salty, had more flavour defects and were brittle in texture. Therefore, they were all downgraded (Table 5).
4 Conclusions
The process of cheese making involves a number of steps, such as heating, acidification and brine salting. These steps cause stress to microorganisms, inhibit their growth and can even lead to their inactivation. The bacterial stress response enables bacteria to survive adverse and fluctuating conditions in their respective habitats. Lactic acid fermentation leads to a drastic drop in the pH value of cheese and induces acidic stress on most microorganisms involved in cheese ripening. Additionally, the fast conversion of available carbohydrates into organic acids results in carbohydrate starvation. The microorganisms present in the starter, adjunct and surface cultures, the growth of NSLAB and the actions of indigenous milk and clotting enzymes all influence the complex biochemical process of cheese ripening. The degradation of arginine by the ADI pathway, decarboxylation of histidine or glutamate into the corresponding biogenic amine and degradation of lactate into 1,2-propandiol by L. buchneri and L. parabuchneri are important metabolic pathways in the response to acidic stress. The growth of these NSLAB in cheese accelerates the increase in pH and promotes the production of CO2 during cheese ripening, thereby affecting the texture, openness and flavour of cheese. Recently, the growth of histamine-producing strains of L. parabuchneri has been associated with serious cheese defects, such as crack formation and a burning taste. However, only a few studies have investigated the metabolic activity of these NSLAB in cheese so far. In the present study, the metabolic stress response varied considerably among the three investigated strains. For example, L. parabuchneri FAM 21731, a histamine-positive strain, produced only small amounts of 1,2-propanediol under acidic conditions, whereas L. parabuchneri FAM 21835, a histamine-negative strain, produced the highest amounts of 1,2-propanediol. This example illustrates that strain-specific properties play an important role in the response to acidic stress. Despite the differences in the individual mechanisms used, the results clearly showed that acidic conditions favoured the degradation of lactate and arginine. An estimation of the amount of CO2 produced revealed that the ADI metabolism and the decarboxylation of glutamic acid were the most important sources of gas production. Although this study clearly demonstrated the importance of the cheese pH in the metabolic activity of strains of L. buchneri and L. parabuchneri during cheese ripening, further studies are needed to understand the role of these two species, which are frequently found in silage and therefore in milk, in the overall quality of cheese.
References
Araque I, Bordons A, Reguant C (2013) Effect of ethanol and low pH on citrulline and ornithine excretion and arc gene expression by strains of Lactobacillus brevis and Pediococcus pentosaceus. Food Microbiol 33:107–113
Bachmann HP, Fröhlich-Wyder MT, Jakob E, Roth E, Wechsler D (2011) In: Fuquay JW et al (eds) Encyclopedia of dairy sciences, volume 1, 2nd edn. San Diego, Academic Press
Bisig W, Guggisberg D, Irmler S, Jakob E, Wechsler D, Fröhlich-Wyder MT (2014) Measurement of CO2 from metabolic activity of adjunct cultures during cheese ripening. In: The ninth cheese symposium 12th & 13th November 2014, Teagasc, Cork, Ireland
Cho YR, Chang JY, Chang HC (2007) Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from Kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechn 17:104–109
Coton E, Rollan GC, Lonvaud-Funel A (1998) Histidine carboxylase of Leuconostoc oenos 9204: purification, kinetic properties, cloning and nucleotide sequence of the hdc gene. J Appl Microbiol 84:143–151
Coton M, Berthier F, Coton E (2008) Rapid identification of the three major species of dairy obligate heterofermenters Lactobacillus brevis, Lactobacillus fermentum and Lactobacillus parabuchneri by species-specific duplex PCR. FEMS Microbiol Lett 284:150–157
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol R 67:429–453
