Abstract
Managed honey bees are experiencing high rates of colony loss, in part due to widespread exposure to agrochemicals and other environmental toxins. The ability to learn about relevant environmental stimuli is an important skill necessary for foraging and navigation, although it is sometimes impaired in bees that have been exposed to toxins or other stressors. Here, we review the effects of anthropogenic toxins (which we divide into five major classes: insecticides, acaricides, biopesticides, other agrochemicals, and other toxins) on learning performance in European honey bees. We discuss the general trends of these studies, including that neurotoxic insecticides are overwhelmingly the most well-studied, and that most studies focus on acute exposure of individual, adult bees to a single toxin. Protocols for field-relevant exposure vary widely among labs, and we make suggestions to aid in the standardization of future studies. We review the relevance of learning studies for toxicological risk assessment, concluding that they are valuable tools for assessing sublethal behavioral effects of toxins. Their inclusion in risk assessment studies would be an improvement over current procedures, which focus largely on lethality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Insect pollinator populations are in decline globally (Potts et al. 2010). Colony loss rates for managed honey bees are also high in the USA (Bruckner et al. 2023) and worldwide (Gray et al. 2022). These losses are happening simultaneously with a global increase in the demand for pollination services (Aizen et al. 2019). The production of fruit crops in the USA is already being limited by a lack of pollinators (Reilly et al. 2020). Future pollinator losses are expected to have devastating economic consequences (Lippert et al. 2021), with fruit, vegetable, and stimulant crops being especially vulnerable (Gallai et al. 2009).
Pollinators face a variety of threats, but the three most commonly implicated as causes of population declines include poor nutrition, parasites and pathogens, and agrochemicals (Goulson et al. 2015). This review will focus on the effects of agrochemicals and other environmental toxins on honey bees, a major area of concern in recent years (Johnson 2015; Siviter et al. 2021). Honey bees in the USA have been exposed to increasingly toxic levels of agrochemicals over the past 30 years (Douglas et al. 2020). Large varieties of pesticides are often found in hive products and bee-collected pollen (Mullin et al. 2010; Ostiguy et al. 2019). In some cases, large amounts of agrochemicals in hives have correlated with colony mortality (Traynor et al. 2016, 2021). While agrochemicals and other toxins can be lethal for bees exposed to high enough doses, sublethal effects can occur at lower doses and cause substantial harm. Sublethal effects are defined as harmful physiological, developmental, or behavioral effects that occur in individuals that have survived exposure to a toxin (Desneux et al. 2006). This review will focus on impaired learning ability, a popular measure of behavioral sublethal effects (Siviter et al. 2018).
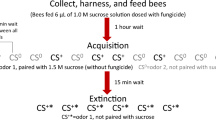
Learning has been studied in honey bees using a variety of methods; however, the proboscis extension reflex (PER) paradigm is the most popular for ecotoxicology studies. It is a form of classical Pavlovian conditioning (Rescorla 1988) which utilizes the natural proboscis extension reflex of the honey bee—that is, a bee will reflexively extend its proboscis when a sugar solution is touched to its antennae (Bitterman et al. 1983; Takeda 1961). In a typical experiment, an odor is used as a conditioned stimulus (CS) while a sugar water solution acts as the unconditioned stimulus (US). Over a series of trials, a bee is exposed to the odor, and then the sugar solution is immediately brought to its antennae, eliciting the PER. A bee that has successfully learned the association will extend its proboscis in response to the odor alone before the sugar solution is delivered (Figure 1a). Testing groups of individuals over a series of trials generates learning curves, which can be compared across treatment groups (such as bees exposed to varying doses of a toxin; see Figure 1b for an example of a toxin causing negative effects on PER learning) (Matsumoto et al. 2012; Smith and Burden 2014). In addition to the acquisition phase, memory is often tested at various time points post-conditioning (1 h, 24 h, 48 h, etc.) by conducting a few trials where the odor is presented without the reinforcing sugar solution. This technique has been adapted in countless ways to address a variety of topics, ranging from the cellular and molecular bases of learning and memory to the ecological associations between flowers and pollinators (Giurfa and Sandoz 2012). The PER paradigm can be adapted to measure visual (Hori et al. 2006) and tactile (Scheiner et al. 1999) associative learning, as well as forms of non-associative conditioning such as habituation (Braun and Bicker 1992) and latent inhibition (Chandra et al. 2010).
a Schematic showing the steps of PER. Over a series of trials, bees are exposed to an odor, and then a sugar water solution is immediately touched to their antennae. Bees that successfully learn the association will extend their proboscis in response to the odor alone, without any exposure to sugar. b Hypothetical acquisition curve for a PER experiment comparing a control group and a group exposed to a toxin. The line represents the percentage of bees showing a learned response (proboscis extension prior to sugar water presentation) for each trial. In this case, the control group achieves a higher learning rate than the treatment group, suggesting that the toxin is negatively impacting learning performance.
Lab-based learning experiments are popular ways to test for behavioral sublethal effects because associative learning is necessary for foraging and navigation, which are critically important for colony function and survival. Bees are central place foragers, which means that they navigate to floral resources outside the colony and bring their products back home (Bell 1990). Successful navigation requires bees to take sensory cues from the environment and integrate these with motor outputs (Buehlmann et al. 2020). Relevant sensory cues can be either visual or olfactory. Bees start their foraging lives by performing a series of orientation flights around the hive, through which they become extensively familiar with the surrounding visual landmarks (Capaldi and Dyer 1999). They use these landmarks to navigate home after foraging over long ranges (Pahl et al. 2011). Olfactory cues are used to navigate at shorter ranges to help bees home in on the correct location of either a floral patch or the colony (Chaffiol et al. 2005). As part of the forager recruitment process (which also includes the waggle dance), bees learn floral odors from their nestmates in the hive. These cues help them navigate to the same floral patch during their own trip (Arenas et al. 2007). This information suggests that olfactory and visual learning experiments may be predictive of foraging and navigation behaviors in the field, and indeed, PER learning performance correlates with real-world foraging performance (Cabirol et al. 2018). Bees are also able to transfer knowledge of odors they have learned in the real world to the PER paradigm in the laboratory (Gerber et al. 1996).
2 Scope of this review
In this review, we focus on European honey bee (Apis mellifera) studies that involve “anthropogenic” toxins, which are present in the environment at higher levels than normal because of human activity. We focus on European honey bees because of their importance to pollination and because they have been subject to the greatest number of studies, enabling comparisons across toxin types. There is a smaller body of work focusing on the effects of toxins in other bee species (e.g., Apis cerana, Bombus spp.), and while we acknowledge the importance of such studies, we choose to limit the scope to Apis mellifera here for coherence, due to space limitations, and because a causal analysis suggests that the same general trends hold true. Anthropogenic toxins can be categorized into five major groups: insecticides, acaricides, biopesticides, other agrochemicals, and other toxins. See Figure 2 for a summary of the effects of each class. A complete list of all included studies can be found in the electronic supplementary material (Online Resource 1) associated with this article, which also contains more details about the methods by which these studies were identified (Online Resource 2). The majority of these studies use the PER paradigm. We also cover studies that utilize other methods for testing associative learning such as T-mazes, shuttle boxes (an apparatus in which an animal usually learns to avoid an electrified floor), and free-flying assays.
Breakdown of studies devoted to each class of toxin. Of the three numbers in parentheses for each class, the first is the number of papers that examined the effects of that toxin class on honey bee learning (also proportional to the size of the box), the second is the number of papers that reported negative effects, and the third is the number that reported negative effects at field-relevant (according to the study’s authors) levels. Insecticides are classified based on the Insecticide Resistance Action Committee (IRAC) mode of action groups. Studies focused on multiple toxins from different classes are counted more than once.
In each section, we note the studies that found effects at field-relevant toxin levels. As we discuss in the “General trends” section below, the term “field-relevant” can be interpreted in a variety of different ways, but for the purposes of this review, we defer to the authors’ judgement regarding whether their dose and exposure protocol qualify as something bees could realistically be exposed to when foraging in the field.
3 Effects of anthropogenic toxins on learning
3.1 Insecticides
Insecticides are pesticides designed specifically to kill insect pests in households and agriculture. Insecticides are sprayed on some bee-pollinated crops and are often found at high levels in hive food stores (Johnson 2015; Mullin et al. 2010; Ostiguy et al. 2019; Traynor et al. 2021). There are 32 major groups of insecticides classified according to their mode of action (Insecticide Resistance Action Committee (IRAC) 2022). Of these 32 groups, nine have been tested for their effects on honey bee learning. Of these nine groups, six have modes of action that interfere with insect nerve or muscle function, two are insect growth regulators, and one affects the midgut (IRAC Group 11, which for the purposes of this review is included in the “Biopesticides” section). Neonicotinoids (IRAC Group 4) and imidacloprid in particular have been the focus of many studies on honey bee learning. In this section, we first summarize the studies concerning imidacloprid and other neonicotinoids and then broaden the scope to include other classes of insecticides.
The effects of imidacloprid (neonicotinoid, IRAC Group 4, nicotinic acetylcholine receptor competitive modulators) on honey bee learning and memory are by far the most well-documented. The majority of PER studies report some negative effects of imidacloprid on learning and/or memory (Decourtye et al. 2003, 2004a,b; Khooshe-Bast et al. 2023; Li et al. 2019; Schwartz et al. 2021), including effects at field-relevant doses (Iqbal et al. 2019; Mengoni Goñalons and Farina 2015, 2018; Mustard et al. 2020; Wright et al. 2015; Yang et al. 2012). A few studies show no effects of imidacloprid alone (Karahan et al. 2015; Williamson and Wright 2013; Williamson et al. 2013). The presence and magnitude of the negative effect can vary based on season, with summer bees being more prone to experiencing negative effects than winter bees (Decourtye et al. 2003), and bee age, with young adult bees being more negatively affected than older bees (Mengoni Goñalons and Farina 2015). Field-relevant larval exposure to imidacloprid also impairs PER learning in adults (Yang et al. 2012). Other forms of learning, including habituation (Guez et al. 2001; Lambin et al. 2001) and free-flying associative learning (Decourtye et al. 2004b), are altered by imidacloprid exposure. Imidacloprid impairs bee performance in an aversive shuttle box assay (Delkash-Roudsari et al. 2020) and in an aversive paradigm that simulates predation (Zhang and Nieh 2015). Some studies also show physiological alterations associated with impaired learning, including changes in cytochrome oxidase activity (Decourtye et al. 2004a) and gene transcription (Li et al. 2019) in the brain.
Other neonicotinoids, including acetamiprid (Thany et al. 2015), clothianidin (Mustard et al. 2020; Piiroinen and Goulson 2016; Tison et al. 2019), dinotefuran (Liu et al. 2019; Mustard et al. 2020), thiacloprid (Begna and Jung 2021; Ke et al. 2023; Li et al. 2023; Tison et al. 2017), and thiamethoxam (Aliouane et al. 2009; Mustard et al. 2020; Papach et al. 2017; Wright et al. 2015) negatively affect PER learning and/or memory, including some compounds at field-relevant levels (Li et al. 2023; Mustard et al. 2020; Piiroinen and Goulson 2016; Wright et al. 2015). A smaller number of studies report no effects of acetamiprid (Aliouane et al. 2009; El Hassani et al. 2008; Schuhmann and Scheiner 2023), clothianidin (Alkassab and Kirchner 2016; Shepherd et al. 2021), and thiamethoxam (El Hassani et al. 2008) on PER learning. In addition to these PER studies, Ludicke and Nieh (2020) report the negative effects of a field-relevant dose of thiamethoxam on a T-maze learning task in which bees choose between sections of the maze illuminated with different colored lights for a food reward. Bartling et al. (2019) also report negative effects of clothanidin on an olfactory shuttle box assay. Newer-generation pesticide groups including sulfoximines and butenolides have the same basic mechanism of action as neonicotinoids. Fewer studies have examined the effects of these chemicals on honey bee PER learning, and the results are mixed. One study reports a negative effect of sulfoxaflor (Cartereau et al. 2022), while another finds no effect (Siviter et al. 2019), and two studies report negative effects of flupyradifurone (Bell et al. 2020; Hesselbach and Scheiner 2018), one at field-relevant levels (Bell et al. 2020).
Insecticides from a number of other classes that target the insect nervous system produce negative effects in PER learning assays. This includes carbamates (IRAC Group 1, acetylcholinesterase inhibitors) (Abramson et al. 1999), organophosphates (IRAC Group 1, acetylcholinesterase inhibitors) (Khooshe-Bast et al. 2023; Li et al. 2017; Urlacher et al. 2016; Weick and Thorn 2002), cyclodiene organochlorines (IRAC Group 2, GABA-gated chloride channel blockers) (Abramson et al. 1999; Decourtye et al. 2005), phenylpyrazoles (IRAC Group 2, GABA-gated chloride channel blockers) (Aliouane et al. 2009; Decourtye et al. 2005; El Hassani et al. 2005, 2009), pyrethroids (IRAC Group 3, sodium channel modulators) (Abramson et al. 1999; Decourtye et al. 2005; Liao et al. 2018; Mamood and Waller 1990; Ramirez-Romero et al. 2005; Taylor et al. 1987; Thany et al. 2015), and pyridine azomethine derivatives (IRAC Group 9, chordotonal organ TRPV channel modulators) (Abramson et al. 2012). Most of these classes have reports of negative effects at field-relevant levels as well, including carbamates (Abramson et al. 1999), organophosphates (Urlacher et al. 2016), cyclodiene organochlorines (Abramson et al. 1999), and pyrethroids (Abramson et al. 1999; Ramirez-Romero et al. 2005). One study reports a positive effect of injection of the organophosphate trichlorfon on PER learning (Shapira et al. 2001). Additionally, fipronil (phenylpyrazole) produces negative effects on PER learning with a tactile instead of olfactory stimulus (Bernadou et al. 2009). Ethion (organophosphate) impairs learning performance in an aversive shuttle box assay (Delkash-Roudsari et al. 2020).
Only a few non-neuroactive or unknown mechanism of action (MOA) insecticides have been tested for effects on honey bee learning. The insect growth regulators diflubenzuron (IRAC Group 15, inhibitors of chitin biosynthesis affecting CHS1) and tebufenozide (IRAC Group 18, ecdysone receptor agonists) both produce negative effects on PER learning at field-relevant levels (Abramson et al. 2004). Additionally, a field-relevant dose of dicofol (unknown MOA) produces negative effects on PER learning (Stone et al. 1997).
3.2 Acaricides
Acaricides are compounds used to control arachnid pests (mites and ticks). Although their mechanisms of action overlap considerably with insecticides, we discuss them separately here because they are widely used directly within honey bee colonies to control the parasitic mite Varroa destructor and thus are often the most commonly found chemicals in samples of wax, bee bread, and honey (Johnson 2015; Mullin et al. 2010; Ostiguy et al. 2019; Traynor et al. 2021). Here, we discuss the effects of synthetic (e.g., coumaphos, fluvalinate, and amitraz, presented according to IRAC classifications) and botanically derived (e.g., formic acid, oxalic acid, thymol) acaricides.
Many synthetic acaricides produce negative effects on PER learning and/or memory, including coumaphos (IRAC Group 1, acetylcholinesterase inhibitors) (Gashout et al. 2020; Weick and Thorn 2002; Williamson and Wright 2013; Williamson et al. 2013), fluvalinate and flumethrin (IRAC Group 3, sodium channel modulators) (Frost et al. 2013; Gashout et al. 2020; Taylor et al. 1987; Wu et al. 2023), and amitraz (IRAC Group 19, octopamine receptor agonists) (Begna and Jung 2021; Gashout et al. 2020), with effects at field-relevant levels reported for coumaphos (Williamson and Wright 2013; Williamson et al. 2013) and fluvalinate (Frost et al. 2013). A few studies report no effects of fluvalinate (Decourtye et al. 2005) and amitraz (Rix and Cutler 2017) on PER learning, and one study reports no effect of fluvalinate on visual/aversive shuttle box learning (Colin et al. 2020).
Some botanically derived acaricides also produce negative effects on PER learning, including formic acid (Gashout et al. 2020) and thymol (Bonnafé et al. 2015, 2018; Khooshe-Bast et al. 2023), with thymol producing negative effects at field-relevant levels (Bonnafé et al. 2018). One study reports that oxalic acid improves PER learning (Schneider et al. 2012b), and another reports the same for formic acid (Bachert and Scheiner 2023). Thymol also alters congruency between olfactory and gustatory stimuli in the PER assay (Chapuy et al. 2019).
3.3 Biopesticides
Biopesticides are agricultural pesticides based on living organisms and/or their products. They can be broken into three broad categories: naturally occurring biochemicals acting through non-toxic mechanisms (botanically derived compounds, essential oils, etc.), microbial entomopathogens (live bacterial or fungal organisms), and plant-incorporated protectants from genetically engineered plants (most commonly, Cry proteins originally from the bacterium Bacillus thuringiensis, Bt). They are often touted as pollinator-friendly alternatives to more traditional, synthetic pesticides, and their use has increased in recent years (Cappa et al. 2022). Here, we focus on Bt Cry toxins first and then broaden the scope to consider a few other biopesticides.
Bt Cry proteins (IRAC Group 11, microbial disruptors of insect midgut membranes) are used to control lepidopteran and coleopteran pests via the production of lesions in the midgut epithelium. These proteins are expressed in the pollen of transgenic plants, which may be collected by bees (Johnson 2015; Picard-Nizou et al. 1997). Most studies report no effects of Bt Cry toxins on PER learning (Dai et al. 2012, 2016; Han et al. 2010). The exception to this is Ramirez-Romero et al. (2008), which reports that Cry1AB alters PER extinction at field-relevant levels. Bt Cry toxins also produce no effects on visual T-maze learning (Han et al. 2010) or free-flying associative learning (Ramirez-Romero et al. 2005).