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus: a review. Proteomics 4:106–122
de Man J, Rogosa M, Sharpe ME (1960) A medium for the cultivation of Lactobacilli. J Appl Microbiol 23:130–135
Dhakal R, Bajpai VK, Baek KH (2012) Production of GABA (gamma-aminobutyric acid) by microorganisms: a review. Braz J Microbiol 43:1230–1241
Fröhlich-Wyder MT, Guggisberg D, Badertscher R, Wechsler D, Wittwer A, Irmler S (2013) The effect of Lactobacillus buchneri and Lactobacillus parabuchneri on the eye formation of semi-hard cheese. Int Dairy J 33:120–128
Guggisberg D, Fröhlich-Wyder MT, Irmler S, Greco M, Wechsler D, Schuetz P (2013) Eye formation in semi-hard cheese: X-ray computed tomography as a non-invasive tool for assessing the influence of adjunct lactic acid bacteria. Dairy Sci Technol 93:135–149
Hufnagel JC, Hofmann T (2008) Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. J Agric Food Chem 56:9190–9199
Isolini D, Grand M, Glättli H (1990) Selektivmedien zum Nachweis von obligat und fakultativ heterofermentativen Laktobazillen [Selective medium for the detection of obligatory and facultative heterofermentative Lactobacilli]. Schweiz Milchwirtsch Forsch 19:57–59
Jakobsen M, Jensen PN (2009) Assessment of carbon dioxide solubility coefficients for semihard cheeses: the effect of temperature and fat content. Eur Food Res Technol 229:287–294
Linares DM, Martin MC, Ladero V, Alvarez MA, Fernandez M (2011) Biogenic amines in dairy products. Crit Rev Food Sci 51:691–703
Linares DM, del Rio B, Ladero V, Martinez N, Fernandez M, Martin MC, Alvarez MA (2012) Factors influencing biogenic amines accumulation in dairy products. Front Microbiol 3, Article 180
Manca de Nadra MC, Nadra Chaud CA, de Ruiz P, Holgado AA, Oliver G (1986) Synthesis of the arginine dihydrolase pathway enzymes in Lactobacillus buchneri. Curr Microbiol 13:261–264
Manca de Nadra MC, Pesce de Ruiz Holgado AA, Oliver G (1988) Arginine dihydrolase pathway in Lactobacillus buchneri—a review. Biochimie 70:367–374
McSweeney PLH (2011) In: Fuquay JW (ed) Encyclopedia of dairy sciences, 2nd edn. San Diego, Academic Press
Molenaar D, Bosscher JS, Tenbrink B, Driessen AJM, Konings WN (1993) Generation of a proton motive force by histidine decarboxylation and electrogenic histidine histamine antiport in Lactobacillus buchneri. J Bacteriol 175:2864–2870
Oude Elferink SJ, Krooneman J, Gottschal JC, Spoelstra SF, Faber F, Driehuis F (2001) Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Appl Environ Microbiol 67:125–132
Park KB, Oh SH (2006) Isolation and characterization of Lactobacillus buchneri strains with high gamma-aminobutyric acid producing capacity from naturally aged cheese. Food Sci Biotechnol 15:86–90
Skeie S, Kieronczyk A, Eidet S, Reitan M, Olsen K, Ostlie H (2008) Interaction between starter bacteria and adjunct Lactobacillus plantarum INF 15D on the degradation of citrate, asparagine and aspartate in a washed-curd cheese. Int Dairy J 18:169–177
Sumner SS, Speckhard MW, Somers EB, Taylor SL (1985) Isolation of histamine-producing Lactobacillus buchneri from Swiss cheese implicated in a food poisoning outbreak. Appl Environ Microbiol 50:1094–1096
Upadhyay VK, McSweeney PLH, Magboul AAA, Fox PF (2004) In: Fox PF et al (eds) Chemistry, physics and microbiology, volume 1, 3rd edn. Elsevier Academic Press, London
Yvon M, Gitton C, Chambellon E, Bergot G, Monnet V (2011) The initial efficiency of the proteolytic system of Lactococcus lactis strains determines their responses to a cheese environment. Int Dairy J 21:335–345
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is part of the Special issue dedicated to the 9th International Cheese Symposium held in Cork, Ireland and organized by Teagasc in collaboration with University College Cork and INRA, 12th & 13th November 2014.
About this article
Cite this article
Fröhlich-Wyder, MT., Bisig, W., Guggisberg, D. et al. Influence of low pH on the metabolic activity of Lactobacillus buchneri and Lactobacillus parabuchneri strains in Tilsit-type model cheese. Dairy Sci. & Technol. 95, 569–585 (2015). https://doi.org/10.1007/s13594-015-0238-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-015-0238-1