A few other biopesticides have been tested for their effects on honey bee learning. Bioganic®, a commercial formulation marketed for household pest control and composed of a mixture of essential oils, produces negative effects on PER and free-flying associative learning at field-relevant levels (Abramson et al. 2006). Soybean Bowman–Birk inhibitor reduces PER learning, while Kunitz Soybean Trypsin inhibitor has no effect (Pham‐Delègue et al. 2000). Cowpea trypsin inhibitor reduces PER and free-flying associative learning (Picard-Nizou et al. 1997). Hv1a/GNA, a fusion protein containing a calcium channel blocker from spider venom, produces no negative effects on PER learning (Nakasu et al. 2014). Beauveria bassiana, an entomopathogenic fungus that infects hosts via cuticle contact and is used in agriculture to control a wide range of pest species, produces no effects on PER associative learning. However, it does alter PER habituation, increasing the number of trials needed for habituation to occur (Carlesso et al. 2020).
3.4 Other agrochemicals
Fungicides are pesticides used to control fungal diseases in agriculture. These chemicals are commonly sprayed on bee-pollinated crops, sometimes during bloom, and have been widely found in various in-hive food stores (Johnson 2015; Mullin et al. 2010; Ostiguy et al. 2019; Traynor et al. 2021). There are 13 major groups of fungicides, targeting a wide range of biochemical processes in fungal cells (Fungicide Resistance Action Committee (FRAC) 2022). Representatives from two out of the 13 groups have been tested for their effects on honey bee learning. The first of these is prochloraz (FRAC code G1, targeting C14-demethylase in sterol biosynthesis), which causes faster extinction of PER learning (Decourtye et al. 2005). A formulation containing two active ingredients meant to interfere with cellular respiration (boscalid, FRAC code C2, targeting succinate dehydrogenase and pyraclostrobin, FRAC code C3, targeting cytochrome bc1 at Qo site) produces negative effects on PER learning and memory at field-relevant levels (DesJardins et al. 2021). However, another formulation containing the active ingredients boscalid and dimoxystrobin (which have the same mechanism of action as pyraclostrobin) produces no effects on PER learning at field-relevant levels (Schuhmann and Scheiner 2023).
Likewise, herbicides are commonly applied to bee-pollinated crops, and a wide variety of compounds have been found in hive food stores (Johnson 2015; Mullin et al. 2010; Ostiguy et al. 2019; Traynor et al. 2021). Only two herbicides, glyphosate (HRAC Group 9/inhibition of enolpyruvyl shikimate phosphate synthase) and paraquat (HRAC Group 22/PS I electron diversion), have been tested for effects on honey bee learning, representing two out of 26 major herbicide groups (Herbicide Resistance Action Committee (HRAC) 2022). Glyphosate produces negative effects on PER learning in young adult bees (Mengoni Goñalons and Farina 2018). It impairs PER learning and memory in foraging-age bees at field-relevant levels (Herbert et al. 2014; Hernández et al. 2021; Luo et al. 2021). One study reports no effect of glyphosate on aversive shuttle box learning (Delkash-Roudsari et al. 2020). A high dose of paraquat also impairs PER learning (Khooshe-Bast et al. 2023).
Adjuvants can either be included in pesticide formulations (formulation adjuvants) or added to tank mixes together with pesticides (spray adjuvants). They are added to enhance the efficacy of the active ingredients. Although widely used, they are often assumed to be inert and are rarely tested for possible effects on pollinators, and their presence usually is not tested for in hive food stores (Mullin et al. 2015). While some studies described above did test the effects of whole formulations, we focus here on the one study that tested adjuvants by themselves, conducted by Ciarlo et al. (2012). This study tested multiple compounds from three major adjuvant classes (organosilicones, nonionic surfactants, and crop oil concentrates). Organosilicones have significant negative effects on PER learning, suggesting they may not be safe for pollinators (Mullin et al. 2016). Nonionic surfactants have slight negative effects, and crop oil concentrates produce no effects.
3.5 Other toxins
A number of metals produce negative effects on PER learning at field-relevant levels, including selenium (Burden et al. 2016), lead (Monchanin et al. 2021a, b), copper, and arsenic (Monchanin et al. 2021b). Manganese and cadmium also produce negative effects at sublethal doses (Khooshe-Bast et al. 2023; Li et al. 2022). Learning is most severely impaired when bees are fed a combination of lead, copper, and arsenic, suggesting additive effects (Monchanin et al. 2021b). These metals may be present in the soil due to mining and industrial operations. They are taken up by plants, resulting in contaminated pollen and nectar, which is then collected by bees (Johnson 2015).
Industrial air pollutants can also produce negative effects on PER learning at field-relevant levels. The concerns regarding these chemicals are twofold. First, there is concern that these pollutants could mask floral volatiles used by honey bees to locate food sources when foraging, impairing odor recognition. This can be tested by mixing an air pollutant with the conditioned stimulus in PER learning, and indeed, this produces negative effects (Leonard et al. 2019). There is also concern that honey bees could be exposed to these chemicals outright, producing sublethal effects, possibly including impaired learning. When bees are exposed before conditioning, both diesel exhaust (Reitmayer et al. 2019) and ozone (Démares et al. 2022) produce negative effects on PER learning.
Microplastics are widely present in water and soil and have been found in honey stores (Alma et al. 2023). One study examined the effects of both acute and chronic consumption of microplastics on PER habituation and associative learning, and it reports no effects (Balzani et al. 2022).
4 General trends
4.1 Neurotoxic insecticides/acaricides are overwhelmingly the most well-studied
Our review found 61 studies focused on compounds known to be toxic to arthropod nervous systems, 11 studies focused on other types of insecticides/acaricides, 10 studies focused on biopesticides, six studies on herbicides, three studies on fungicides, one study on spray adjuvants, five studies on metals, three studies on air pollutants, and one study on microplastics (some studies are counted more than once because they tested compounds from more than one of these classes). Most of the compounds tested produced some sort of negative effect, so importantly, direct neurotoxicity is not a prerequisite for negative effects on learning.
4.2 Many studies use acute, individual exposure rather than chronic and/or colony-level exposure
We found 46 studies that used acute, individual exposure (feeding individual bees a known amount of a toxin one time before conditioning or testing). This is compared to 23 studies that fed toxins to groups of adults in cages. In this case, a set concentration was usually provided in a sugar solution ad libitum over a period of several days. Ten studies exposed entire colonies to the toxin; this usually involved providing contaminated nectar or pollen over a period of days or weeks and then capturing adult foragers for learning tests. The remaining studies used a combination of methods or less-common approaches. Each of these approaches comes with benefits and drawbacks. For example, an acute/individual exposure paradigm provides the benefit of being able to control the exact amount of toxin received and the life stage of the individual while testing short-term effects. While both acute and chronic exposure can occur in field settings, chronic, colony-level exposure is likely more often experienced by pollinating bees.
4.3 Most studies look only at active ingredients, rather than formulations
Among agrochemical studies (not including adjuvants, genetically modified crops, or other biopesticides), 15 focused on commercial formulations, while 64 focused on active ingredients only. There are likely benefits and drawbacks to both approaches here as well. Focusing on active ingredients only may be prudent sometimes because the compounds themselves may become disassociated with their formulations by the time bees are exposed to them (e.g., in contaminated hive food stores). However, bees that directly feed on pollen or nectar from flowers that have been sprayed by agrochemicals experience exposure to full formulations. Also, it would be good to carefully test adjuvants and co-formulants on their own, as these can also impair learning (Ciarlo et al. 2012).
4.4 Most studies only focus on adult exposure
We found two studies that exposed larvae to toxins (and then tested their learning capacities when they became adults), as opposed to 78 studies that exposed and tested adults and 10 studies that exposed entire colonies (and thus were not specific about which life stage was exposed). However, in this case, work done on other bee species shows the importance of working with other life stages. Tan et al. (2015, 2017) found negative effects of the insecticides imidacloprid and flupyradifurone on Asian honey bees (Apis cerana) exposed as larvae, while Smith et al. (2020) found negative effects of larval exposure to imidacloprid on bumble bees (Bombus terrestris). Exposure across a combination of life stages may be more relevant to field conditions and can lead to greater impairments than when bees are only exposed as adults (DesJardins et al. 2021); this should be a focus area for future studies.
4.5 Only a few studies have tested for additive or synergistic effects of multiple compounds on learning
Some studies have found negative effects of combinations of insecticides and acaricides on PER learning (Begna and Jung 2021; Colin et al. 2020; Williamson and Wright 2013). One study found that a mixture of the neonicotinoid insecticide imidacloprid and the herbicide glyphosate impairs PER learning (Mengoni Goñalons and Farina 2018). One study found that a mixture of four organophosphate insecticides produces no effect on learning (Al Naggar et al. 2015), while another also found no effect of a mixture of the fungicides boscalid and dimoxystrobin and the neonicotinoid acetamiprid (Schuhmann and Scheiner 2023). Another intriguing area of study has focused on synergistic effects between parasite/pathogen and pesticide exposure. The insecticides flupyradifurone and clothianidin impair PER learning when bees are also exposed to Nosema cerenae (Bell et al. 2020; Piiroinen and Goulson 2016), and imidacloprid and Varroa destructor interact to produce negative effects on learning (Schwartz et al. 2021). As bees are likely to be exposed to complex mixtures of agrochemicals, other toxins, pathogens, and parasites inside hives (Mullin et al. 2010; Ostiguy et al. 2019), there should be an increased focus on testing the effects of field-relevant interactions among chemicals and other stressors.
4.6 The underlying mechanisms are not always clear
Some studies report the results of additional experiments meant to determine the physiological mechanisms underlying impaired learning. For example, imidacloprid alters metabolism and cholinergic signaling in the mushroom bodies (the centers of learning and memory in the insect brain) (Decourtye et al. 2004a). However, the mechanisms underlying impaired learning performance are not always clear, especially in the case of compounds not designed to be neuroactive. Moreover, there is a distinction between impaired learning performance and impaired learning per se. Altered performance in a learning assay may be reflective of broader issues with sensory processing, motor function, and/or motivation instead of (or in addition to) impairments in the neural processes involved in learning and memory (Muth and Leonard 2019). The studies discussed in the preceding section do not always make this distinction. Some do show concurrent abnormalities in sucrose responsiveness (Aliouane et al. 2009; Begna and Jung 2021; Carlesso et al. 2020; El Hassani et al. 2005, 2008; Frost et al. 2013; Herbert et al. 2014; Hesselbach and Scheiner 2018; Luo et al. 2021; Mengoni Goñalons and Farina 2015, 2018), which suggest that broader sensory, motor, and/or motivational effects may play an important role. Ideally, future studies should also seek to determine underlying physiological mechanisms, with closer attention paid to potential sensory, motor, and motivation-related explanations.
4.7 Methodological details vary widely between labs, which highlights the need to exercise caution when comparing results
Even a relatively “standardized” procedure such as PER (Barascou et al. 2021) is prone to methodological variations that can alter the outcome; previous reviews have raised this concern and called for more standardization across labs (Frost et al. 2012; Matsumoto et al. 2012; Smith and Burden 2014). Examples of such methodological variations include the number of trials during the acquisition phase, whether differential (requiring bees to differentiate between two conditioned stimuli) or absolute conditioning is used, the duration of the intertrial intervals, whether and when memory is tested, colony history (such as past acaricide treatments), the subspecies or genetic strain of honey bees tested, the ages of tested bees, and season during which the experiment was conducted. These factors need to be considered when comparing results across labs. Additionally, investigators should strive to adopt a more standardized set of methods. Previous reviews on the topic (De Stefano et al. 2014; Frost et al. 2012; Matsumoto et al. 2012; Smith and Burden 2014) offer detailed suggestions for standardizing PER procedures; we suggest referring to those when deciding on which procedures to adopt.
4.8 Many studies claim to expose bees to a dose or concentration that is field-relevant but define that in different ways
We found 45 studies that did not claim to test a dose or concentration bees would be likely to encounter in the real world; these studies usually just picked a sublethal dose/concentration by choosing a set fraction of the LD-50 or LC-50. Forty-seven studies claimed some degree of field relevance for their chosen dose or concentration, although the definition of that term varied. Some used the dose or concentration recommended by a governing body or manufacturer to control a particular pest. However, this is unlikely to be the concentration that a honey bee will be exposed to, unless sprayed directly. Some chose a dose or concentration that had been previously found in hive food products (e.g., bee bread, honey) or in bee bodies. Concentrations in bee bread likely underestimate the concentrations in collected pollen, since pollen is mixed with honey and salivary secretions, and agrochemicals in the body are diluted and possibly metabolized or excreted by the bee. Some chose a dose or concentration that had been found in nectar or pollen from treated crops. This seems to be the best approach, though for estimating a field-relevant dose, an effort should be made to estimate the consumption rate of the tainted pollen and/or nectar over time in the field.
When deciding on an exposure protocol that is field-relevant, factors that should be carefully considered include whether to use acute or chronic exposure, whether to expose individuals or groups (in cages or whole colonies), the life stage(s) during which exposure and testing occur, the administration method (oral via nectar or pollen, topical, etc.), and the dose itself. Our recommendation for creating a truly field-relevant exposure protocol is to think carefully about methodological details and create an “exposure scenario” in which the combination of variables mimics a situation that bees may encounter in the field. For example, a field-relevant dose could be drawn from a study that measured pesticide residues in treated flowers, nectar, and/or bee-collected pollen (e.g., Graham et al. 2022). In this case, it may make more sense to focus on feeding a series of doses corresponding to the length of the blooming period to individual adult foragers(e.g., Linguadoca et al. 2021), directly mimicking a scenario in which they are foraging on treated crops. If drawing the field-relevant dose from a study that measured levels of toxins in hive food stores such as bee bread (e.g., Mullin et al. 2010; Ostiguy et al. 2019; Traynor et al. 2021), it would make sense to expose whole colonies over a longer period of time, perhaps via contaminated pollen patties, mimicking a scenario in which a generation of bees consumes contaminated food as larvae and young adults. Whatever the protocol is, each factor should be explicitly described and justified.
5 Significance of learning studies in toxin risk assessment
Many of the compounds discussed above that impair learning performance in the lab also produce negative effects on foraging and navigation behaviors in the field. Some neonicotinoids reduce the number of foraging trips made by individual foragers (Ohlinger et al. 2022; Schneider et al. 2012a; Tison et al. 2020). Neonicotinoids can also increase the duration of foraging trips, with treated bees taking longer to return to the hive than controls (Schneider et al. 2012a; Yang et al. 2008). Neonicotinoids can also impair homing ability, with neonicotinoid-exposed bees returning at lower rates than controls in experiments (Fischer et al. 2014; Henry et al. 2012). The phenylpyrazole insecticide fipronil also decreases foraging trips (Decourtye et al. 2011). Pyrethroid insecticides can also produce negative effects, with deltamethrin impairing homing ability (Van Dame et al. 1995) and fluvalinate altering duration of foraging trips (Colin et al. 2021). In addition to these insecticides, the herbicide glyphosate (Sol Balbuena et al. 2015) increases the average duration of homing flights in treated bees when compared to controls. Ultimately, it has been suggested that bees are so vulnerable to environmental stressors because central place foraging requires relatively advanced cognitive abilities, which can be negatively affected even at low doses of toxins (Klein et al. 2017).
Given that lab-based learning experiments seem to be relevant to bee behaviors in the real world (Henry et al. 2015), the question becomes, should they be used when assessing pesticide risks to bees, and if so, how? There have been calls to include more nuanced testing for the sublethal effects of pesticides on bees (Barascou et al. 2021; Decourtye et al. 2013; Fisher 2021). Overall, lab-based learning studies are a relatively easy way to quantify behavioral sublethal effects of environmental toxins in honey bees, as they typically require less resources than field studies and allow for more control over several variables important for studies of learning and memory (Smith and Burden 2014). Additionally, this level of experimental control allows for more detailed physiological and genetic investigation of the effects of toxin treatments. Despite this, some have called the ecological relevance of these experiments into question (Barascou et al. 2021). Studies that show the negative effects of toxins on foraging and navigation behaviors in the field might be more likely to get the attention of policymakers. PER assays would perhaps be most effective if they were incorporated into lower-tier risk assessments with explicitly standardized procedures and field-relevant exposure protocols. This would be an improvement over current lower-tier studies, which mostly focus on LD50s (Barascou et al. 2021). If PER experiments produce negative effects, then higher tiers could include foraging and/or navigation experiments in the field. This approach should be explored as a possible way to incorporate important behavioral sublethal effects into risk assessment procedures.
6 Conclusion
About 90 studies have examined the effects of anthropogenic toxins on honey bee learning, with most but not all finding negative effects of a variety of agrochemicals, biopesticides, metals, and air pollutants. Learning experiments are a relatively easy way to test for behavioral sublethal effects, and these studies, when carefully planned and executed, can correlate with negative effects on foraging and navigation behaviors in the field. These studies should be further incorporated into procedures for toxin risk assessment. A more diverse set of toxins should also be assessed for behavioral sublethal effects. In particular, relatively few studies have focused on fungicides and metals, although bees may even more likely to be exposed to them than they are insecticides, since fungicides are sometimes sprayed during bloom. There should also be a greater emphasis on testing for effects with field-relevant doses and exposure protocols; this would increase the relevance of these learning studies to toxin risk assessment.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
References
Abramson CI, Aquino IS, Ramalho FS, Price JM (1999) The effect of insecticides on learning in the Africanized honey bee (Apis mellifera L.). Arch Environ Con Tox 37:529–535. https://doi.org/10.1007/s002449900548
Abramson CI, Squire J, Sheridan A, Mulder PG (2004) The effect of insecticides considered harmless to honey bees (Apis mellifera): proboscis conditioning studies by using the insect growth regulators tebufenozide and diflubenzuron. Environ Entomol 33:378–388. https://doi.org/10.1603/0046-225X-33.2.378
Abramson CI, Singleton JB, Wilson MK, Wanderley PA, Ramalho FS, Michaluk LM (2006) The effect of an organic pesticide on mortality and learning in Africanized honey bees (Apis mellifera L.) in Brasil. Am J Environ Sci 2:33–40. https://doi.org/10.3844/ajessp.2006.33.40
Abramson CI, Sokolowski MBC, Brown EA, Pilard S (2012) The effect of pymetrozine (Plenum WG-50®) on proboscis extension conditioning in honey bees (Apis mellifera: Hybrid var. Buckfast). Ecotox Environ Safe 78:287–295. https://doi.org/10.1016/j.ecoenv.2011.11.038
Aizen MA, Aguiar S, Biesmeijer JC, Garibaldi LA, Inouye DW, Jung C, Martins DJ, Medel R, Morales CL, Ngo H, Pauw A, Paxton RJ, Sáez A, Seymour CL (2019) Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob Change Biol 25:3516–3527. https://doi.org/10.1111/gcb.14736
Al Naggar Y, Wiseman S, Sun J, Cutler GC, Aboul-Soud M, Naiem E, Mona M, Seif A, Giesy JP (2015) Effects of environmentally-relevant mixtures of four common organophosphorus insecticides on the honey bee (Apis mellifera L.). J Insect Physiol 82:85–91. https://doi.org/10.1016/j.jinsphys.2015.09.004
Aliouane Y, El Hassani AK, Gary V, Armengaud C, Lambin M, Gauthier M (2009) Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environ Toxicol Chem 28:113–122. https://doi.org/10.1897/08-110.1
Alkassab AT, Kirchner WH (2016) Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology 25:1000–1010. https://doi.org/10.1007/s10646-016-1657-3
Alma AM, de Groot GS, Buteler M (2023) Microplastics incorporated by honeybees from food are transferred to honey, wax and larvae. Environ Pollut 320:121078. https://doi.org/10.1016/j.envpol.2023.121078
Arenas A, Fernández VM, Farina WM (2007) Floral odor learning within the hive affects honeybees’ foraging decisions. Naturwissenschaften 94:218–222. https://doi.org/10.1007/s00114-006-0176-0
Bachert A, Scheiner R (2023) The ant’s weapon improves honey bee learning performance. Sci Rep 13:8399. https://doi.org/10.1038/s41598-023-35540-7
Balzani P, Galeotti G, Scheggi S, Masoni A, Santini G, Baracchi D (2022) Acute and chronic ingestion of polyethylene (PE) microplastics has mild effects on honey bee health and cognition. Environ Pollut 305:119318. https://doi.org/10.1016/j.envpol.2022.119318
Barascou L, Brunet J-L, Belzunces L, Decourtye A, Henry M, Fourrier J, Le Conte Y, Alaux C (2021) Pesticide risk assessment in honeybees: toward the use of behavioral and reproductive performances as assessment endpoints. Chemosphere 276:130134. https://doi.org/10.1016/j.chemosphere.2021.130134
Bartling MT, Vilcinskas A, Lee K-Z (2019) Sub-lethal doses of clothianidin inhibit the conditioning and biosensory abilities of the Western Honeybee Apis mellifera. InSects 10:340. https://doi.org/10.3390/insects10100340
Begna T, Jung C (2021) Effects of sequential exposures of sub-lethal doses of amitraz and thiacloprid on learning and memory of honey bee foragers, Apis mellifera. J Asia-Pac Entomol 24:77–83. https://doi.org/10.1016/j.aspen.2021.03.012
Bell HC, Montgomery CN, Benavides JE, Nieh JC (2020) Effects of Nosema ceranae (Dissociodihaplophasida: Nosematidae) and flupyradifurone on olfactory learning in honey bees, Apis mellifera (Hymenoptera: Apidae). J Insect Sci 20:29. https://doi.org/10.1093/jisesa/ieaa130
Bell WJ (1990) Central place foraging. In WJ Bell (ed), Searching behaviour: the behavioural ecology of finding resources. Springer Netherlands, pp 171–187. https://doi.org/10.1007/978-94-011-3098-1_12
Bernadou A, Démares F, Couret-Fauvel T, Sandoz JC, Gauthier M (2009) Effect of fipronil on side-specific antennal tactile learning in the honeybee. J Insect Physiol 55:1099–1106. https://doi.org/10.1016/j.jinsphys.2009.08.019
Bitterman ME, Menzel R, Fietz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119. https://doi.org/10.1037/0735-7036.97.2.107
Bonnafé E, Drouard F, Hotier L, Carayon J-L, Marty P, Treilhou M, Armengaud C (2015) Effect of a thymol application on olfactory memory and gene expression levels in the brain of the honeybee Apis mellifera. Environ Sci Pollut R 22:8022–8030. https://doi.org/10.1007/s11356-014-2616-2
Bonnafé E, Alayrangues J, Hotier L, Massou I, Renom A, Souesme G, Marty P, Allaoua M, Treilhou M, Armengaud C (2018) Monoterpenoid-based preparations in beehives affect learning, memory, and gene expression in the bee brain. Environ Toxicol Chem 36:337–345. https://doi.org/10.1002/etc.3527
Braun G, Bicker G (1992) Habituation of an appetitive reflex in the honeybee. J Neurophysiol 67:588–598. https://doi.org/10.1152/jn.1992.67.3.588
Bruckner S, Wilson M, Aurell D, Rennich K, vanEngelsdorp D, Steinhauer N, Williams GR (2023) A national survey of managed honey bee colony losses in the USA: results from the Bee Informed Partnership for 2017–18, 2018–19, and 2019–20. J Apicult Res 62:429–443. https://doi.org/10.1080/00218839.2022.2158586
Buehlmann C, Mangan M, Graham P (2020) Multimodal interactions in insect navigation. Anim Cogn 23:1129–1141. https://doi.org/10.1007/s10071-020-01383-2
Burden CM, Elmore C, Hladun KR, Trumble JT, Smith BH (2016) Acute exposure to selenium disrupts associative conditioning and long-term memory recall in honey bees (Apis mellifera). Ecotox Environ Safe 127:71–79. https://doi.org/10.1016/j.ecoenv.2015.12.034
Cabirol A, Cope AJ, Barron AB, Devaud J-M (2018) Relationship between brain plasticity, learning and foraging performance in honey bees. PLoS ONE 13:e0196749. https://doi.org/10.1371/journal.pone.0196749
Capaldi EA, Dyer FC (1999) The role of orientation flights on homing performance in honeybees. J Exp Biol 202:1655–1666. https://doi.org/10.1242/jeb.202.12.1655
Cappa F, Baracchi D, Cervo R (2022) Biopesticides and insect pollinators: detrimental effects, outdated guidelines, and future directions. Sci Total Environ 837:155714. https://doi.org/10.1016/j.scitotenv.2022.155714
Carlesso D, Smargiassi S, Sassoli L, Cappa F, Cervo R, Baracchi D (2020) Exposure to a biopesticide interferes with sucrose responsiveness and learning in honey bees. Sci Rep 10:11929. https://doi.org/10.1038/s41598-020-76852-2
Cartereau A, Pineau X, Lebreton J, Mathé-Allainmat M, Taillebois E, Thany SH (2022) Impairments in learning and memory performances associated with nicotinic receptor expression in the honeybee Apis mellifera after exposure to a sublethal dose of sulfoxaflor. PLoS ONE 17:e0272514. https://doi.org/10.1371/journal.pone.0272514
Chaffiol A, Laloi D, Pham-Delègue M-H (2005) Prior classical olfactory conditioning improves odour-cued flight orientation of honey bees in a wind tunnel. J Exp Biol 208:3731–3737. https://doi.org/10.1242/jeb.01796
Chandra SBC, Wright GA, Smith BH (2010) Latent inhibition in the honey bee, Apis mellifera: is it a unitary phenomenon? Anim Cogn 13:805–815. https://doi.org/10.1007/s10071-010-0329-6
Chapuy C, Ribbens L, Renou M, Dacher M, Armengaud C (2019) Thymol affects congruency between olfactory and gustatory stimuli in bees. Sci Rep 9:7752. https://doi.org/10.1038/s41598-019-43614-8
Ciarlo TJ, Mullin CA, Frazier JL, Schmehl DR (2012) Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE 7:e40848. https://doi.org/10.1371/journal.pone.0040848
Colin T, Plath JA, Klein S, Vine P, Devaud J-M, Lihoreau M, Meikle WG, Barron AB (2020) The miticide thymol in combination with trace levels of the neonicotinoid imidacloprid reduces visual learning performance in honey bees (Apis mellifera). Apidologie 51:499–509. https://doi.org/10.1007/s13592-020-00737-6
Colin T, Forster CC, Westacott J, Wu X, Meikle WG, Barron AB (2021) Effects of late miticide treatments on foraging and colony productivity of European honey bees (Apis mellifera). Apidologie 52:474–492. https://doi.org/10.1007/s13592-020-00837-3
Dai P-L, Zhou W, Zhang J, Cui H-J, Wang Q, Jiang W-Y, Sun J-H, Wu Y-Y, Zhou T (2012) Field assessment of Bt cry1Ah corn pollen on the survival, development and behavior of Apis mellifera ligustica. Ecotox Environ Safe 79:232–237. https://doi.org/10.1016/j.ecoenv.2012.01.005
Dai P-L, Jia H-R, Geng L-L, Diao Q-Y (2016) Bt toxin Cry1Ie causes no negative effects on survival, pollen consumption, or olfactory learning in worker honey bees (Hymenoptera: Apidae). J Econ Entomol 109:1028–1033. https://doi.org/10.1093/jee/tow088
De Stefano LA, Stepanov II, Abramson CI (2014) The first order transfer function in the analysis of agrochemical data in honey bees (Apis Mellifera L.): proboscis extension reflex (PER) studies. InSects 5:167–198. https://doi.org/10.3390/insects5010167
Decourtye A, Lacassie E, Pham-Delègue M-H (2003) Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag Sci 59:269–278. https://doi.org/10.1002/ps.631
Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S, Gauthier M, Pham-Delègue M-H (2004a) Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic Biochem Phys 78:83–92. https://doi.org/10.1016/j.pestbp.2003.10.001
Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue M-H (2004b) Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotox Environ Safe 57:410–419. https://doi.org/10.1016/j.ecoenv.2003.08.001
Decourtye A, Devillers J, Genecque E, Menach KL, Budzinski H, Cluzeau S, Pham-Delègue M-H (2005) Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch Environ Con Tox 48:242–250. https://doi.org/10.1007/s00244-003-0262-7
Decourtye A, Devillers J, Aupinel P, Brun F, Bagnis C, Fourrier J, Gauthier M (2011) Honeybee tracking with microchips: a new methodology to measure the effects of pesticides. Ecotoxicology 20:429–437. https://doi.org/10.1007/s10646-011-0594-4
Decourtye A, Henry M, Desneux N (2013) Overhaul pesticide testing on bees. Nature 497:188. https://doi.org/10.1038/497188a
Delkash-Roudsari S, Chicas-Mosier AM, Goldansaz SH, Talebi-Jahromi K, Ashouri A, Abramson CI (2020) Assessment of lethal and sublethal effects of imidacloprid, ethion, and glyphosate on aversive conditioning, motility, and lifespan in honey bees (Apis mellifera L.). Ecotox Environ Safe 204:111108. https://doi.org/10.1016/j.ecoenv.2020.111108
Démares F, Gibert L, Creusot P, Lapeyre B, Proffit M (2022) Acute ozone exposure impairs detection of floral odor, learning, and memory of honey bees, through olfactory generalization. Sci Total Environ 827:154342. https://doi.org/10.1016/j.scitotenv.2022.154342
DesJardins NS, Fisher A, Ozturk C, Fewell JH, DeGrandi-Hoffman G, Harrison JF, Smith BH (2021) A common fungicide, Pristine®, impairs olfactory associative learning performance in honey bees (Apis mellifera). Environ Pollut 288:117720. https://doi.org/10.1016/j.envpol.2021.117720
Desneux N, Decourtye A, Delpuech J-M (2006) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Douglas MR, Sponsler DB, Lonsdorf EV, Grozinger CM (2020) County-level analysis reveals a rapidly shifting landscape of insecticide hazard to honey bees (Apis mellifera) on US farmland. Sci Rep 10:797. https://doi.org/10.1038/s41598-019-57225-w
El Hassani AK, Dacher M, Gauthier M, Armengaud C (2005) Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol Biochem B 82:30–39. https://doi.org/10.1016/j.pbb.2005.07.008
El Hassani AK, Dacher M, Gary V, Lambin M, Gauthier M, Armengaud C (2008) Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch Environ Con Tox 54:653–661. https://doi.org/10.1007/s00244-007-9071-8
El Hassani AK, Dupuis JP, Gauthier M, Armengaud C (2009) Glutamatergic and GABAergic effects of fipronil on olfactory learning and memory in the honeybee. Invertebr Neurosci 9:91. https://doi.org/10.1007/s10158-009-0092-z
Fischer J, Müller T, Spatz A-K, Greggers U, Grünewald B, Menzel R (2014) Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 9:e91364. https://doi.org/10.1371/journal.pone.0091364
Fisher A (2021) Protect pollinators—reform pesticide regulations. Nature 595:172. https://doi.org/10.1038/d41586-021-01818-x
Frost EH, Shutler D, Hillier NK (2012) The proboscis extension reflex to evaluate learning and memory in honeybees (Apis mellifera): some caveats. Naturwissenschaften 99:677–686. https://doi.org/10.1007/s00114-012-0955-8
Frost EH, Shutler D, Hillier NK (2013) Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J Exp Biol 216:2931–2938. https://doi.org/10.1242/jeb.086538
Fungicide Resistance Action Committee (2022) FRAC Code List©*2022: fungal control agents sorted by cross-resistance pattern and mode of action (including coding for FRAC Groups on product labels). https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2022--final.pdf?sfvrsn=b6024e9a_2. Accessed 27 May 2023
Gallai N, Salles J-M, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821. https://doi.org/10.1016/j.ecolecon.2008.06.014
Gashout HA, Guzman-Novoa E, Goodwin PH, Correa-Benítez A (2020) Impact of sublethal exposure to synthetic and natural acaricides on honey bee (Apis mellifera) memory and expression of genes related to memory. J Insect Physiol 121:104014. https://doi.org/10.1016/j.jinsphys.2020.104014
Gerber B, Geberzahn N, Hellstern F, Klein J, Kowalksy O, Wüstenberg D, Menzel R (1996) Honey bees transfer olfactory memories established during flower visits to a proboscis extension paradigm in the laboratory. Anim Behav 52:1079–1085. https://doi.org/10.1006/anbe.1996.0255
Giurfa M, Sandoz J-C (2012) Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Memory 19:54–66. https://doi.org/10.1101/lm.024711.111
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. https://doi.org/10.1126/science.1255957
Graham KK, Milbrath MO, Zhang Y, Baert N, McArt S, Isaacs R (2022) Pesticide risk to managed bees during blueberry pollination is primarily driven by off-farm exposures. Sci Rep 12:7189. https://doi.org/10.1038/s41598-022-11156-1
Gray A et al (2022) Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: the combined effects of operation size, migration and queen replacement. J Apicult Res 62:204–210. https://doi.org/10.1080/00218839.2022.2113329
Guez D, Suchail S, Gauthier M, Maleszka R, Belzunces LP (2001) Contrasting effects of imidacloprid on habituation in 7- and 8-day-old honeybees (Apis mellifera). Neurobiol Learn Mem 76:183–191. https://doi.org/10.1006/nlme.2000.3995
Han P, Niu C-Y, Lei C-L, Cui J-J, Desneux N (2010) Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 19:1612–1619. https://doi.org/10.1007/s10646-010-0546-4
Henry M, Béguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350. https://doi.org/10.1126/science.1215039
Henry M, Cerrutti N, Aupinel P, Decourtye A, Gayrard M, Odoux J-F, Pissard A, Rüger C, Bretagnolle V (2015) Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. P R Soc B 282:20152110. https://doi.org/10.1098/rspb.2015.2110
Herbert LT, Vázquez DE, Arenas A, Farina WM (2014) Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. J Exp Biol 217:3457–3464. https://doi.org/10.1242/jeb.109520
Herbicide Resistance Action Committee (2022) HRAC Mode of Action Classification 2022. https://hracglobal.com/files/HRAC_MOA_Poster_January_6_2022.pdf. Accessed 27 May 2023
Hernández J, Riveros AJ, Amaya-Márquez M (2021) Sublethal doses of glyphosate impair olfactory memory retention, but not learning in the honey bee (Apis mellifera scutellata). J Insect Conserv 25:683–694. https://doi.org/10.1007/s10841-021-00335-6
Hesselbach H, Scheiner R (2018) Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Sci Rep 8:4954. https://doi.org/10.1038/s41598-018-23200-0
Hori S, Takeuchi H, Arikawa K, Kinoshita M, Ichikawa N, Sasaki M, Kubo T (2006) Associative visual learning, color discrimination, and chromatic adaptation in the harnessed honeybee Apis mellifera L. J Comp Physiol A 192:691–700. https://doi.org/10.1007/s00359-005-0091-4
Insecticide Resistance Action Committee. (2022). Mode of Action Classification. https://irac-online.org/documents/moa-brochure/. Accessed 27 May 2023
Iqbal J, Alqarni AS, Raweh HSA (2019) Effect of sub-lethal doses of imidacloprid on learning and memory formation of indigenous Arabian bee (Apis mellifera jemenitica Ruttner) Adult Foragers. Neotrop Entomol 48:373–380. https://doi.org/10.1007/s13744-018-0651-2
Johnson RM (2015) Honey Bee Toxicology. Annu Rev Entomol 60:415–434. https://doi.org/10.1146/annurev-ento-011613-162005
Karahan A, Çakmak I, Hranitz JM, Karaca I, Wells H (2015) Sublethal imidacloprid effects on honey bee flower choices when foraging. Ecotoxicology 24:2017–2025. https://doi.org/10.1007/s10646-015-1537-2
Ke L, Chen X, Dai P, Liu Y-J (2023) Chronic larval exposure to thiacloprid impairs honeybee antennal selectivity, learning and memory performances. Front Physiol 14. https://doi.org/10.3389/fphys.2023.1114488
Khooshe-Bast Z, Sahebzadeh N, Tahmasbi G, Haddadi M, Khani A (2023) Effects of octopamine on memory retention under chemical stress: a behavioral study on honey bees. J Apicult Res. https://doi.org/10.1080/00218839.2023.2186018
Klein S, Cabirol A, Devaud J-M, Barron AB, Lihoreau M (2017) Why bees are so vulnerable to environmental stressors. Trends Ecol Evol 32:268–278. https://doi.org/10.1016/j.tree.2016.12.009
Lambin M, Armengaud C, Raymond S, Gauthier M (2001) Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch Insect Biochem 48:129–134. https://doi.org/10.1002/arch.1065
Leonard RJ, Vergoz V, Proschogo N, McArthur C, Hochuli DF (2019) Petrol exhaust pollution impairs honey bee learning and memory. Oikos 128:264–273. https://doi.org/10.1111/oik.05405
Li Z, Yu T, Chen Y, Heerman M, He J, Huang J, Nie H, Su S (2019) Brain transcriptome of honey bees (Apis mellifera) exhibiting impaired olfactory learning induced by a sublethal dose of imidacloprid. Pestic Biochem Phys 156:36–43. https://doi.org/10.1016/j.pestbp.2019.02.001
Li Z, Qiu Y, Li J, Wan K, Nie H, Su S (2022) Chronic cadmium exposure induces impaired olfactory learning and altered brain gene expression in honey bees (Apis mellifera). InSects 13:988. https://doi.org/10.3390/insects13110988
Li A, Yin L, Ke L, Diao Q-Y, Wu Y, Dai P, Liu Y-J (2023) Thiacloprid impairs honeybee worker learning and memory with inducing neuronal apoptosis and downregulating memory-related genes. Sci Total Environ 885:163820. https://doi.org/10.1016/j.scitotenv.2023.163820
Li Z, Li M, Huang J, Ma C, Xiao L, Huang Q, Zhao Y, Nie H, Su S (2017) Effects of sublethal concentrations of chlorpyrifos on olfactory learning and memory performances in two bee species, Apis mellifera and Apis cerana. Sociobiology 64:174–181. https://doi.org/10.13102/sociobiology.v64i2.1385
Liao C, He X, Wang Z, Barron AB, Zhang B, Zeng Z, Wu X (2018) Short-term exposure to lambda-cyhalothrin negatively affects the survival and memory-related characteristics of worker bees Apis mellifera. Arch Environ Con Tox 75:59–65. https://doi.org/10.1007/s00244-018-0514-1
Linguadoca A, Rizzi C, Villa S, Brown MJF (2021) Sulfoxaflor and nutritional deficiency synergistically reduce survival and fecundity in bumblebees. Sci Total Environ 795:148680. https://doi.org/10.1016/j.scitotenv.2021.148680
Lippert C, Feuerbacher A, Narjes M (2021) Revisiting the economic valuation of agricultural losses due to large-scale changes in pollinator populations. Ecol Econ 180:106860. https://doi.org/10.1016/j.ecolecon.2020.106860
Liu S, Liu Y, He F, Zhang H, Li X, Tan H (2019) Enantioselective olfactory effects of the neonicotinoid dinotefuran on honey bees (Apis mellifera L.). J Agr Food Chem 67:12105–12116. https://doi.org/10.1021/acs.jafc.9b04851
Ludicke JC, Nieh JC (2020) Thiamethoxam impairs honey bee visual learning, alters decision times, and increases abnormal behaviors. Ecotox Environ Safe 193:110367. https://doi.org/10.1016/j.ecoenv.2020.110367
Luo QH, Gao J, Guo Y, Liu C, Ma YZ, Zhou ZY, Dai PL, Hou CS, Wu YY, Diao QY (2021) Effects of a commercially formulated glyphosate solutions at recommended concentrations on honeybee (Apis mellifera L.) behaviours. Sci Rep 11:2115. https://doi.org/10.1038/s41598-020-80445-4
Mamood A, Waller GD (1990) Recovery of learning responses by honeybees following a sublethal exposure to permethrin. Physiol Entomol 15:55–60. https://doi.org/10.1111/j.1365-3032.1990.tb00492.x
Matsumoto Y, Menzel R, Sandoz J-C, Giurfa M (2012) Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Meth 211:159–167. https://doi.org/10.1016/j.jneumeth.2012.08.018
Mengoni Goñalons C, Farina WM (2015) Effects of sublethal doses of imidacloprid on young adult honeybee behaviour. PLoS ONE 10:e0140814. https://doi.org/10.1371/journal.pone.0140814
Mengoni Goñalons C, Farina WM (2018) Impaired associative learning after chronic exposure to pesticides in young adult honey bees. J Exp Biol 221:176644. https://doi.org/10.1242/jeb.176644
Monchanin C, Blanc-Brude A, Drujont E, Negahi MM, Pasquaretta C, Silvestre J, Baqué D, Elger A, Barron AB, Devaud J-M, Lihoreau M (2021a) Chronic exposure to trace lead impairs honey bee learning. Ecotox Environ Safe 212:112008. https://doi.org/10.1016/j.ecoenv.2021.112008
Monchanin C, Drujont E, Devaud J-M, Lihoreau M, Barron AB (2021b) Metal pollutants have additive negative effects on honey bee cognition. J Exp Biol 224:241869. https://doi.org/10.1242/jeb.241869
Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, vanEngelsdorp D, Pettis JS (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5:e9754. https://doi.org/10.1371/journal.pone.0009754
Mullin CA, Chen J, Fine JD, Frazier MT, Frazier JL (2015) The formulation makes the honey bee poison. Pestic Biochem Phys 120:27–35. https://doi.org/10.1016/j.pestbp.2014.12.026
Mullin CA, Fine JD, Reynolds RD, Frazier MT (2016) Toxicological risks of agrochemical spray adjuvants: organosilicone surfactants may not be safe. Front Public Health 4. https://doi.org/10.3389/fpubh.2016.00092
Mustard JA, Gott A, Scott J, Chavarria NL, Wright GA (2020). Honeybees fail to discriminate floral scents in a complex learning task after consuming a neonicotinoid pesticide. J Exp Biol 223. https://doi.org/10.1242/jeb.217174
Muth F, Leonard AS (2019) A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Sci Rep 9:4764. https://doi.org/10.1038/s41598-019-39701-5
Nakasu EYT, Williamson SM, Edwards MG, Fitches EC, Gatehouse JA, Wright GA, Gatehouse AMR (2014) Novel biopesticide based on a spider venom peptide shows no adverse effects on honeybees. P R Soc B 281:20140619. https://doi.org/10.1098/rspb.2014.0619
Ohlinger BD, Schürch R, Durzi S, Kietzman PM, Silliman MR, Couvillon MJ (2022) Honey bees (Hymenoptera: Apidae) decrease foraging but not recruitment after neonicotinoid exposure. J Insect Sci 22:16. https://doi.org/10.1093/jisesa/ieab095
Ostiguy N, Drummond FA, Aronstein K, Eitzer B, Ellis JD, Spivak M, Sheppard WS (2019) Honey bee exposure to pesticides: a four-year nationwide study. InSects 10:13. https://doi.org/10.3390/insects10010013
Pahl M, Zhu H, Tautz J, Zhang S (2011) Large scale homing in honeybees. PLOS ONE 6:e19669. https://doi.org/10.1371/journal.pone.0019669
Papach A, Fortini D, Grateau S, Aupinel P, Richard F-J (2017) Larval exposure to thiamethoxam and American foulbrood: effects on mortality and cognition in the honey bee Apis mellifera. J Apicult Res 56:475–486. https://doi.org/10.1080/00218839.2017.1332541
Pham-Delègue M-H, Girard C, Métayer ML, Picard-Nizou A-L, Hennequet C, Pons O, Jouanin L (2000) Long-term effects of soybean protease inhibitors on digestive enzymes, survival and learning abilities of honeybees. Entomol Exp Appl 95:21–29. https://doi.org/10.1046/j.1570-7458.2000.00637.x
Picard-Nizou AL, Grison R, Olsen L, Pioche C, Arnold G, Pham-Delegue MH (1997) Impact of proteins used in plant genetic engineering: toxicity and behavioral study in the honeybee. J Econ Entomol 90:1710–1716. https://doi.org/10.1093/jee/90.6.1710
Piiroinen S, Goulson D (2016) Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honeybees and bumblebees. P R Soc B 283:20160246. https://doi.org/10.1098/rspb.2016.0246
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Ramirez-Romero R, Chaufaux J, Pham-Delègue M-H (2005) Effects of Cry1Ab protoxin, deltamethrin and imidacloprid on the foraging activity and the learning performances of the honeybee Apis mellifera, a comparative approach. Apidologie 36:601–611. https://doi.org/10.1051/apido:2005039
Ramirez-Romero R, Desneux N, Decourtye A, Chaffiol A, Pham-Delègue M-H (2008) Does Cry1Ab protein affect learning performances of the honey bee Apis mellifera L. (Hymenoptera, Apidae)? Ecotox Environ Safe 70:327–333. https://doi.org/10.1016/j.ecoenv.2007.12.002
Reilly JR et al (2020) Crop production in the USA is frequently limited by a lack of pollinators. P R Soc B 287:20200922. https://doi.org/10.1098/rspb.2020.0922
Reitmayer CM, Ryalls JMW, Farthing E, Jackson CW, Girling RD, Newman TA (2019) Acute exposure to diesel exhaust induces central nervous system stress and altered learning and memory in honey bees. Sci Rep 9:5793. https://doi.org/10.1038/s41598-019-41876-w
Rescorla RA (1988) Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci 11:329–352. https://doi.org/10.1146/annurev.ne.11.030188.001553
Rix RR, Cutler GC (2017) Acute exposure to worst-case concentrations of amitraz does not affect honey bee learning, short-term memory, or hemolymph octopamine levels. J Econ Entomol 110:127–132. https://doi.org/10.1093/jee/tow250
Scheiner R, Erber J, Page RE (1999) Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.). J Comp Physiol A 185:1–10. https://doi.org/10.1007/s003590050360
Schneider CW, Tautz J, Grünewald B, Fuchs S (2012a) RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7:e30023. https://doi.org/10.1371/journal.pone.0030023
Schneider S, Eisenhardt D, Rademacher E (2012b) Sublethal effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae): changes in behaviour and longevity. Apidologie 43:218–225. https://doi.org/10.1007/s13592-011-0102-0
Schuhmann A, Scheiner R (2023) A combination of the frequent fungicides boscalid and dimoxystrobin with the neonicotinoid acetamiprid in field-realistic concentrations does not affect sucrose responsiveness and learning behavior of honeybees. Ecotox Environ Safe 256:114850. https://doi.org/10.1016/j.ecoenv.2023.114850
Schwartz KR, Minor H, Magro C, McConnell J, Capani J, Griffin J, Doebel H (2021) The neonicotinoid imidacloprid alone alters the cognitive behavior in Apis mellifera L. and the combined exposure of imidacloprid and Varroa destructor mites synergistically contributes to trial attrition. J Apicult Res 60:431–438. https://doi.org/10.1080/00218839.2020.1866233
Shapira M, Thompson CK, Soreq H, Robinson GE (2001) Changes in neuronal acetylcholinesterase gene expression and division of labor in honey bee colonies. J Mol Neurosci 17:1–12. https://doi.org/10.1385/JMN:17:1:1
Shepherd S, Lima MAP, Oliveira EE, Sharkh SM, Aonuma H, Jackson CW, Newland PL (2021) Sublethal neonicotinoid exposure attenuates the effects of electromagnetic fields on honey bee flight and learning. Environmental Advances 4:100051. https://doi.org/10.1016/j.envadv.2021.100051
Siviter H, Koricheva J, Brown MJF, Leadbeater E (2018) Quantifying the impact of pesticides on learning and memory in bees. J Appl Ecol 55:2812–2821. https://doi.org/10.1111/1365-2664.13193
Siviter H, Scott A, Pasquier G, Pull CD, Brown MJF, Leadbeater E (2019) No evidence for negative impacts of acute sulfoxaflor exposure on bee olfactory conditioning or working memory. PeerJ 7:e7208. https://doi.org/10.7717/peerj.7208
Siviter H, Bailes EJ, Martin CD, Oliver TR, Koricheva J, Leadbeater E, Brown MJF (2021) Agrochemicals interact synergistically to increase bee mortality. Nature 596:389–392. https://doi.org/10.1038/s41586-021-03787-7
Smith BH, Burden CM (2014) A proboscis extension response protocol for investigating behavioral plasticity in insects: application to basic, biomedical, and agricultural research. JoVE-J vis Exp 91:e51057. https://doi.org/10.3791/51057
Smith DB, Arce AN, Ramos Rodrigues A, Bischoff PH, Burris D, Ahmed F, Gill RJ (2020) Insecticide exposure during brood or early-adult development reduces brain growth and impairs adult learning in bumblebees. P R Soc B 287:20192442. https://doi.org/10.1098/rspb.2019.2442
Sol Balbuena M, Tison L, Hahn M-L, Greggers U, Menzel R, Farina WM (2015) Effects of sublethal doses of glyphosate on honeybee navigation. J Exp Biol 218:2799–2805. https://doi.org/10.1242/jeb.117291
Stone JC, Abramson CI, Price JM (1997) Task-dependent effects of dicofol (Kelthane) on learning in the honey bee (Apis mellifera). B Environ Contam Tox 58:177–183. https://doi.org/10.1007/s001289900317
Takeda K (1961) Classical conditioned response in the honey bee. J Insect Physiol 6:168–179. https://doi.org/10.1016/0022-1910(61)90060-9
Tan K, Chen W, Dong S, Liu X, Wang Y, Nieh JC (2015) A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci Rep 5:10989. https://doi.org/10.1038/srep10989
Tan K, Wang C, Dong S, Li X, Nieh JC (2017) The pesticide flupyradifurone impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci Rep 7:17772. https://doi.org/10.1038/s41598-017-18060-z
Taylor KS, Waller GD, Crowder LA (1987) Impairment of a classical conditioned response of the honey bee (Apis mellifera L.) by sublethal doses of synthetic pyrethroid insecticides. Apidologie 18:243–252. https://doi.org/10.1051/apido:19870304
Thany SH, Bourdin CM, Graton J, Laurent AD, Mathé-Allainmat M, Lebreton J, Le Questel J-Y (2015) Similar comparative low and high doses of deltamethrin and acetamiprid differently impair the retrieval of the proboscis extension reflex in the forager honey bee (Apis mellifera). InSects 6:805–814. https://doi.org/10.3390/insects6040805
Tison L, Holtz S, Adeoye A, Kalkan Ö, Irmisch NS, Lehmann N, Menzel R (2017) Effects of sublethal doses of thiacloprid and its formulation Calypso® on the learning and memory performance of honey bees. J Exp Biol 220:3695–3705. https://doi.org/10.1242/jeb.154518
Tison L, Rößner A, Gerschewski S, Menzel R (2019) The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotox Environ Safe 180:139–145. https://doi.org/10.1016/j.ecoenv.2019.05.007
Tison L, Duer A, Púčiková V, Greggers U, Menzel R (2020) Detrimental effects of clothianidin on foraging and dance communication in honey bees. PLoS ONE 15:e0241134. https://doi.org/10.1371/journal.pone.0241134
Traynor KS, Pettis JS, Tarpy DR, Mullin CA, Frazier JL, Frazier M, vanEngelsdorp D (2016) In-hive pesticide exposome: assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci Rep 6:33207. https://doi.org/10.1038/srep33207
Traynor KS, Tosi S, Rennich K, Steinhauer N, Forsgren E, Rose R, Kunkel G, Madella S, Lopez D, Eversole H, Fahey R, Pettis J, Evans JD, vanEngelsdorp D (2021) Pesticides in honey bee colonies: establishing a baseline for real world exposure over seven years in the USA. Environ Pollut 279:116566. https://doi.org/10.1016/j.envpol.2021.116566
Urlacher E, Monchanin C, Rivière C, Richard F-J, Lombardi C, Michelsen-Heath S, Hageman KJ, Mercer AR (2016) Measurements of chlorpyrifos levels in forager bees and comparison with levels that disrupt honey bee odor-mediated learning under laboratory conditions. J Chem Ecol 42:127–138. https://doi.org/10.1007/s10886-016-0672-4
Van Dame R, Meled M, Colin M-E, Belzunces LP (1995) Alteration of the homing-flight in the honey bee Apis mellifera L. Exposed to sublethal dose of deltamethrin. Environ Toxicol Chem 14:855–860. https://doi.org/10.1002/etc.5620140517
Weick J, Thorn RS (2002) Effects of acute sublethal exposure to coumaphos or diazinon on acquisition and discrimination of odor stimuli in the honey bee (Hymenoptera: Apidae). J Econ Entomol 95:227–236. https://doi.org/10.1603/0022-0493-95.2.227
Williamson SM, Wright GA (2013) Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J Exp Biol 216:1799–1807. https://doi.org/10.1242/jeb.083931
Williamson SM, Baker DD, Wright GA (2013) Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee Apis mellifera. Invertebr Neurosci 13:63–70. https://doi.org/10.1007/s10158-012-0144-7
Wright GA, Softley S, Earnshaw H (2015) Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci Rep 5:15322. https://doi.org/10.1038/srep15322
Wu X, Li Z, Yang H, He X, Yan W, Zeng Z (2023) The adverse impact on lifespan, immunity, and forage behavior of worker bees (Apis mellifera Linnaeus 1758) after exposure to flumethrin. Sci Total Environ 858:160146. https://doi.org/10.1016/j.scitotenv.2022.160146
Yang E-C, Chuang YC, Chen YL, Chang LH (2008) Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol 101:1743–1748. https://doi.org/10.1603/0022-0493-101.6.1743
Yang E-C, Chang H-C, Wu W-Y, Chen Y-W (2012) Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 7:e49472. https://doi.org/10.1371/journal.pone.0049472
Zhang E, Nieh JC (2015) The neonicotinoid imidacloprid impairs honey bee aversive learning of simulated predation. J Exp Biol 218:3199–3205. https://doi.org/10.1242/jeb.127472
Funding
This work was supported by the US Department of Agriculture (grant numbers 2017-68004-26322 and 2022-67013-36285).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of the review. NSD defined the scope, performed the literature review, and wrote the first draft of the manuscript. All authors revised the manuscript, and all read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: James Nieh
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
DesJardins, N.S., Harrison, J.F. & Smith, B.H. The effects of anthropogenic toxins on honey bee learning: Research trends and significance. Apidologie 54, 59 (2023). https://doi.org/10.1007/s13592-023-01040-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01040-w